Evaluation of Bioactive Compounds and Antioxidant and Cytotoxic Effects of Oil and Pulp without Açaí Fat (Euterpe oleracea) Obtained by Supercritical Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Sample Preparation
2.2. Characterization of the Lyophilized Pulp
2.2.1. Average Particle Diameter
2.2.2. True Density, Apparent Density, and Bed Porosity
2.2.3. Centesimal Composition
2.3. Supercritical Extraction
2.4. Characterization of Obtained Products
2.4.1. Total Phenolic Compounds Content
2.4.2. Total Anthocyanins Content
2.4.3. Carotenoids Content
2.4.4. Antioxidant Activity
DPPH Method (2,2-diphenyl-1-picryl-hydrazyl)
ABTS Method (2,2′-azine-bis 3-ethylbenzoline-6-sulfonic Acid)
Ferric Reducing Antioxidant Power Assay (FRAP)
2.5. Evaluation of Cytotoxic Activity
2.5.1. In Vitro Cell Culture
2.5.2. MTT Essay
2.6. Statistical Analysis of Results
3. Results and Discussion
3.1. Characterization and Pretreatment of Raw Material
3.2. Determination of Global Income
3.3. Phenolic Compounds Content
3.4. Total Anthocyanins Content
3.5. Total Carotenoids Content
3.6. DPPH ASSAY
3.7. ABTS ASSAY
3.8. FRAP METHOD
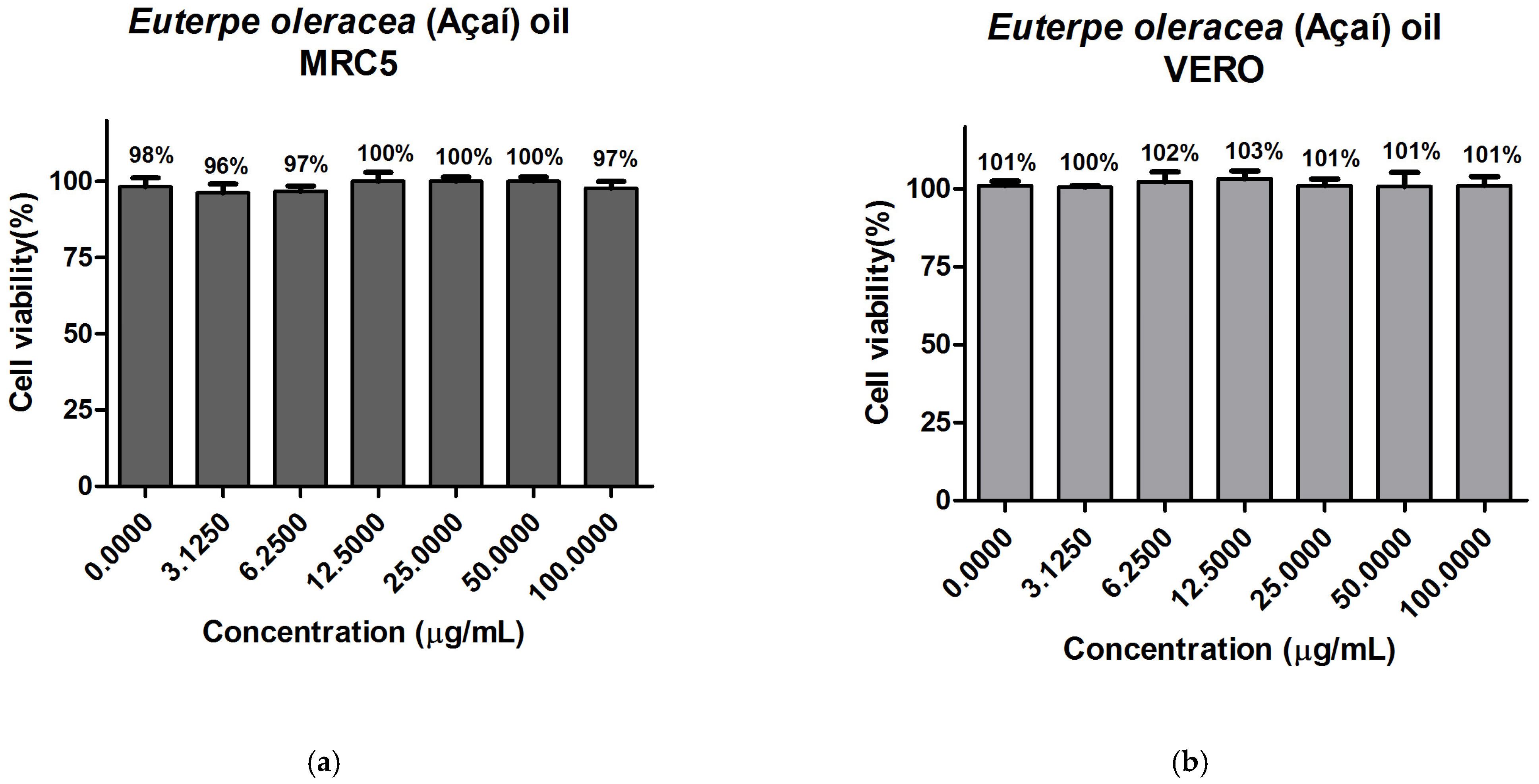
3.9. In Vitro Evaluation of Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siqueira, A.D.; Brondizio, E.S. Local food preference and global markets. Perspectives on açai fruit as terroir and a Geographic Indicator product. Appetite 2011, 56, 544. [Google Scholar] [CrossRef]
- Barbosa, J.R.; De Carvalho, R.N., Jr. Food sustainability trends—How to value the açaí production chain for the development of food inputs from its main bioactive ingredients? Trends Food Sci. Technol. 2022, 124, 86–95. [Google Scholar] [CrossRef]
- de Lima Yamaguchi, K.K.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; Da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, G.R.; Fragoso, M.F.; Borguini, R.G.; De Araújo Santiago, M.C.P.; Fernandes, A.A.H.; Barbisan, L.F. Protective effects of spray-dried açaí (Euterpe oleracea Mart) fruit pulp against initiation step of colon carcinogenesis. Food Res. Int. 2015, 77, 432–440. [Google Scholar] [CrossRef]
- Nascimento, R.J.S.D.; Couri, S.; Antoniassi, R.; Freitas, S.P. Fatty acids composition of açaí pulp oil obtained by enzymatic technology and hexane. Rev. Bras. Frutic. 2008, 30, 498–502. [Google Scholar] [CrossRef]
- Cavalcante, P.; Cedrim, A.S.; Marinho, E.; Barros, A.; Do Nascimento, T.G.; Amélio, P.C. Antioxidant properties of açaí (Euterpe oleraceae Mart.) in the metbolic syndrome. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic composition and antioxidant activity of açaí (Euterpe oleraceae Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef]
- de Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.P.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Euterpe oleraceae Mart. (AÇAÍ) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef]
