Microcapsule Preparation and Properties of Flavonoid Extract from Immature Citrus reticulata ‘Chachiensis’ Peel
Abstract
1. Introduction
2. Materials, Instruments, and Methods
2.1. Materials and Instruments
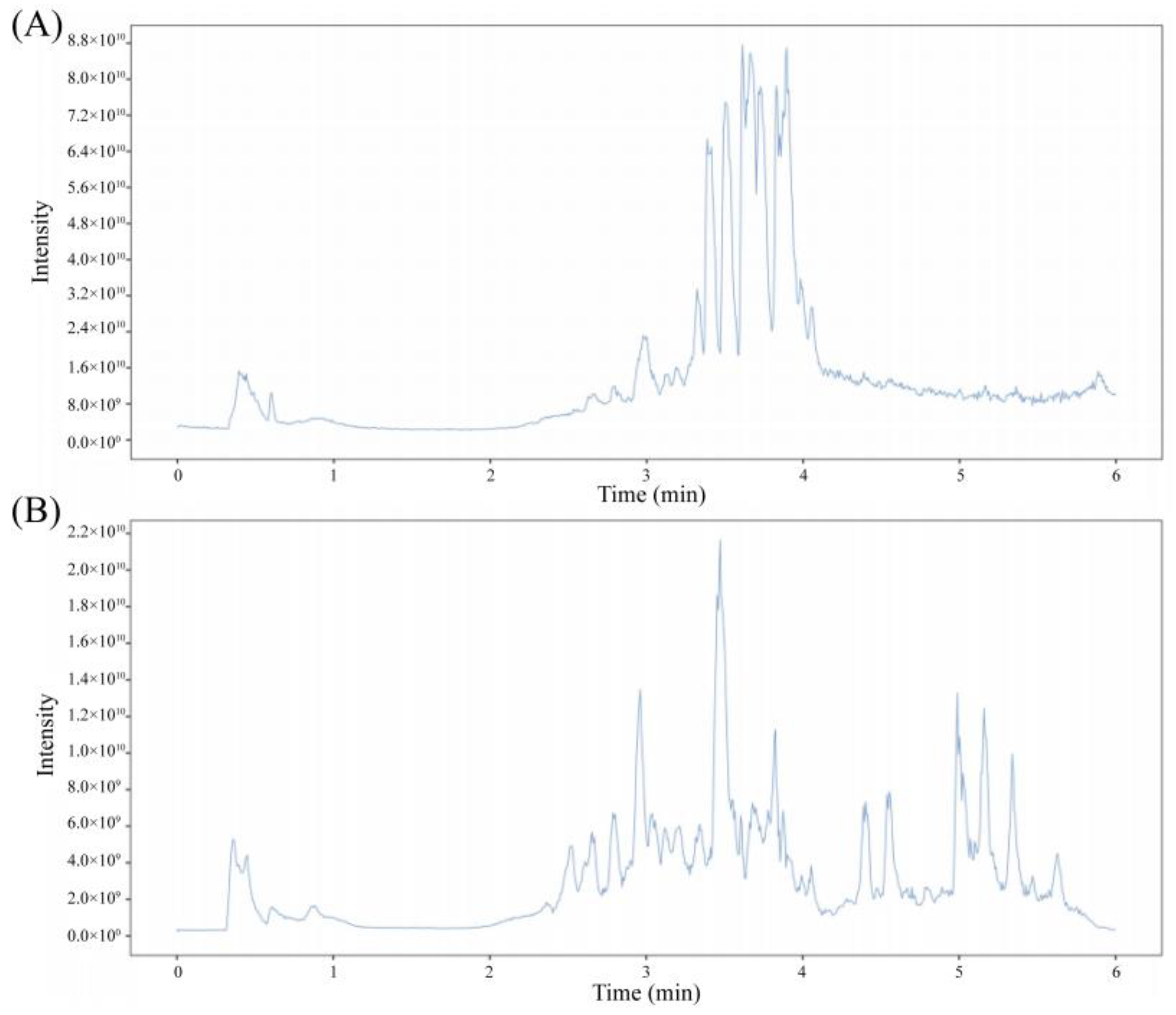
2.2. UHPLC-OE-MS Detection Method and Conditions
2.3. Preparation and Wall Material Screening of CCE Microcapsules
2.4. Determination of Flavonoid Content and EE of CCE Microcapsules
2.4.1. Determination of Flavonoid Content
2.4.2. Determination of EE
2.5. Single-Factor Experiments with PPMD-CCE
2.6. PPMD-CCE Response Surface Experiment
2.7. Characterization of CCE Microcapsules
2.7.1. Measurement of Particle Size and Zeta Potential
2.7.2. Color Measurement
2.7.3. Moisture Content
2.7.4. Water Solubility Index
2.7.5. Bulk Density
2.7.6. Liquidity
2.7.7. Hygroscopicity
2.7.8. Scanning Electron Microscopy (SEM)
2.7.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.7.10. X-ray Diffraction (XRD)
2.7.11. Differential Scanning Calorimetry (DSC)
2.8. Antioxidant Capacity of CCE Microcapsules
2.8.1. DPPH Radical Scavenging
2.8.2. ABTS Radical Scavenging
2.9. The Simulated Gastrointestinal Digestion Experiment
2.10. Storage Stability of CCE Microcapsules
2.10.1. Temperature Stability
2.10.2. Light Stability
2.11. Statistical Analysis
3. Results and Discussion
3.1. UHPLC-OE-MS Detection Results
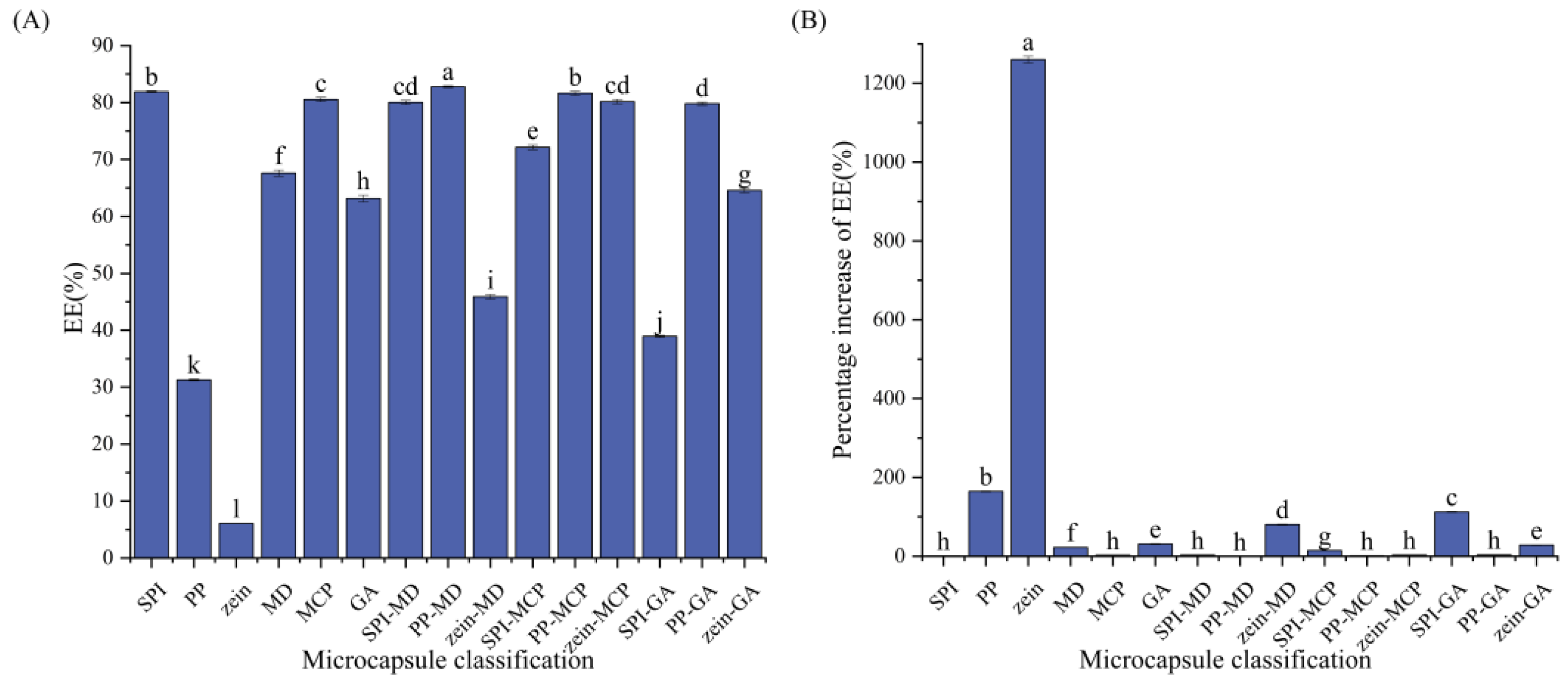
3.2. Determination of the CCE Microcapsule Wall Material
3.3. PPMD-CCE Microcapsule Preparation Process Optimization
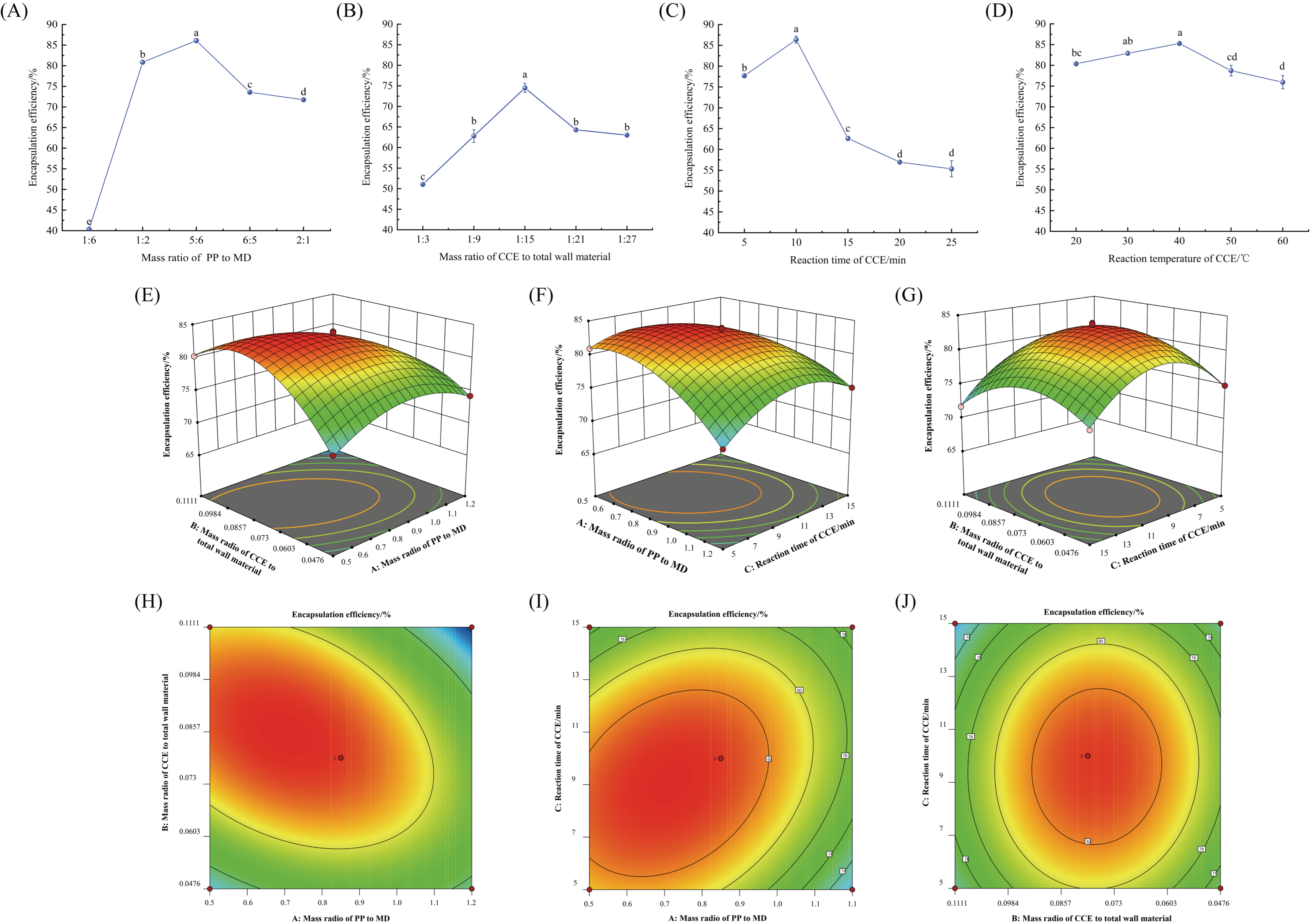
3.3.1. Single-Factor Experiment
3.3.2. Response Surface Optimization Test
3.3.3. Optimization and Verification of the PPMD-CCE Preparation Process
3.4. The Physicochemical Properties of CCE Microcapsules
3.4.1. Particle Size and the Zeta Potential
3.4.2. Color
3.4.3. Other Physicochemical Properties
3.5. Characterization of the CCE and Its Microcapsules
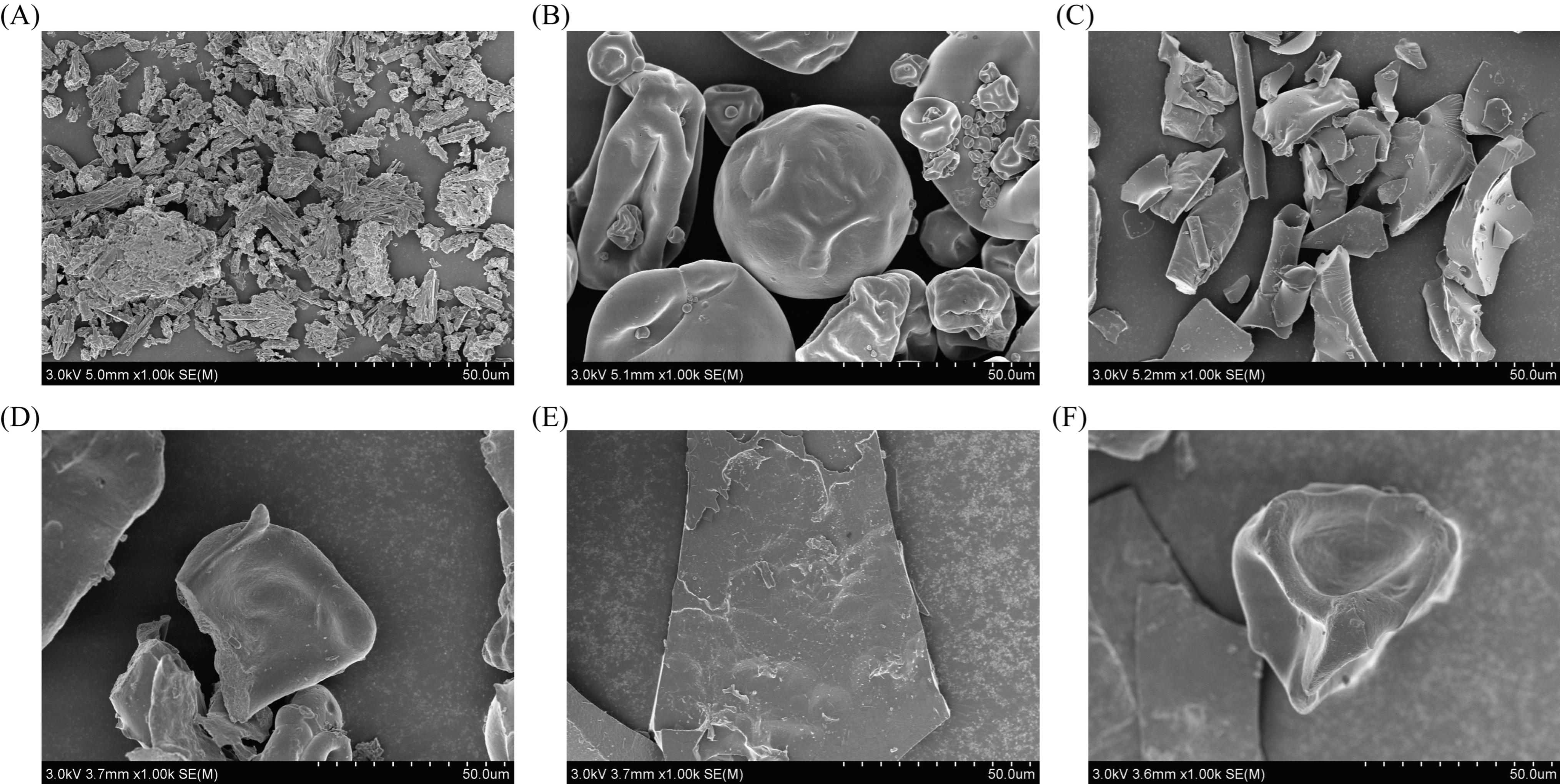
3.5.1. SEM
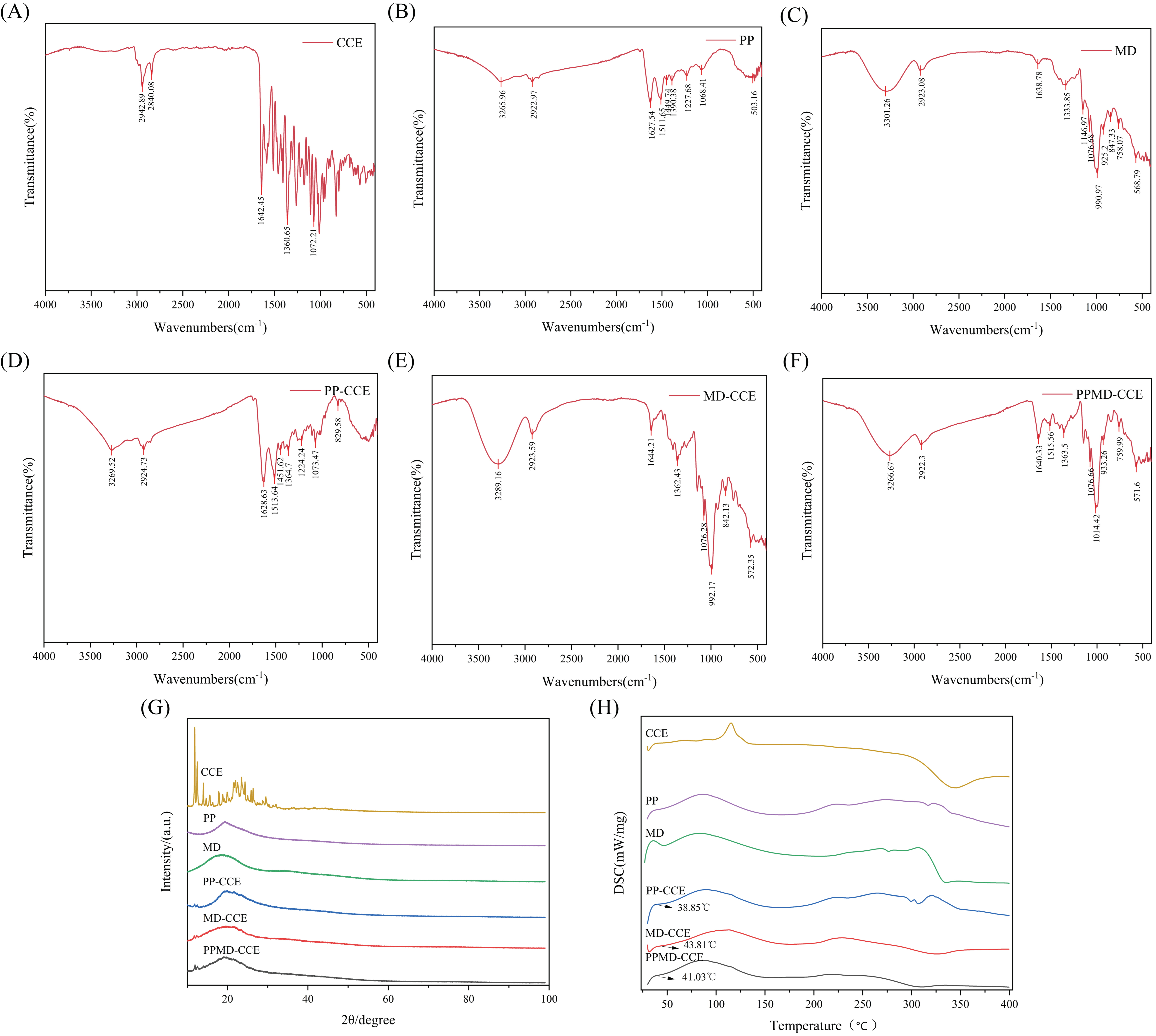
3.5.2. FTIR Spectroscopy Analysis
3.5.3. XRD Analysis
3.5.4. DSC Analysis
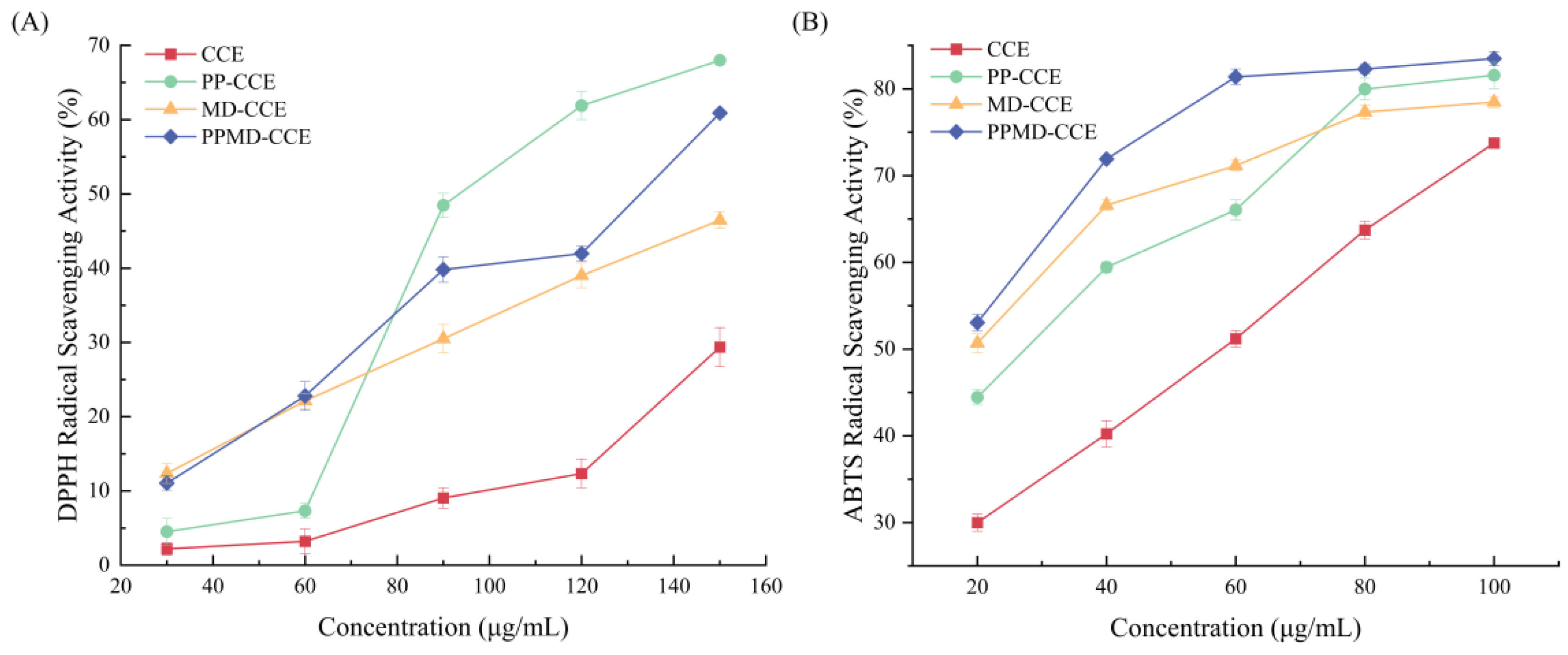
3.6. In Vitro Antioxidant Capacity of CCE and Its Microcapsules
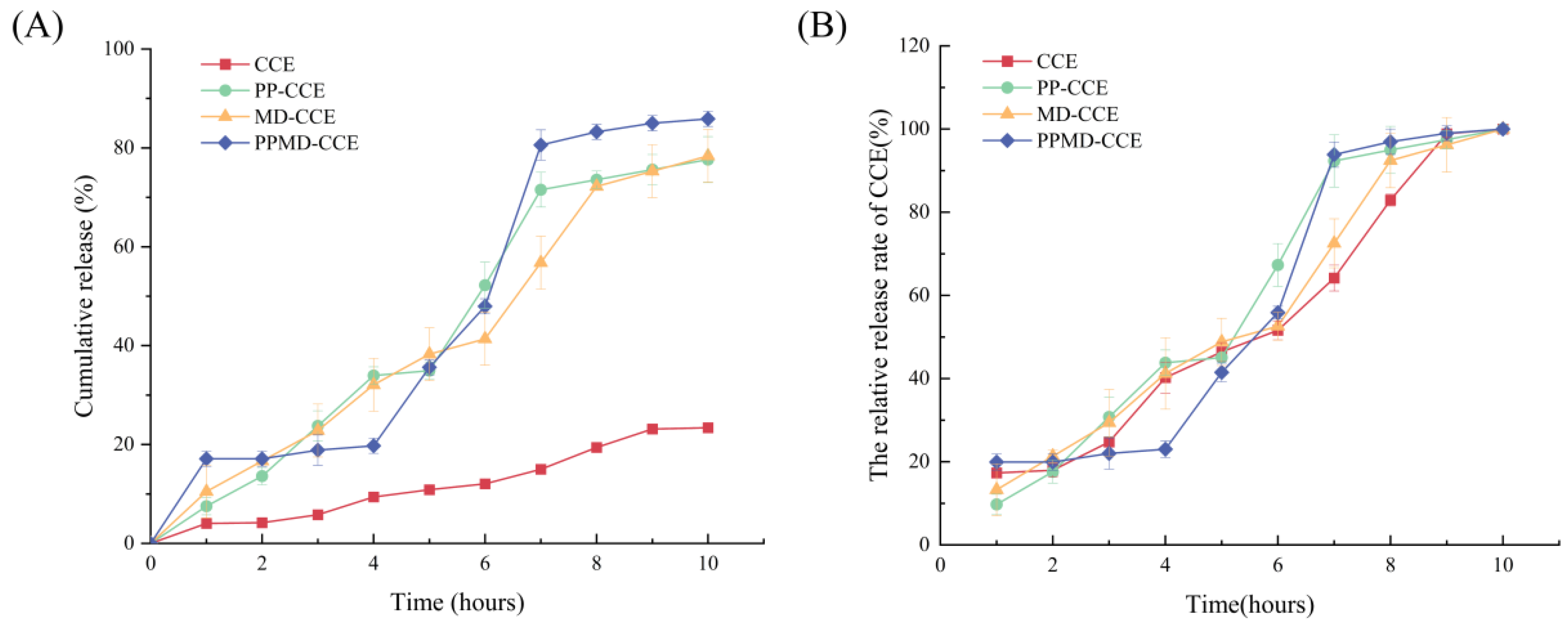
3.7. In Vitro Gastrointestinal Digestion and Release of CCE and Its Microcapsules
3.8. Storage Stability of CCE and Its Microcapsules
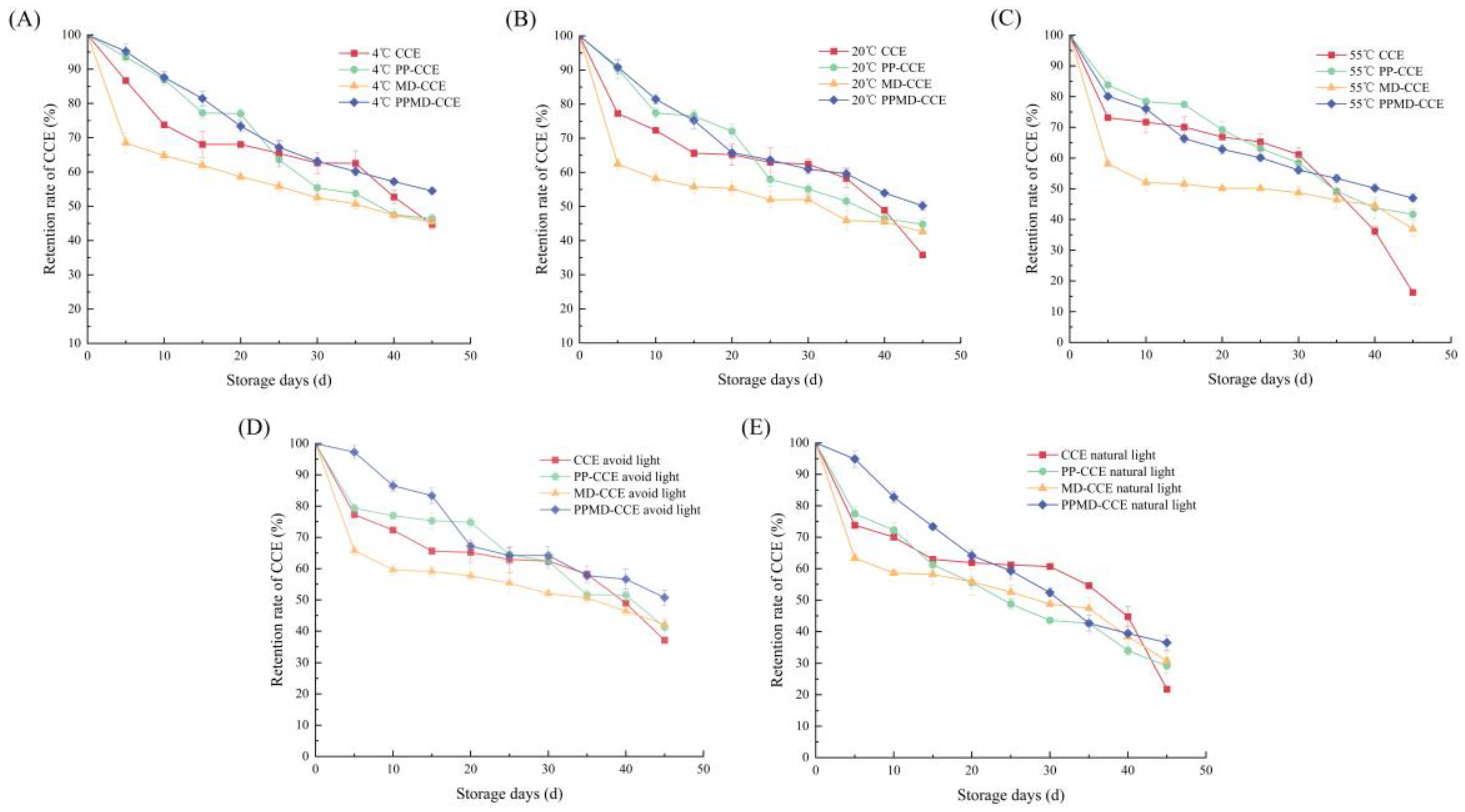
3.8.1. Temperature Stability Experiment Results
