Antioxidant and Anti-Atherosclerosis Activities of Hydrolyzed Jellyfish Collagen and Its Conjugate with Black Jelly Mushroom Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Effect of Various Enzymatic Hydrolysis Processes on Yield, α-Amino Group Content, and Antioxidant Activities of Hydrolyzed Collagen (HC) from Jellyfish
2.3.1. Protease Activity Assay
2.3.2. Preparation of HC by Various Enzymatic Hydrolysis Processes
Yield
The α-Amino Group Content (α-AGC)
Antioxidant Activities (AAs)
Amino Acid Composition
2.4. Preparation of Black Jelly Mushroom Extract (BJME)
2.5. Preparation of HC-BJME Conjugate
2.5.1. Analyses
Fourier Transform Infrared (FTIR) Spectroscopy
Size Exclusion Chromatography
2.6. Impacts of HC and HC-BJME Conjugate on Cell Viability and Prevention of Cholesterol-Induced Endothelial Cell Injury
2.6.1. Cell Culture
2.6.2. Cell Viability Measurement and Hoechst33342 Staining
2.6.3. Prevention of Cholesterol-Induced Endothelial Cell Injury
Effect of Cholesterol on Endothelial Injury Induction
Anti-Atherosclerotic Potential of HC and Conjugate
2.7. Statistical Analysis
3. Results
3.1. Effect of Hydrolyzed Collagen (HC) from Jellyfish Prepared Using Various Processes on Yield, α-Amino Group Content (α-AGC), and Antioxidant Activities
3.1.1. Yield
3.1.2. α-AGC
3.1.3. Antioxidant Activities (AAs)
3.2. Amino Acid Composition of the Selected HC
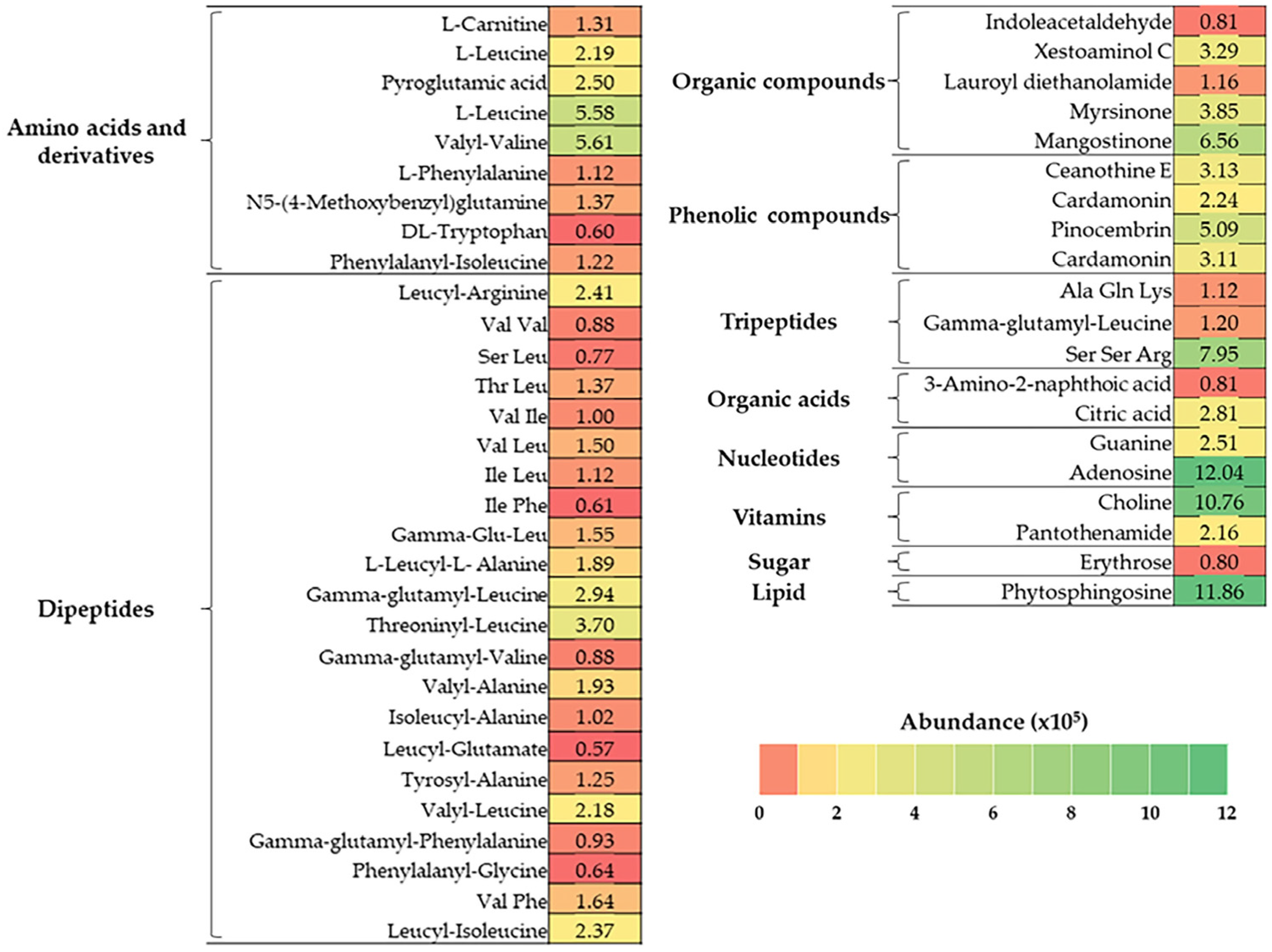
3.3. Identification of the Compounds in BJME Using LC-QTOF-MS
3.4. Characteristics of the HC-BJME Conjugate Prepared Using BJME at Different Levels
3.4.1. Surface Hydrophobicity and Antioxidant Activities (AAs)
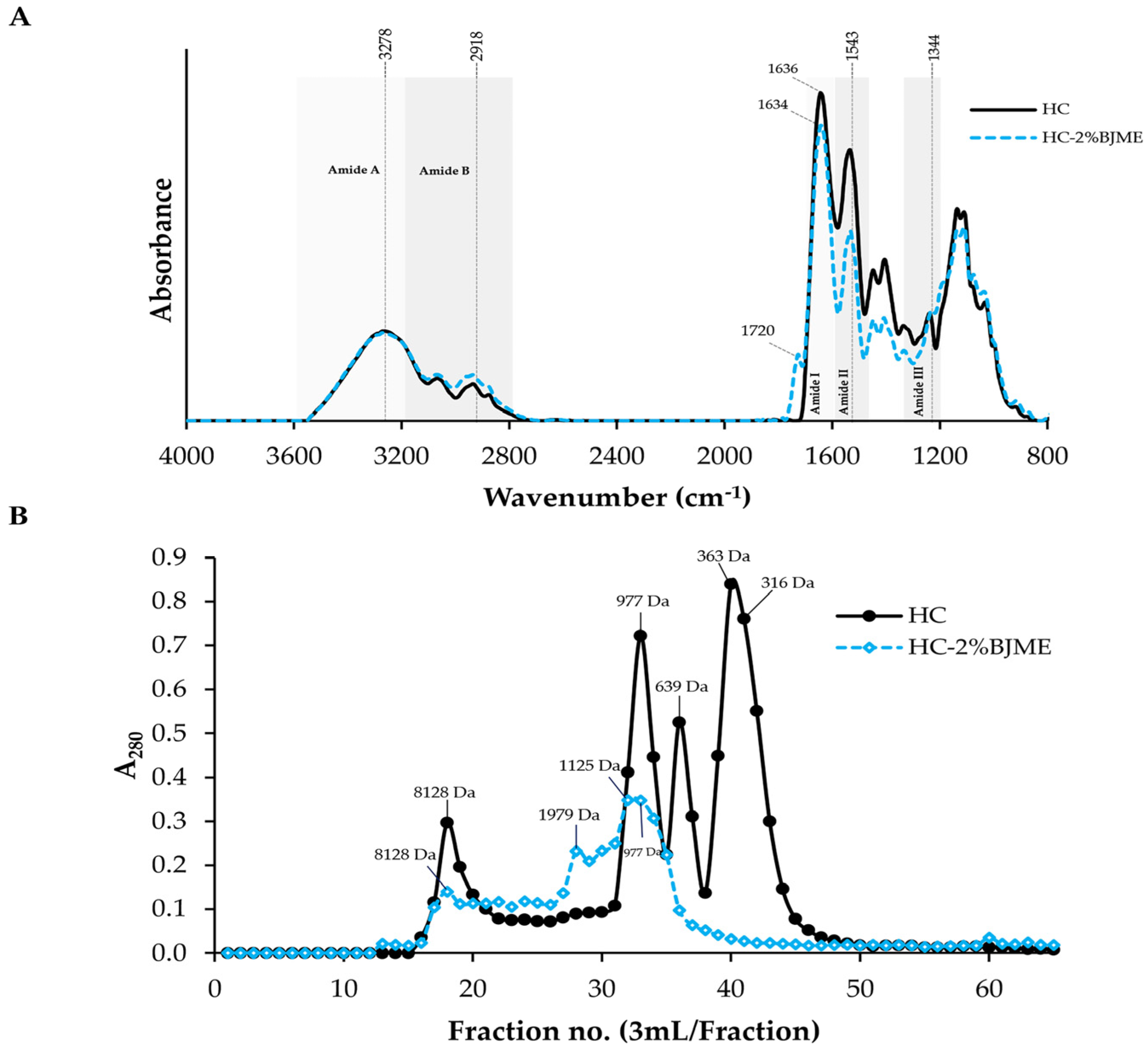
3.4.2. FTIR Spectra
3.4.3. Size Distribution
3.5. Cytotoxicity of HC and HC-2%BJME Conjugate
3.6. Comparative Study of the Preventive Ability against Cholesterol-Induced Endothelial Injury of HC and HC-BJME Conjugate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| a-AGC | Alpha-amino group content. |
| AAs | Antioxidant activities |
| ABTS-RSA | ABTS radical scavenging activity |
| BJME | Black jelly mushroom extract |
| DH | Degree of hydrolysis |
| DM | Dry matter |
| DMEM | Dulbecco’s modified Eagle medium |
| DPPH-RSA | DPPH radical scavenging activity |
| EC | Endothelial cell |
| FBS | Fetal bovine serum |
| FRAP | Ferric reducing antioxidant power |
| FTIR | Fourier transform infrared spectroscopy |
| HC | Hydrolyzed collagen |
| LC-QTOF-MS | Liquid chromatography–quadrupole time-of-flight–mass spectrophotometry |
| LDLs | Low-density lipoproteins |