- Filho, W.E.M.; Almeida-Souza, F.; Vale, A.A.M.; Victor, E.C.; Rocha, M.C.B.; Silva, G.X.; Teles, A.M.; Nascimento, F.R.F.; Moragas-Tellis, C.J.; Chagas, M.D.d.S.; et al. Antitumor Effect of Açaí (Euterpe oleracea Mart.) Seed Extract in LNCaP Cells and in the solid Ehrlich Carcinoma Model. Cancers 2023, 15, 2544. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, E.; Espada-Bellido, C.; Carrera, M.; Palma, J.A.; Álvarez, J.; Ayuso, G.F. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 1: Pressurized Liquid Extraction. Agronomy 2020, 10, 183. [Google Scholar] [CrossRef]
- Da Silveira, T.F.F.; Godoy, H.T. Non-Anthocyanin Phenolic Compounds in Açaí (Euterpe oleracea Mart.) Juice by Ultrahigh-Performance Liquid Chromatography-Diode Array Detector (UHPLC-DAD): A Multivariate Optimization. J. Chromatogr. Sci. 2019, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pscual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS mediated TNF-α and IL-6 production through inhibiting NF-jB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.S.; Musgrave, I.F.; Harvey, B.S.; Smid, S.D. Acaí (Euterpe oleracea Mart.) berry extract exerts neuroprotective effects against amyloidexposure in vitro. Neurosci. Lett. 2013, 556, 221–226. [Google Scholar] [CrossRef]
- Costa, H.C.B.; Silva, D.O.; Vieira, L.G.M. Physical properties of açai-berry pulp and kinetics study of its anthocyanin thermal degradation. J. Food Eng. 2018, 239, 104–113. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef]
- Torma, P.d.C.R.; Carvalho, A.V.; Flores, S.H.; Augusti, P.R.; Rios, A.O. Characterization, bioactive compounds and antioxidant potential of açaí (Euterpe oleracea) genotypes. J. Agric. Food Chem. 2019, 15, 637–647. [Google Scholar]
- Gale, C.R.; Ashurst, H.E.; Powers, H.J.; Martyn, C.N. Antioxidant vitamin status and carotid atherosclerosis in the elderly. Am. J. Clin. Nutr. 2001, 74, 402–408. [Google Scholar] [CrossRef][Green Version]
- Osganian, S.K.; Stampfer, M.J.; Rimm, E.; Spiegelman, D.; Manson, J.E.; Willett, W.C. Dietary carotenoids and risk of coronary artery disease in women. Am. J. Clin. Nutr. 2003, 77, 1390–1399. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barrandon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-carotene supplementation and cancer risk: A systematic review and metaanalysis of randomized controlled trials. Int. J. Cancer. 2010, 127, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Renzi, L.M. Carotenoids. Adv. Nutr. 2013, 4, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Kuspradini, H.; Rosiarto, A.M.; Putri, A.S.; Kusuma, I.W. Antioxidant and toxicity properties of anthocyanin extracted from red flower of four tropical shrubs. Nusant. Biosci. 2016, 8, 135–140. [Google Scholar] [CrossRef]