3.8.2. Light Stability Experiment Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, X.; Chen, J.; Chen, J.; Li, A.; Li, Q.; Bai, L.; Zhang, Q.; Wu, H.; Liu, W. Identification and Quality Evaluation of Citrus reticulata Cv. Chachiensis Varieties Based on SNP Markers. Arab. J. Chem. 2023, 16, 104948. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, R.; Zhao, Z.; Zheng, G.; Xu, X.; Yang, D. Extraction Process and Method Validation for Bioactive Compounds from Citrus reticulata Cv. Chachiensis: Application of Response Surface Methodology and HPLC-DAD. ACTA Chromatogr. 2021, 33, 270–280. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of Flavonoid and Antioxidant Activity of Fruit Tissues from 27 Chinese Local Citrus Cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Zhang, R.; Zhao, Z.; Xu, X.; Chen, B.; Yang, D.; Zheng, G. Characterization of Antioxidant Compounds Extracted from Citrus reticulata Cv. Chachiensis Using UPLC-Q-TOF-MS/MS, FT-IR and Scanning Electron Microscope. J. Pharm. Biomed. Anal. 2021, 192, 113683. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lu, S. Citri Reticulatae Pericarpium (Chenpi): Botany, Ethnopharmacology, Phytochemistry, and Pharmacology of a Frequently Used Traditional Chinese Medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef]
- Li, P.; Yao, X.; Zhou, Q.; Meng, X.; Zhou, T.; Gu, Q. Citrus Peel Flavonoid Extracts: Health-Beneficial Bioactivities and Regulation of Intestinal Microecology in Vitro. Front. Nutr. 2022, 9, 888745. [Google Scholar] [CrossRef]
- Navarro-Martinez, P.; Noguera-Artiaga, L.; Sanchez-Bravo, P.; Rosas-Burgos, E.C.; Perez-Lopez, A.J.; Carbonell-Barrachina, A.A. Development and Characterization of Liquors Prepared with an Underutilized Citrus By-Product, the Peel. Eur. Food Res. Technol. 2019, 245, 41–50. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. Legume Proteins Are Smart Carriers to Encapsulate Hydrophilic and Hydrophobic Bioactive Compounds and Probiotic Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1250–1279. [Google Scholar] [CrossRef]
- Islam, F.; Ali, Y.A.; Imran, A.; Afzaal, M.; Zahra, S.M.; Fatima, M.; Saeed, F.; Usman, I.; Shehzadi, U.; Mehta, S.; et al. Vegetable Proteins as Encapsulating Agents: Recent Updates and Future Perspectives. Food Sci. Nutr. 2023, 11, 1705–1717. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Li, Y. Pea Protein Composition, Functionality, Modification, and Food Applications: A Review. Adv. Food Nutr. Res. 2022, 101, 71–127. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Wang, S.; Li, B.; Liang, H. Nanoparticle Encapsulation Strategy: Leveraging Plant Exine Capsules Used as Secondary Capping for Oral Delivery. J. Agric. Food Chem. 2019, 67, 8168–8176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Wang, J.; Cui, S.W.; Wang, S.; Wang, B.; Zhang, N.; Yang, X.; Li, J.; Wang, H. Fabrication, Characterization, and Lipid-Lowering Effects of Naringenin-Zein-Sodium Caseinate-Galactosylated Chitosan Nanoparticles. Int. J. Biol. Macromol. 