| MCA | Metal chelating activity |
| OH | Hydroxyl |
| SPI | Soy protein isolate |
| VE-cadherin | Vascular endothelial cadherin |
References
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of lipid accumulation and inflammation in atherosclerosis: Focus on molecular and cellular mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.E.; Gupta, R.M. The role of endothelial cells in atherosclerosis: Insights from genetic association studies. Am. J. Pathol. 2024, 194, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; Van Hinsbergh, V.W.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors—A review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional vascular endothelium as a driver of atherosclerosis: Emerging insights into pathogenesis and treatment. Front. Pharmacol. 2021, 12, 787541. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Vestweber, D. Mechanisms ensuring endothelial junction integrity beyond VE-cadherin. Front. Physiol. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sigala, F.; Vourliotakis, G.; Georgopoulos, S.; Kavantzas, N.; Papalambros, E.; Agapitos, M.; Bastounis, E. Vascular endothelial cadherin expression in human carotid atherosclerotic plaque and its relationship with plaque morphology and clinical data. Eur. J. Vasc. Endovasc. Surg. 2003, 26, 523–528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from porcine lipase-defatted seabass skin: Antioxidant, fibroblast cell proliferation, and collagen production activities. J. Food Biochem. 2019, 43, e12825. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Zhao, Y.-Q.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Eight collagen peptides from hydrolysate fraction of Spanish mackerel skins: Isolation, identification, and in vitro antioxidant activity evaluation. Mar. Drugs 2019, 17, 224. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Sukketsiri, W.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from defatted sea bass skin and its conjugate with epigallocatechin gallate: In vitro antioxidant, anti-inflammatory, wound-healing and anti-obesity activities. Food Biosci. 2021, 43, 101303. [Google Scholar] [CrossRef]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Qiu, Y.-T.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Novel antioxidant collagen peptides of Siberian sturgeon (Acipenser baerii) cartilages: The preparation, characterization, and cytoprotection of H2O2-damaged human umbilical vein endothelial cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, P.G.; Suh, J.H.; Pegg, R.B.; Chen, J.; Solval, K.M. The emergence of jellyfish collagen: A comprehensive review on research progress, industrial applications, and future opportunities. Trends Food Sci. 2023, 141, 104206. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Lueyot, A.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wonganu, B.; Wongsa-Ngasri, P.; Charoenlappanit, S.; Roytrakul, S.; Thumthanaruk, B. Influence of collagen and some proteins on gel properties of jellyfish gelatin. PLoS ONE 2021, 16, e0253254. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Wang, X.; Yu, H.; Li, R.; Geng, H.; Xing, R.; Liu, S.; Li, P. Jellyfish peptide as an alternative source of antioxidant. Antioxidants 2023, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Upata, M.; Siriwoharn, T.; Makkhun, S.; Yarnpakdee, S.; Regenstein, J.M.; Wangtueai, S. Tyrosinase inhibitory and antioxidant activity of enzymatic protein hydrolysate from jellyfish (Lobonema smithii). Foods 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Powell, L.C.; Matin, N.; Mearns-Spragg, A.; Thornton, C.A.; Khan, I.M.; Francis, L.W. Jellyfish collagen: A biocompatible collagen source for 3D scaffold fabrication and enhanced chondrogenicity. Mar. Drugs 2021, 19, 405. [Google Scholar] [CrossRef]
- Costa, R.; Capillo, G.; Albergamo, A.; Li Volsi, R.; Bartolomeo, G.; Bua, G.; Ferracane, A.; Savoca, S.; Gervasi, T.; Rando, R.; et al. A multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca. Mar. Drugs 2019, 17, 172. [Google Scholar] [CrossRef]
- Socrier, L.; Quéro, A.; Verdu, M.; Song, Y.; Molinié, R.; Mathiron, D.; Pilard, S.; Mesnard, F.; Morandat, S. Flax phenolic compounds as inhibitors of lipid oxidation: Elucidation of their mechanisms of action. Food Chem. 2019, 274, 651–658. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, X.; Zhang, L.; Wang, X.; Xu, Y.; Qin, Z.; Liu, G.; Wang, Q.; Tian, K.; Lim, K.S.; et al. The functional role of lipoproteins in atherosclerosis: Novel directions for diagnosis and targeting therapy. Aging Dis. 2022, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Luo, Y.-C.; Ji, B.-P.; Li, B.; Su, W.; Xiao, Z.-L.; Zhang, G.-Z. Hypocholesterolemic effects of Auricularia auricula ethanol extract in ICR mice fed a cholesterol-enriched diet. J. Food Technol. 2011, 48, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Sukketsiri, W.; Aluko, R.E.; Benjakul, S. In vitro antioxidant and wound-healing activities of hydrolyzed collagen from defatted Asian sea bass skin as influenced by different enzyme types and hydrolysis processes. RSC Adv. 2021, 11, 18144–18151. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Sukketsiri, W.; Battino, M.; Benjakul, S. Conjugate between hydrolyzed collagen from defatted seabass skin and epigallocatechin gallate (EGCG): Characteristics, antioxidant activity and in vitro cellular bioactivity. RSC Adv. 2021, 11, 2175–2184. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Chotphrethipong, L.; Benjakul, S.; Hutamekalin, P.; Champoochana, N.; Senphan, T.; Nalinanon, S. Pulsed electric field-assisted extraction of Djenkol (Archidendron pauciflorum) peel: Characterization, suppression of intracellular ROS generation and inflammatory cytokines in LPS-activated RAW264. 7 macrophage cells. Appl. Food Res. 2024, 4, 100428. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Cryoprotective effect of gelatin hydrolysate from blacktip shark skin on surimi subjected to different freeze-thaw cycles. LWT 2012, 47, 437–442. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of hydrolyzed collagen from defatted sea bass skin on proliferation and differentiation of preosteoblast MC3T3-E1 cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Chanvorachote, P.; Reudhabibadh, R.; Singh, A.; Benjakul, S.; Roytrakul, S.; Hutamekalin, P. Chitooligosaccharide from Pacific white shrimp shell chitosan ameliorates inflammation and oxidative stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 pathways in LPS-induced RAW264.7 macrophage cells. Foods 2023, 12, 2740. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Esparza-Espinoza, D.M.; del Carmen Santacruz-Ortega, H.; Plascencia-Jatomea, M.; Aubourg, S.P.; Salazar-Leyva, J.A.; Rodríguez-Felix, F.; Ezquerra-Brauer, J.M. Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity. Fishes 2023, 8, 246. [Google Scholar] [CrossRef]
- Dogan, A.; Siyakus, G.; Severcan, F. FTIR spectroscopic characterization of irradiated hazelnut (Corylus avellana L.). Food Chem. 2007, 100, 1106–1114. [Google Scholar] [CrossRef]
- Broder, T.; Blodau, C.; Biester, H.; Knorr, K.-H. Peat decomposition records in three pristine ombrotrophic bogs in southern Patagonia. Biogeosciences 2012, 9, 1479–1491. [Google Scholar] [CrossRef]
- Okcu, G.; Ayhan, K.; Altuntas, E.G.; Vural, N.; Poyrazoglu, E.S. Determination of phenolic acid decarboxylase produced by lactic acid bacteria isolated from shalgam (şalgam) juice using green analytical chemistry method. LWT—Food Sci. Technol. 2016, 66, 615–621. [Google Scholar] [CrossRef]