- Jovanović Galović, A.; Jovanović Lješković, N.; Vidović, S.; Vladić, J.; Jojić, N.; Ilić, M.; Srdić Rajić, T.; Kojić, V.; Jakimov, D. The Effects of Resveratrol-Rich Extracts of Vitis vinifera Pruning Waste on HeLa, MCF-7 and MRC-5 Cells: Apoptosis, Autophagia and Necrosis Interplay. Pharmaceutics 2022, 14, 2017. [Google Scholar] [CrossRef]
- Vijayarathna, S.; Sasidharan, S. Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pac. J. Trop. Biomed. 2012, 2, 826–829. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Jee, Y.; Kim, H.-J.; Laneri, S.; Gedara, K.; Maheshika, A.; Jayasinghe, K.; Do, S.G.; et al. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri TNF-α/IFN-γ-stimulated human dermal fibroblasts via regulating Nrf2/HO-1 and NF-κB/MAPK pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef]
- Hong, S.L.; Lee, G.S.; Syed Abdul Rahman, S.N.; Ahmed Hamdi, O.A.; Awang, K.; Aznam Nugroho, N.; Abd Malek, S.N. Essential oil content of the rhizome of Curcuma purpurascens Bl. (Temu Tis) and its antiproliferative effect on selected human carcinoma cell lines. Sci. World J. 2014, 2014, 397430. [Google Scholar] [CrossRef]
- Hussein, H.A.; Mohamad, H.; Ghazaly, M.M.; Laith, A.A.; Abdullah, M.A. Cytotoxic effects of Tetraselmis suecica chloroform extracts with silver nanoparticle co-application on MCF-7, 4 T1, and Vero cell lines. J. Appl. Phycol. 2020, 32, 127–143. [Google Scholar] [CrossRef]
- Maul, A.A. Current Status and future of supercritical extraction. Biotechnol. Sci. Dev. 1999, 11, 42–46. [Google Scholar]
- Machida, H.; Takesue, M.; Smith, R.L. Green chemical processes with fluidos supercritical fluids: Properties, materials, separation and energy. J. Supercrit. Fluids 2011, 60, 2–15. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Batista, C.D.C.R.; De Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; Souza, A.P.D.S.; Machado, N.T.; De Carvalho, R.N. Supercritical CO2 extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2016, 107, 364–369. [Google Scholar] [CrossRef]
- Silva, M.P.; Cunha, V.M.B.; Sousa, S.H.B.; Menezes, E.G.O.; Bezerra, P.D.N.; de Farias Neto, J.T.; Filho, G.N.R.; Araújo, M.E.; De Carvalho, R.N. Supercritical CO2 extraction of lyophilized Açaí (Euterpe oleracea Mart.) pulp oil from three municipalities in the state of Pará, Brazil. J. CO2 Util. 2019, 31, 226–234. [Google Scholar] [CrossRef]
- Wagner, Z.; Pavlíček, J. Vapour-liquid equilibrium in the carbon dioxide—p-cymene system at high pressure. Fluid. Phase Equilib. 1993, 90, 135–141. [Google Scholar] [CrossRef]
- Boeira, L.S.; Freitas, P.H.B.; Uchôa, N.R.; Bezerra, J.A.; Cád, S.V.; Duvoisin, S.J.; Albuquerque, P.M.; Mar, J.M.; Ramos, A.S.; Machado, M.B.; et al. Chemical and sensorial characterization of a novel alcoholic beverage produced with native acai (Euterpe precatoria) from different regions of the Amazonas state. LWT 2020, 117, 108–632. [Google Scholar] [CrossRef]
- ASAE S319.3; Method of Determining and Expressing Fineness of Feed Materials by Sieving. ASAE Standards: St. Joseph, MI, USA, 1998; p. 547.