2023, 230, 123150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cao, W.; Li, L.; Ren, A.; Ang, Y.; Chen, J.; Bhandari, B.; Wang, Z.; Ren, X.; Ren, G.; et al. Effects of Different Proteins and Maltodextrin Combinations as Wall Material on the Characteristics of Cornus Officinalis Flavonoids Microcapsules. Front. Nutr. 2022, 9, 1007863. [Google Scholar] [CrossRef] [PubMed]
- Konare, M.A.; Condurache, N.N.; Togola, I.; Pacularu-Burada, B.; Diarra, N.; Stanciuc, N.; Rapeanu, G. Valorization of Bioactive Compounds from Two Underutilized Wild Fruits by Microencapsulation in Order to Formulate Value-Added Food Products. Plants 2023, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.P.; Shivhare, U.S. Microencapsulation by Complex Coacervation Using Whey Protein Isolates and Gum Acacia: An Approach to Preserve the Functionality and Controlled Release of β-Carotene. Food Bioprocess Technol. 2015, 8, 1635–1644. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of Fingered Citron Extract with Gum Arabic, Modified Starch, Whey Protein, and Maltodextrin Using Spray Drying. Int. J. Biol. Macromol. 2020, 152, 1125–1134. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Grace, M.H.; Xiong, J.; Lila, M.A. Spray-drying Microencapsulation of Blackcurrant and Cocoa Polyphenols Using Underexplored Plant-based Protein Sources. J. Food Sci. 2023, 88, 2665–2678. [Google Scholar] [CrossRef]
- Wang, M.; Gao, F.; Zheng, H.; Zhang, T.; Guo, M. Microencapsulation of Ginsenosides Using Polymerised Whey Protein (PWP) as Wall Material and Its Application in Probiotic Fermented Milk. Int. J. Food Sci. Technol. 2017, 52, 1009–1017. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Zhang, W.; Kou, G.; Zhou, Z. Physical Stability and Antioxidant Activity of Citrus Flavonoids in Arabic Gum-Stabilized Microcapsules: Modulation of Whey Protein Concentrate. Food Hydrocoll. 2018, 77, 588–597. [Google Scholar] [CrossRef]
- He, Y.; Ye, Z.; Liu, X.; Wei, Z.; Qiu, F.; Li, H.-F.; Zheng, Y.; Ouyang, D. Can Machine Learning Predict Drug Nanocrystals? J. Control. Release 2020, 322, 274–285. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, W.; Huang, Y. Encapsulation of Tartary Buckwheat Flavonoids and Application to Yoghurt. J. Microencapsul. 2020, 37, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, T.; Drvenica, I.; Kalusevic, A.; Marijanovic, Z.; Jerkovic, I.; Muzek, M.N.; Bratanic, A.; Skroza, D.; Zoric, Z.; Pedisic, S.; et al. Influences of Freeze- and Spray-Drying vs. Encapsulation with Soy and Whey Proteins on Gastrointestinal Stability and Antioxidant Activity of Mediterranean Aromatic Herbs. Int. J. Food Sci. Technol. 2021, 56, 1582–1596. [Google Scholar] [CrossRef]