- Islam, M.S.; Wang, H.; Admassu, H.; Noman, A.; Ma, C.; Fu, A. Degree of hydrolysis, functional and antioxidant properties of protein hydrolysates from Grass Turtle (Chinemys reevesii) as influenced by enzymatic hydrolysis conditions. Food Sci. Nutr. 2021, 9, 4031–4047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Xie, H.; Liu, Z.; Rakariyatham, K.; Yu, C.; Shahidi, F.; Zhou, D. Antioxidant activity and functional properties of Alcalase-hydrolyzed scallop protein hydrolysate and its role in the inhibition of cytotoxicity in vitro. Food Chem. 2021, 344, 128566. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Kim, J.M.; Liceaga, A.M.; Yoon, K.Y. Purification and identification of an antioxidant peptide from perilla seed (Perilla frutescens) meal protein hydrolysate. Food Sci. Nutr. 2019, 7, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [PubMed]
- Khong, N.M.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Zhang, C.; Liu, C. Angiotensin converting enzyme (ACE) inhibitory, antihypertensive and antihyperlipidaemic activities of protein hydrolysates from Rhopilema esculentum. Food Chem. 2012, 134, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Woonnoi, W.; Chotphruethipong, L.; Tanasawet, S.; Benjakul, S.; Sutthiwong, N.; Sukketsiri, W. Hydrolyzed collagen from salmon skin increases the migration and filopodia formation of skin keratinocytes by activation of FAK/Src pathway. Pol. J. Food Nutr. Sci. 2021, 71, 323–332. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Q.; Lu, Q. Purification, structural analysis, and stability of antioxidant peptides from purple wheat bran. BMC Chem. 2020, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiong, Y.L.; Cao, Y.; True, A.D. Interfacial properties of whey protein foams as influenced by preheating and phenolic binding at neutral pH. Food Hydrocoll. 2018, 82, 379–387. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Jumina; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An update on the anticancer activity of xanthone derivatives: A review. Pharmaceuticals 2021, 14, 1144. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.; Kijjoa, A. Naturally-occurring xanthones: Recent developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A novel natural compound with versatile pharmacological and biological activities. Biomed. Res. Int. 2013, 2013, 379850. [Google Scholar] [CrossRef]