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists. (Method 958.06); AOAC: Arlington, MA, USA, 1995; Chapter 39; p. 21. [Google Scholar]
- Adolfo Lutz Institute. Analytical Standards of the Adolfo Lutz Institute. v.1: Chemical and Physical Methods for Food Analysis, 3rd. ed.; IMESP: São Paulo, Brazil, 1985; pp. 49–50. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1–F2. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis, (n.d.).; International Food Policy Research Institute: Washington, DC, USA, 2004. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; De Brito, E.S.; De Morais, S.M.; Sampaio, C.d.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Scientific: Methodology: Determination of total antioxidant activity in fruits by the iron reduction method de (FRAP). Tech. Not. 2006, 125, 4. [Google Scholar]
- Weisenthal, L.M. Differential Staining Cytotoxicity Assay: A Review. Cancer Cell Cult. Methods Protoc. 2011, 731, 259–283. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef] [PubMed]
- Zabot, G.L.; Moraes, M.N.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds from botanic matrices: Experimental data, process parameters and economic evaluation. Recent. Pat. Eng. 2012, 6, 182–206. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; Da Silva, M.P.; de Sousa, S.H.B.; Bezerra, P.D.N.; Menezes, E.G.O.; Da Silva, N.J.N.; Da Silva Banna, D.A.D.; Araújo, M.E.; De Carvalho, R.N., Jr. Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Freitas, D.G.C.; Moretti, R.H. Characterization and sensorial evaluation of functional cereal bar. Food Sci. Technol. 2006, 26, 318–324. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-Lucas, R.; Bejaoui, M.A.; Benlloch, M.; Fernández-Escobar, R. Global warming effects on yield and fruit maturation of olive trees growing under field conditions. Sci. Hortic. 2019, 249, 162–167. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Menezes, E.G.O.; Freitas, L.C.; Andrade, E.H.d.A.; Costa, R.M.R.; Júnior, J.O.C.S.; De Carvalho, R.N., Jr. Supercritical CO2 extraction of uxi (Endopleura uchi) oil: Global yield isotherms, fatty acid profile, functional quality and thermal stability. J. Supercrit. Fluids 2020, 165, 104932. [Google Scholar] [CrossRef]
- Pires, F.C.S.; Silva, A.P.D.S.; Salazar, M.D.L.A.R.; Da Costa, W.A.; da Costa, H.S.C.; Lopes, A.S.; Rogez, H.; De Carvakho, R.N., Jr. Determination of process parameters and bioactive properties of the murici pulp (Byrsonima crassifolia) extracts obtained by supercritical extraction. J. Supercrit. Fluids 2019, 146, 128–135. [Google Scholar] [CrossRef]
- Menezes, E.G.O.; Barbosa, J.R.; Pires, F.C.S.; Ferreira, M.C.R.; Silva, A.P.d.S.; Siqueira, L.M.M.; De Carvalho, R.N., Jr. Development of a new scale-up equation to obtain Tucumã-of-Pará (Astrocaryum vulgare Mart.) oil rich in carotenoids using supercritical CO2 as solvent. J. Supercrit. Fluids 2022, 181, 105481. [Google Scholar] [CrossRef]