- Bandekar, J. Amide Modes and Protein Conformation. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Lin, J.; Liu, Y. Inclusion Complexation Behaviors of 3-Tigloyl-Azadirachtol with β-Cyclodextrin Derivatives. Curr. Pharm. Anal. 2008, 4, 176–182. [Google Scholar] [CrossRef]
- Nikolic, V.; Stankovic, M.; Kapor, A.; Nikolic, L.; Cvetkovic, D.; Stamenković, J. Allylthiosulfinate: Beta-Cyclodextrin Inclusion Complex: Preparation, Characterization and Microbiological Activity. Pharmazie 2004, 59, 845–848. [Google Scholar]
- Nallamuthu, I.; Ponnusamy, V.; Smruthi, M.R.; Khanum, F. Formulation of Naringin Encapsulation in Zein/Caseinate Biopolymers and Its Anti-Adipogenic Activity in 3T3-L1 Pre-Adipocytes. J. Clust. Sci. 2020, 32, 1649–1662. [Google Scholar] [CrossRef]
- Pool, H.; Quintanar, D.; Figueroa, J.D.D.; Bechara, J.E.H.; Mcclements, D.J.; Mendoza, S. Polymeric Nanoparticles as Oral Delivery Systems for Encapsulation and Release of Polyphenolic Compounds: Impact on Quercetin Antioxidant Activity & Bioaccessibility. Food Biophys. 2012, 7, 276–288. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Jiang, K.-M.; Xie, X.-G.; Jin, Y.; Lin, J. Water-Soluble Inclusion Complexes of Trans-Polydatin by Cyclodextrin Complexation: Preparation, Characterization and Bioactivity Evaluation. J. Mol. Liq. 2016, 219, 592–598. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of Curcumin Using Coconut Milk Whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; Gutierrez, N.; Sanchez, P.; Romero, A.; Luis Valverde, J. Smart Microcapsules Containing Nonpolar Chemical Compounds and Carbon Nanofibers. Chem. Eng. J. 2012, 181, 813–822. [Google Scholar] [CrossRef]
- Hu, Y.; Kou, G.; Chen, Q.; Li, Y.; Zhou, Z. Protection and Delivery of Mandarin (Citrus reticulate Blanco) Peel Extracts by Encapsulation of Whey Protein Concentrate Nanoparticles. LWT—Food Sci. Technol. 2019, 99, 24–33. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Corke, H.; Gul, K.; Gan, R.-Y.; Fang, Y. The Health Benefits, Functional Properties, Modifications, and Applications of Pea (Pisum sativum L.) Protein: Current Status, Challenges, and Perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of Phenolic Extraction from Averrhoa Carambola Pomace by Response Surface Methodology and Its Microencapsulation by Spray and Freeze Drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, J.R.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; de Menezes, C.R.; da Rosa, C.S. Microencapsulation of Anthocyanin Compounds Extracted from Blueberry (Vaccinium spp.) by Spray Drying: Characterization, Stability and Simulated Gastrointestinal Conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Yang, J.; Sagis, L.M.C. Interfacial Behavior of Plant Proteins—Novel Sources and Extraction Methods. Curr. Opin. Colloid Interface Sci. 2021, 56, 101499. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of Wheat, Soybean, Rice, and Pea Protein Properties for Effective Applications in Food Products. J. Food Biochem. 2020, 44, e13157. [Google Scholar] [CrossRef]