- Nawaz, J.; Rasul, A.; Shah, M.A.; Hussain, G.; Riaz, A.; Sarfraz, I.; Zafar, S.; Adnan, M.; Khan, A.H.; Selamoglu, Z. Cardamonin: A new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020, 250, 117591. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V. Application of FTIR spectroscopy for quantitative analysis of blood serum: A preliminary study. Diagnostics 2021, 11, 2391. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.C.; Zhang, H.; Niu, P.F.; Shi, L.S.; Yang, X.Y.; Meng, Y.H.; Wang, X.Y.; Gong, T.; Guo, Y.R. Fabrication of a novel antioxidant emulsifier through tuning the molecular interaction between soy protein isolates and young apple polyphenols. Food Chem. 2023, 420, 136110. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhang, Y.; Liang, R.; Zhong, F.; Ma, J. Beta-carotene chemical stability in nanoemulsions was improved by stabilized with beta-lactoglobulin–catechin conjugates through free radical method. J. Agric. Food Chem. 2015, 63, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Liu, Y.; Cui, L.; Fu, L.; Li, B. Various bioactive peptides in collagen hydrolysate from Salmo salar skin and the combined inhibitory effects on atherosclerosis in vitro and in vivo. Food Res. Int. 2022, 157, 111281. [Google Scholar] [CrossRef] [PubMed]
- Resink, T.J.; Joshi, M.; Kyriakakis, E. Cadherins and cardiovascular disease. Swiss Med. Wkly. 2009, 139, 122. [Google Scholar] [CrossRef]
- Richard, L.F.; Dahms, T.E.; Webster, R.O. Adenosine prevents permeability increase in oxidant-injured endothelial monolayers. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H35–H42. [Google Scholar] [CrossRef]


| Samples | Yield (%) | α-AGC (mmol Gly/g dry HC) | Antioxidant Activities | |||
|---|---|---|---|---|---|---|
| DPPH-RSA (µmol TE/g dry HC) | ABTS-RSA (µmol TE/g dry HC) | FRAP (µmol TE/g dry HC) | MCA (µmol EE/g dry HC) | |||
| P-0.2 | 16.05 ± 0.49 e,C | 0.03 ± 0.00 f,C | 3.21 ± 0.09 d,B | 0.51 ± 0.06 c,B | 0.31 ± 0.06 c,A | 0.16 ± 0.01 d,C |
| P-0.3 | 20.62 ± 1.14 d,B | 0.08 ± 0.00 e,B | 3.15 ± 0.05 d,B | 0.91 ± 0.02 b,A | 0.27 ± 0.03 c,A | 0.21 ± 0.01 c,B |
| P-0.4 | 26.01 ± 0.15 c,A | 0.12 ± 0.00 d,A | 3.65 ± 0.08 c,A | 0.92 ± 0.07 b,A | 0.24 ± 0.00 c,A | 0.27 ± 0.01 b,A |
| A-0.2 | 29.62 ± 0.66 b,B | 0.27 ± 0.00 c,C | 3.81 ± 0.04 b,B | 0.99 ± 0.00 b,B | 0.26 ± 0.01 c,C | 0.27 ± 0.00 b,B |
| A-0.3 | 30.14 ± 0.58 ab,AB | 0.29 ± 0.00 b,B | 3.85 ± 0.12 b,B | 1.14 ± 0.00 a,A | 0.38 ± 0.06 b,B | 0.28 ± 0.00 b,B |
| A-0.4 | 31.05 ± 0.57 a,A | 0.30 ± 0.00 a,A | 4.18 ± 0.05 a,A | 1.16 ± 0.00 a,A | 0.50 ± 0.02 a,A | 0.45 ± 0.00 a,A |
| Amino Acid | Content (Residues/1000 Residues) |
|---|---|
| Alanine (Ala) | 91.99 |