- Terefe, N.S.; Kleintschek, T.; Gamage, T.; Fanning, K.J.; Netzel, G.; Versteeg, C.; Netzel, M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov. Food Sci. Emerg. Tecnol. 2013, 19, 57–65. [Google Scholar] [CrossRef]
- Pires, F.C.S.; De Oliveira, J.C.; Menezes, E.G.O.; Silva, A.P.D.S.; Ferreira, M.C.R.; Siqueira, L.M.M.; Almada-Vilhena, A.O.; Pieczarka, J.C.; Nagamachi, C.Y.; De Carvalho, R.N., Jr. Bioactive compounds and evaluation of antioxidant, cytotoxic and cytoprotective effects of murici pulp extracts (Byrsonima crassifolia) obtained by supercritical extraction in hepg2 cells treated with H2O2. Foods 2021, 10, 737. [Google Scholar] [CrossRef]
- Gueffai, A.; Gonzalez-Serrano, D.J.; Christodoulou, M.C.; Orellana-Palacios, J.C.; Ortega, M.L.S.; Ouldmoumna, A.; Kiari, F.Z.; Ioannou, G.D.; Kapnissi-Christodoulou, C.P.; Moreno, A.; et al. Phenolics from Defatted Black Cumin Seeds (Nigella sativa L.): Ultrasound-Assisted Extraction Optimization, Comparison, and Antioxidant Activity. Biomolecules 2022, 12, 1311. [Google Scholar] [CrossRef]
- Shim, S.Y.; Lee, Y.E.; Song, H.Y.; Lee, M. P-Hydroxybenzoic Acid β-d-Glucosyl Ester and Cimidahurinine with Antimelanogenesis and Antioxidant Effects from Pyracantha angustifolia via Bioactivity-Guided Fractionation. Antioxidants 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gołębiewska, E.; Mazur, L.; Lewandowska, H.; Pruszyński, M.; Świderski, G.; Wyrwas, M.; Pawluczuk, N.; Lewandowski, W. Crystal structure, spectroscopic characterization, antioxidant and cytotoxic activity of new Mg(II) and Mn(II)/Na(I) complexes of isoferulic acid. Materials 2021, 14, 3236. [Google Scholar] [CrossRef]
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542. [Google Scholar] [CrossRef]
- Reginaldo, J.; Amador, E.C.D.M.; Barrientos, M.S.; Pinto, K.H.P.; Janner, D.E.; Guerra, G.P. Effect of p-cumaric acid on locomotor activity in drosophila melanogaster, proceedings of the int. Teach. Res. Ext. Salon 2019, 27. Available online: https://periodicos.unipampa.edu.br/index.php/SIEPE/article/view/101331 (accessed on 19 November 2022).
- Maisch, N.A.; Bereswill, S.; Heimesaat, M.M. Antibacterial effects of vanilla ingredients provide novel treatment options for infections with multidrug-resistant bacteria—A recent literature review. Eur. J. Microbiol. Immunol. 2022, 12, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Schoonees, A.; Visser, J.; Musekiwa, A.; Volmink, J. Pycnogenol® (extract of French maritime pine bark) for the treatment of chronic disorders. Cochrane Database Syst. Rev. 2012, 9, CD008294. [Google Scholar] [CrossRef] [PubMed]
- Bondam, A.F.; Da Silveira, D.D.; Dos Santos, J.P.; Hoffmann, J.F. Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, J.; Li, Y.; Luo, H.; Chen, S.; Zeng, X.; Han, Z. Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances. Foods 2022, 11, 2166. [Google Scholar] [CrossRef]
- Anthocyanin: Foods, Benefits, Side Effects, and Supplements, (n.d.). Available online: https://www.healthline.com/nutrition/anthocyanin#side-effects (accessed on 19 November 2022).