- Ducel, V.; Richard, J.; Popineau, Y.; Boury, F. Adsorption Kinetics and Rheological Interfacial Properties of Plant Proteins at the Oil-Water Interface. Biomacromolecules 2004, 5, 2088–2093. [Google Scholar] [CrossRef]
- Tang, J.; Wichers, H.J.; Hettinga, K.A. Heat-Induced Unfolding Facilitates Plant Protein Digestibility during in Vitro Static Infant Digestion. Food Chem. 2022, 375, 131878. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Vongsvivut, J.; Ye, Q.; Selomulya, C. On Surface Composition and Stability of β-Carotene Microcapsules Comprising Pea/Whey Protein Complexes by Synchrotron-FTIR Microspectroscopy. Food Chem. 2023, 426, 136565. [Google Scholar] [CrossRef]
- Yao, X.; Xiang, S.; Nie, K.; Gao, Z.; Jiang, F. Whey Protein Isolate/Gum Arabic Intramolecular Soluble Complexes Improving the Physical and Oxidative Stabilities of Conjugated Linoleic Acid Emulsions. RSC Adv. 2016, 6, 14635–14642. [Google Scholar] [CrossRef]
- Febrianta, H.; Yunianto, V.D.; Nurwantoro, N.; Bintoro, V.P. Freeze-Drying Microencapsulation of Turmeric (Curcuma longa L.) Using an Amorphous Matrix of Maltodextrin, Modified Cassava Flour and Skim Milk. Ann. Univ. Dunarea de Jos Galati. Fascicle VI Food Technol. 2020, 44, 26–42. [Google Scholar] [CrossRef]
- Kopjar, M.; Buljeta, I.; Orkovi, I.; Kelemen, V.; Imunovi, J.; Pichler, A. Plant-Based Proteins as Encapsulating Materials for Glucosyl-Hesperidin. Int. J. Food Sci. Technol. 2022, 57, 728–737. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Sharayei, P.; Zomorodi, S.; Ramaswamy, H.S. Physicochemical and Phytochemical Characterization and Storage Stability of Freeze-Dried Encapsulated Pomegranate Peel Anthocyanin and In Vitro Evaluation of Its Antioxidant Activity. Food Bioprocess Technol. 2019, 12, 199–210. [Google Scholar] [CrossRef]
- Hayes, M.; Ferruzzi, M.G. Update on the Bioavailability and Chemopreventative Mechanisms of Dietary Chlorophyll Derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The Relative Contribution of the Small and Large Intestine to the Absorption and Metabolism of Rutin in Man. Free Radic. Res. 2006, 40, 1035. [Google Scholar] [CrossRef]
- Wang, S.; Ye, X.; Sun, Y.; Liang, J.; Yue, P.; Gao, X. Nanocomplexes Derived from Chitosan and Whey Protein Isolate Enhance the Thermal Stability and Slow the Release of Anthocyanins in Simulated Digestion and Prepared Instant Coffee. Food Chem. 2021, 336, 127707. [Google Scholar] [CrossRef]