| Arginine (Arg) | 55.95 |
| Asparatic acid (Asp) | 66.88 |
| Cysteine (Cys) | 2.71 |
| Glutamic acid (Glu) | 84.73 |
| Glycine (Gly) | 334.89 |
| Histidine (His) | 24.97 |
| Isoleucine (Ile) | 18.11 |
| Leucine (Leu) | 30.98 |
| Lysine (Lys) | 29.09 |
| Hydroxylysine (Hylys) | 34.19 |
| Methionine (Met) | 3.70 |
| Phenylalanine (Phe) | 6.40 |
| Hydroxyproline (Hyp) | 49.90 |
| Proline (Pro) | 70.74 |
| Serine (Ser) | 30.31 |
| Threonine (Thr) | 29.20 |
| Tyrosine (Tyr) | 4.93 |
| Valine (Val) | 29.84 |
| Tryptophan (Trp) | 0.48 |
| Total | 1000.00 |
| Imino acid (Hyp + Pro) | 120.64 |
| Samples | Surface Hydrophobicity | Antioxidant Activities | |||
|---|---|---|---|---|---|
| DPPH-RSA (µmol TE/g dry HC) | ABTS-RSA (µmol TE/g dry HC) | FRAP (µmol TE/g dry HC) | MCA (µmol EE/g dry HC) | ||
| HC | 99.85 ± 4.25 e | 4.18 ± 0.05 d | 1.16 ± 0.01 d | 0.50 ± 0.02 e | 0.45 ± 0.00 c |
| OHC | 110.56 ± 6.90 d | 2.30 ± 0.02 e | 0.82 ± 0.02 e | 0.34 ± 0.00 f | 0.50 ± 0.04 c |
| HC-1%BJME | 130.15 ± 9.60 c | 9.07 ± 0.20 b | 3.44 ± 0.02 c | 1.39 ± 0.00 d | 2.78 ± 0.18 b |
| HC-2%BJME | 207.29 ± 1.62 a | 10.69 ± 0.14 a | 3.73 ± 0.01 a | 1.76 ± 0.02 a | 3.57 ± 0.15 a |
| HC-3%BJME | 194.23 ± 1.26 b | 8.15 ± 0.15 c | 3.50 ± 0.04 b | 1.51 ± 0.01 c | 3.54 ± 0.12 a |
| HC-4%BJME | 185.24 ± 5.91b | 7.97 ± 0.07 c | 3.52 ± 0.03 b | 1.54 ± 0.02 b | 2.88 ± 0.21 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binlateh, T.; Hutamekalin, P.; Benjakul, S.; Chotphruethipong, L. Antioxidant and Anti-Atherosclerosis Activities of Hydrolyzed Jellyfish Collagen and Its Conjugate with Black Jelly Mushroom Extract. Foods 2024, 13, 2463. https://doi.org/10.3390/foods13152463
Binlateh T, Hutamekalin P, Benjakul S, Chotphruethipong L. Antioxidant and Anti-Atherosclerosis Activities of Hydrolyzed Jellyfish Collagen and Its Conjugate with Black Jelly Mushroom Extract. Foods. 2024; 13(15):2463. https://doi.org/10.3390/foods13152463
Chicago/Turabian StyleBinlateh, Thunwa, Pilaiwanwadee Hutamekalin, Soottawat Benjakul, and Lalita Chotphruethipong. 2024. "Antioxidant and Anti-Atherosclerosis Activities of Hydrolyzed Jellyfish Collagen and Its Conjugate with Black Jelly Mushroom Extract" Foods 13, no. 15: 2463. https://doi.org/10.3390/foods13152463
APA StyleBinlateh, T., Hutamekalin, P., Benjakul, S., & Chotphruethipong, L. (2024). Antioxidant and Anti-Atherosclerosis Activities of Hydrolyzed Jellyfish Collagen and Its Conjugate with Black Jelly Mushroom Extract. Foods, 13(15), 2463. https://doi.org/10.3390/foods13152463