- Zha, J.; Koffas, M.A.G. Production of anthocyanins in metabolically engineered microorganisms: Current status and perspectives. Synth. Syst. Biotechnol. 2017, 2, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Chan, C.F.; Lien, C.Y.; Lai, Y.C.; Huang, C.L.; Liao, W.C. Influence of purple sweet potato extracts on the UV absorption properties of a cosmetic cream. Cosmet. Sci. 2010, 5, 333–341. [Google Scholar]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Orticulture 2022, 2, 3. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Pott, A.; Filiú, W.F.d.O.; Caires, A.R.L.; Michels, F.S.; Maróstica, M.R., Jr.; Santos, N.M.S.; Nunes, Â.A.; Oliveira, L.C.S.; et al. Characterization of Buriti (Mauritia flexuosa) Pulp Oil and the Effect of Its Supplementation in an In Vivo Experimental Model. Nutrients 2022, 14, 2547. [Google Scholar] [CrossRef]
- Monteiro, S.F.; Costa, E.L.N.; Ferreira, R.S.B.; Chisté, R.C. Simultaneous extraction of carotenoids and phenolic compounds from pulps of orange and yellow peach palm fruits (Bactris gasipaes) by ultrasound-assisted extraction. Food Sci. Technol. 2021, 42, e34021. [Google Scholar] [CrossRef]
- Alexandre, D.; Cunha, R.L.; Hubinger, M.D. Preservation of the açaí pulp through the application of obstacles. Food Sci. Tecnol. 2004, 24, 114–119. [Google Scholar] [CrossRef]
- Martins, G.R.; Guedes, D.; De Paula, U.L.M.; De Oliveira, M.D.S.P.; Lutterbach, M.T.S.; Reznik, L.Y.; Sérvulo, E.F.C.; Alviano, C.S.; Da Silva, A.J.R.; Alviano, D.S. Açaí (Euterpe oleracea Mart.) Seed Extracts from Different Varieties: A Source of Proanthocyanidins and Eco-Friendly Corrosion Inhibition Activity. Molecules 2021, 26, 3433. [Google Scholar] [CrossRef]
- Duncan, C.E. Factors Influencing the Stability and Marketability of a Novel, Phytochemical-Rich Oil from the Açai Palm Fruit (Euterpe oleracea Mart.) a Dissertation. 2010. Available online: https://hdl.handle.net/1969.1/ETD-TAMU-2010-12-8789 (accessed on 19 November 2022).
- Arct, J.; Mieloch, M. β-carotene in skin care. Pol. J. Cosmetol. 2016, 19, 206–213. Available online: https://www.researchgate.net/publication/347513828 (accessed on 19 November 2022).
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, F.M.; Borguini, R.G.; Monique, A.; Machado, R.; Miranda, A.C.; Gôuvea, S.; Pacheco, S.; Godoy, R.L.d.O. Carotenoids content in nutricosmetics products: Evaluation of product adequacy and quality. Rev. Inst. Adolfo Lutz 2013, 72, 249–254. [Google Scholar] [CrossRef][Green Version]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Application of different chemical methods to determine antioxidant activity in fruit pulp. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N.Y. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Iris, F.F.; Benzie, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Roberta, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Sena, C.; Santos, O.V.; Da Costa, W.A.; Rodrigues, A.M.C.; De Carvalho, R.N., Jr. Extraction of bacaba (Oenocarpus bacaba) oil with supercritical CO2: Global yield isotherms, fatty acid composition, functional quality, oxidative stability, spectroscopic profile and antioxidant activity. Grasas Aceites 2018, 69, e246. [Google Scholar] [CrossRef]
- Melo, E.A.; Maciel, M.I.S.; Lima, V.L.A.G.; Leal, F.L.L.; Caetano, A.C.S.; Nascimento, R.J. Antioxidant capacity of vegetables commonly consumed. Food Sci. Technol. 2006, 26, 639–644. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; De Brito, E.S.; Pérez-jiménez, J.; Saura-calixto, F.; Mancini-filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Borges, G.S.C.; Gonzaga, L.V.; Jardini, F.A.; Filho-mancini, J.; Heller, M.; Micke, G.; Costa, A.C.O.; Fett, R. Protective effect of Euterpe edulis M. on Vero cell culture and antioxidant evaluation based on phenolic composition using HPLC-ESI-MS/MS. Food Res. Int. 2013, 51, 363–369. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Elisia, I.; Popovich, D.G.; Hu, C.; Kitts, D.D. Evaluation of viability assays for anthocyanins in cultured cells. Phytochem. Anal. 2008, 19, 479–486. [Google Scholar] [CrossRef]
- Đurđević, S.; Šavikin, K.; Živković, J.; Böhm, V.; Stanojković, T.; Damjanović, A.; Petrović, S. Antioxidant and cytotoxic activity of fatty oil isolated by supercritical fluid extraction from microwave pretreated seeds of wild growing Punica granatum L. J. Supercrit. Fluids. 2018, 133, 225–232. [Google Scholar] [CrossRef]
- Ghafar, S.A.A.; Ismail, M.; Yazan, L.S.; Fakurazi, S.; Ismail, N.; Chan, K.W.; Tahir, P.M. Cytotoxic activity of kenaf seed oils from supercritical carbon dioxide fluid extraction towards human colorectal cancer (HT29) cell lines, Evidence-Based Complement. Altern. Med. 2013, 2013, 549705. [Google Scholar] [CrossRef]
- Elgndi, M.A.; Filip, S.; Pavlić, B.; Vladić, J.; Stanojković, T.; Žižak, Ž.; Zeković, Z. Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. Supercrit. Fluids. 2017, 128, 128–137. [Google Scholar] [CrossRef]
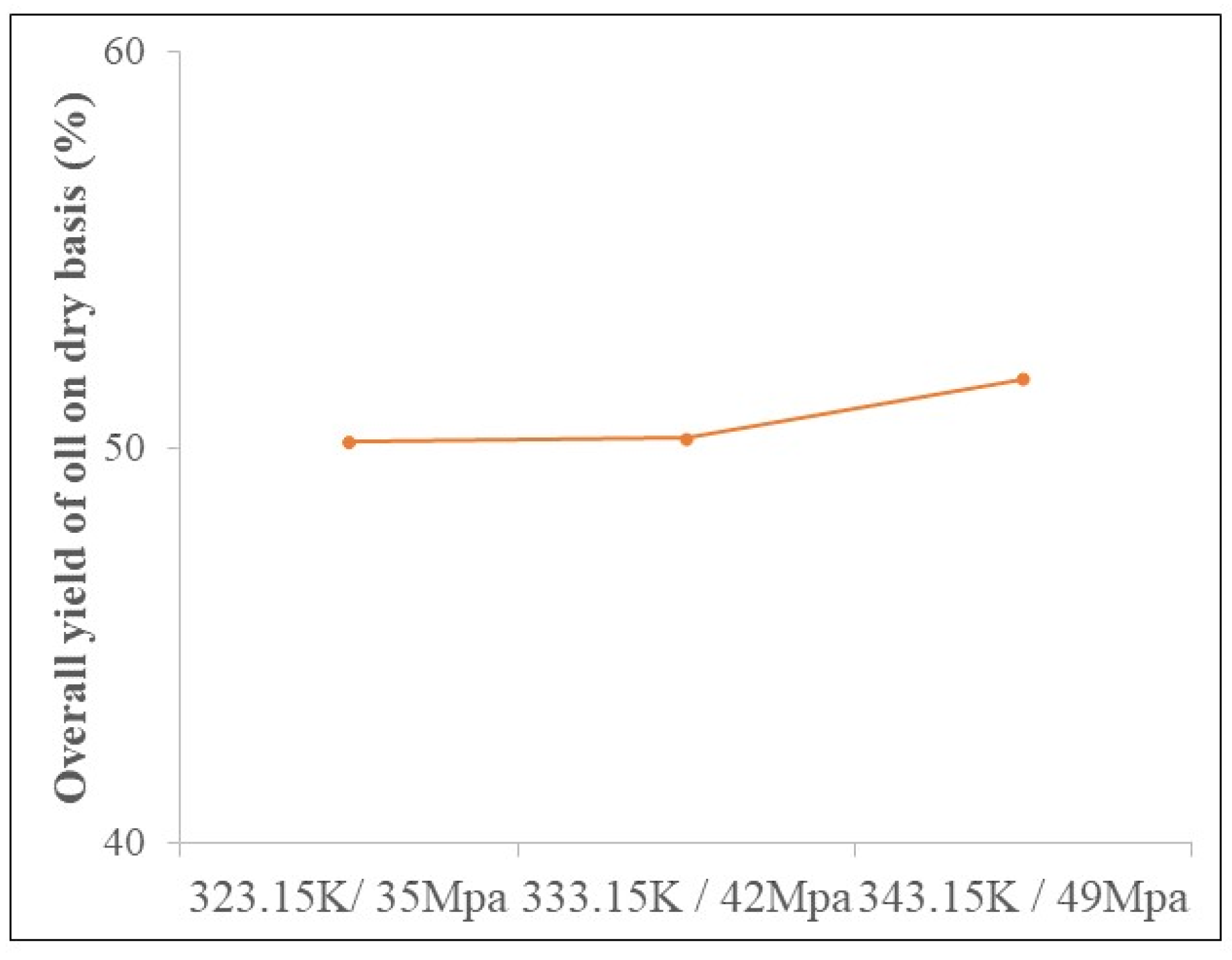

| Sample | T (K) | P (MPa) | Yield (% d.b.) | Phenolic Compounds (mg GAE/g d.b.) | Anthocyanin (mg/100 g d.b.) | Carotenoids (µg/g d.b.) | DPPH (μmol TE/g d.b.) | ABTS (μmol TE/g d.b.) | FRAP (Ferrous Sulfate μm/g d.b.) |
|---|---|---|---|---|---|---|---|---|---|