- Tchabo, W.; Kaptso, G.K.; Ngolong, N.G.L.; Wang, K.; Bao, G.; Ma, Y.; Wang, X.; Mbofung, C.M. In Vitro Assessment of the Effect of Microencapsulation Techniques on the Stability, Bioaccessibility and Bioavailability of Mulberry Leaf Bioactive Compounds. Food Biosci. 2022, 47, 101461. [Google Scholar] [CrossRef]
- Yadav, K.; Bajaj, R.K.; Mandal, S.; Mann, B. Encapsulation of Grape Seed Extract Phenolics Using Whey Protein Concentrate, Maltodextrin and Gum Arabica Blends. J. Food Sci. Technol. 2020, 57, 426–434. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In Vitro Gastrointestinal Digestion and Fecal Fermentation Reveal the Effect of Different Encapsulation Materials on the Release, Degradation and Modulation of Gut Microbiota of Blueberry Anthocyanin Extract. Food Res. Int. Ott. Ont. 2020, 132, 109098. [Google Scholar] [CrossRef]
- Sarkar, A.; Horne, D.S.; Singh, H. Pancreatin-Induced Coalescence of Oil-in-Water Emulsions in an in Vitro Duodenal Model. Int. Dairy J. 2010, 20, 589–5976. [Google Scholar] [CrossRef]
- Barbosa, A.E.G.; Bastos, L.P.H.; Garcia-Rojas, E.E. Complex Coacervates Formed between Whey Protein Isolate and Carboxymethylcellulose for Encapsulation of β-Carotene from Sacha Inchi Oil: Stability, In Vitro Digestion and Release Kinetics. Food Biophys. 2021, 16, 293–305. [Google Scholar] [CrossRef]
- Giovagnoli-Vicuña, C.; Briones-Labarca, V.; Romero, M.S.; Giordano, A.; Pizarro, S. Effect of Extraction Methods and In Vitro Bio-Accessibility of Microencapsulated Lemon Extract. Molecules 2022, 27, 4166. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.A.; Cano-Higuita, D.M.; de Oliveira, R.A.; Telis, V.R.N. Influence of Different Combinations of Wall Materials on the Microencapsulation of Jussara Pulp (Euterpe edulis) by Spray Drying. Food Chem. 2016, 212, 1–9. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Spray Freeze Drying Method for Microencapsulation of Lactobacillus Plantarum. J. Food Eng. 2015, 166, 95–103. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of Wall Materials on the Spray Drying Efficiency, Powder Properties and Stability of Bioactive Compounds in Tamarillo Juice Microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Alhelli, A.M.; Hussin, A.S.M. Influence of Different Combinations of Wall Materials on Encapsulation of Nigella Sativa Oil by Spray Dryer. J. Food Process. Eng. 2021, 44, e13639. [Google Scholar] [CrossRef]

| Factor | Coding Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Mass ratio of PP to MD (A) | 1:2 | 5:6 | 6:5 |
| Mass ratio of CCE to total wall material (B) | 1:9 | 1:15 | 1:21 |
| CCE response time (C) | 5 min | 10 min | 15 min |
| Pound Name | CAS | RT/min | m/z | Peak Area | Proportion of Peak Area in Flavonoids/% |
|---|---|---|---|---|---|
| Hexamethylquercetagetin | 1251-84-9 | 219.7 | 403.1383 | 92,268,427,012 | 79.7813 |
| 3′-Hydroxy-7,8,4′,5′-tetramethoxyflavone | 133342-98-0 | 213.4 | 359.1121 | 8,889,119,462 | 7.6861 |
| Heptamethoxyflavone | 1178-24-1 | 221 | 433.1487 | 2,596,011,951 | 2.2447 |
| Hesperetin 7-neohesperidoside | 13241-33-3 | 177.4 | 611.1962 | 1,472,503,392 | 1.2732 |
| Hesperidin | 520-26-3 | 177.4 | 611.1962 | 1,472,503,392 | 1.2732 |
| Narirutin | 14259-46-2 | 176.2 | 579.1725 | 731,371,913.4 | 0.6324 |
| Hesperetin 7-O-glucoside | 31712-49-9 | 182.3 | 463.125 | 674,188,098.5 | 0.5829 |
| Pectolinarigenin | 520-12-7 | 182.1 | 315.0859 | 628,925,109.1 | 0.5438 |
| Isosakuranetin | 231.3 | 285.0769 | 458,444,185.7 | 0.3964 | |
| Citromitin | 212.6 | 405.154 | 433,063,308.9 | 0.3745 |
| Wall Material | EE/% | Percentage Increase in EE/% | 95% CI |
|---|---|---|---|
| SPI | 81.90 ± 0.17 b | 1.080 ± 0.28 h | [81.49, 82.32] |
| PP | 31.29 ± 0.15 k | 164.55 ± 1.24 b | [30.92, 31.67] |
| zein | 6.09 ± 0.03 l | 1260.16 ± 8.43 a | [6.02, 6.15] |
| MD | 67.59 ± 0.55 f | 22.49 ± 0.85 f | [66.23, 68.96] |
| MCP | 80.54 ± 0.38 c | 2.79 ± 0.32 h | [79.60, 81.47] |
| GA | 63.14 ± 0.60 h | 31.12 ± 1.29 e | [61.66, 64.62] |
| SPI-MD | 80.02 ± 0.36 cd | 3.46 ± 0.38 h | [79.13, 80.90] |
| PP-MD | 82.79 ± 0.15 a | control group | [82.41, 83.17] |
| zein-MD | 45.89 ± 0.38 i | 80.41 ± 1.27 d | [44.95, 46.84] |
| SPI-MCP | 72.16 ± 0.41 e | 14.73 ± 0.68 g | [71.13, 73.19] |
| PP-MCP | 81.62 ± 0.36 b | 1.43 ± 0.55 h | [80.73, 82.52] |
| zein-MCP | 80.17 ± 0.38 cd | 3.26 ± 0.68 h | [79.22, 81.13] |
| SPI-GA | 38.93 ± 0.20 j | 112.68 ± 1.41 c | [38.42, 39.43] |
| PP-GA | 79.81 ± 0.26 d | 3.73 ± 0.51 h | [79.17, 80.45] |
| zein-GA | 64.57 ± 0.39 g | 28.23 ± 0.99 e | [63.60, 65.52] |
| Run | Factor A (Mass Ratio of PP to MD) | Factor B (Mass Ratio of CCE to Total Wall Material) | Factor C (CCE Response Time) | EE/% |
|---|---|---|---|---|
| 1 | 0 | 1 | 1 | 71.72 |
| 2 | −1 | 0 | 1 | 75.36 |
| 3 | 0 | 0 | 0 | 83.95 |
| 4 | 0 | 0 | 0 | 83.29 |
| 5 | 1 | 1 | 0 | 74.28 |
| 6 | 0 | 1 | −1 | 73.68 |
| 7 | 1 | 0 | −1 | 72.21 |
| 8 | −1 | 1 | 0 | 72.76 |
| 9 | 0 | 0 | 0 | 83.82 |
| 10 | 0 | −1 | −1 | 74.97 |
| 11 | −1 | −1 | 0 | 80.29 |
| 12 | 0 | 0 | 0 | 82.27 |
| 13 | −1 | 0 | −1 | 80.95 |
| 14 | 0 | −1 | 1 | 74.39 |
| 15 | 1 | 0 | 1 | 75.09 |
| 16 | 0 | 0 | 0 | 83.21 |
| 17 | 1 | −1 | 0 | 69.22 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 389.73 | 9 | 43.30 | 145.78 | <0.0001 | significant |
| A | 43.06 | 1 | 43.06 | 144.96 | <0.0001 | **** |