| Lyophilized Pulp | - | - | - | 77.51 ± 0.54 d | 693.85 ± 2.03 b | - | 289.08 ± 6.83 d | 385.92 ± 8.90 d | 500.35 ± 8.66 c |
| Fat-free Pulp | 323.15 | 35 | n.d | 150.20 ± 1.32 a | 1330.50 ± 11.05 a | n.d | 414.99 ± 5.02 a | 554.53 ± 7.68 c | 746.25 ± 3.82 a |
| 333.15 | 42 | n.d | 109.14 ± 0.72 b | 1324.82 ± 55.44 a | n.d | 388.93 ± 4.65 b | 589.74 ± 0.00 b | 726.15 ± 6.79 a | |
| 343.15 | 49 | n.d | 96.43 ± 0.46 c | 1384.39 ± 64.72 a | n.d | 362.71 ± 5.07 c | 644.23 ± 7.23 a | 653.13 ± 9.97 b | |
| Oils | 323.15 | 35 | 50.14 ± 0.01 b | n.d | n.d | 250.40 ± 2.10 b | 2.02 ± 0.07 b | 2.05 ± 0.04 c | 9.95 ± 0.89 b |
| 333.15 | 42 | 50.24 ± 0.97 b | n.d | n.d | 246.22 ± 2.51 c | 2.40 ± 0.11 a | 2.24 ± 0.11 b | 5.50 ± 9.97 c | |
| 343.15 | 49 | 51.74 ± 0.06 a | n.d | n.d | 277.09 ± 3.65 a | 2.55 ± 0.14 a | 2.60 ± 0.05 a | 15.25 ± 1.69 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siqueira, L.M.M.; Campos, A.L.d.B.S.; Pires, F.C.S.; Ferreira, M.C.R.; Silva, A.P.d.S.e.; Menezes, E.G.O.; Ramos, I.N.d.F.; Khayat, A.S.; Rêgo, J.d.A.R.d.; Carvalho Junior, R.N.d. Evaluation of Bioactive Compounds and Antioxidant and Cytotoxic Effects of Oil and Pulp without Açaí Fat (Euterpe oleracea) Obtained by Supercritical Extraction. Foods 2024, 13, 2819. https://doi.org/10.3390/foods13172819
Siqueira LMM, Campos ALdBS, Pires FCS, Ferreira MCR, Silva APdSe, Menezes EGO, Ramos INdF, Khayat AS, Rêgo JdARd, Carvalho Junior RNd. Evaluation of Bioactive Compounds and Antioxidant and Cytotoxic Effects of Oil and Pulp without Açaí Fat (Euterpe oleracea) Obtained by Supercritical Extraction. Foods. 2024; 13(17):2819. https://doi.org/10.3390/foods13172819
Chicago/Turabian StyleSiqueira, Letícia Maria Martins, Ana Luiza de Barros Souza Campos, Flávia Cristina Seabra Pires, Maria Caroline Rodrigues Ferreira, Ana Paula de Souza e Silva, Eduardo Gama Ortiz Menezes, Ingryd Nayara de Farias Ramos, André Salim Khayat, José de Arimateia Rodrigues do Rêgo, and Raul Nunes de Carvalho Junior. 2024. "Evaluation of Bioactive Compounds and Antioxidant and Cytotoxic Effects of Oil and Pulp without Açaí Fat (Euterpe oleracea) Obtained by Supercritical Extraction" Foods 13, no. 17: 2819. https://doi.org/10.3390/foods13172819
APA StyleSiqueira, L. M. M., Campos, A. L. d. B. S., Pires, F. C. S., Ferreira, M. C. R., Silva, A. P. d. S. e., Menezes, E. G. O., Ramos, I. N. d. F., Khayat, A. S., Rêgo, J. d. A. R. d., & Carvalho Junior, R. N. d. (2024). Evaluation of Bioactive Compounds and Antioxidant and Cytotoxic Effects of Oil and Pulp without Açaí Fat (Euterpe oleracea) Obtained by Supercritical Extraction. Foods, 13(17), 2819. https://doi.org/10.3390/foods13172819