| B | 5.17 | 1 | 5.17 | 17.40 | 0.0042 | ** |
| C | 3.45 | 1 | 3.45 | 11.60 | 0.0114 | * |
| AB | 39.63 | 1 | 39.63 | 133.41 | <0.0001 | **** |
| AC | 17.94 | 1 | 17.94 | 60.38 | 0.0001 | **** |
| BC | 0.4761 | 1 | 0.4761 | 1.60 | 0.2460 | |
| A2 | 50.96 | 1 | 50.96 | 171.57 | <0.0001 | **** |
| B2 | 136.39 | 1 | 136.39 | 459.18 | <0.0001 | **** |
| C2 | 64.92 | 1 | 64.92 | 218.54 | <0.0001 | **** |
| Residual | 2.08 | 7 | 0.2970 | |||
| Lack of Fit | 0.3176 | 3 | 0.1059 | 0.2404 | 0.8644 | |
| Pure Error | 1.76 | 4 | 0.4404 | |||
| Cor Total | 391.81 | 16 |
| Formulation | Particle Size/μm | PDI | Zeta Potential/mV |
|---|---|---|---|
| PP-CCE | 12.2333 ± 0.0929 a | 0.5371 ± 0.0133 b | −29.0867 ± 0.6601 a |
| MD-CCE | 9.9567 ± 0.0681 c | 0.4583 ± 0.0220 c | −52.2267 ± 0.3403 b |
| PPMD-CCE | 10.2733 ± 0.1966 b | 0.5877 ± 0.0216 a | −55.1167 ± 0.7333 c |
| Formulation | L* | a* | b* |
|---|---|---|---|
| CCE | 53.38 ± 0.02 d | 4.72 ± 0.04 a | 18.39 ± 0.05 d |
| PP-CCE | 58.10 ± 0.02 c | 4.77 ± 0.03 a | 21.95 ± 0.03 a |
| MD-CCE | 63.65 ± 0.02 b | 2.74 ± 0.03 b | 19.17 ± 0.03 b |
| PPMD-CCE | 64.02 ± 0.01 a | 2.54 ± 0.02 c | 18.86 ± 0.01 c |
| Formulation | MC/% | WSI/% | WAI/% | BD/g/cm−3 | Liquidity/° | Hygroscopicity/% |
|---|---|---|---|---|---|---|
| PP-CCE | 5.6167 ± 0.6167 b | 14.80 ± 0.0800 c | 4.52 ± 0.0794 a | 0.3896 ± 0.0043 a | 29.4784 ± 0.6910 b | 15.4767 ± 0.0252 a |
| MD-CCE | 4.8900 ± 0.8253 b | 86.48 ± 0.7110 a | 0.28 ± 0.0265 c | 0.3822 ± 0.0042 a | 39.6437 ± 0.7148 a | 15.0533 ± 0.0306 b |
| PPMD-CCE | 6.9467 ± 0.2250 a | 49.02 ± 0.0640 b | 2.06 ± 0.0300 b | 0.3261 ± 0.0031 b | 38.3254 ± 0.5791 a | 15.0900 ± 0.0300 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Q.; Wu, S.; Liu, Y.; Chen, J.; Li, T.; Su, D. Microcapsule Preparation and Properties of Flavonoid Extract from Immature Citrus reticulata ‘Chachiensis’ Peel. Foods 2024, 13, 3096. https://doi.org/10.3390/foods13193096
Zhang X, Li Q, Wu S, Liu Y, Chen J, Li T, Su D. Microcapsule Preparation and Properties of Flavonoid Extract from Immature Citrus reticulata ‘Chachiensis’ Peel. Foods. 2024; 13(19):3096. https://doi.org/10.3390/foods13193096
Chicago/Turabian StyleZhang, Xinyi, Qili Li, Sisi Wu, Yan Liu, Jiaxu Chen, Tao Li, and Donglin Su. 2024. "Microcapsule Preparation and Properties of Flavonoid Extract from Immature Citrus reticulata ‘Chachiensis’ Peel" Foods 13, no. 19: 3096. https://doi.org/10.3390/foods13193096
APA StyleZhang, X., Li, Q., Wu, S., Liu, Y., Chen, J., Li, T., & Su, D. (2024). Microcapsule Preparation and Properties of Flavonoid Extract from Immature Citrus reticulata ‘Chachiensis’ Peel. Foods, 13(19), 3096. https://doi.org/10.3390/foods13193096






