Gastrodin Alleviates DSS-Induced Colitis in Mice through Strengthening Intestinal Barrier and Modulating Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
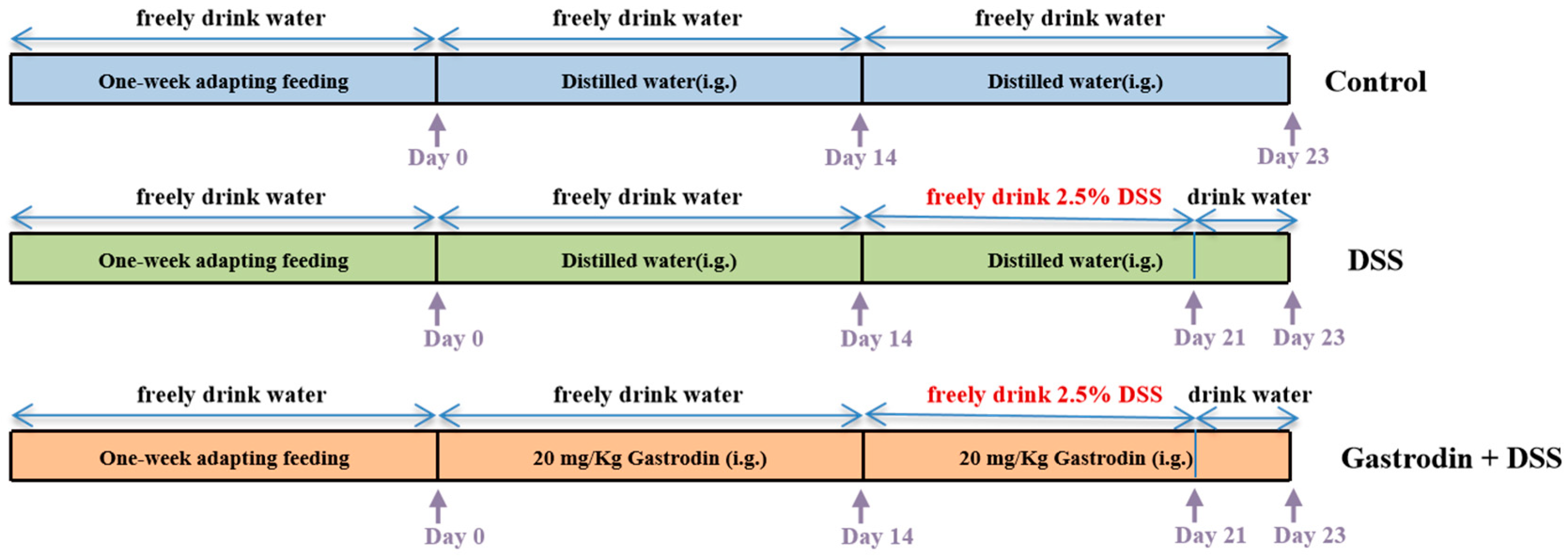
2.2. Animals and Experimental Design
2.3. Histopathological Evaluation and PAS Staining
2.4. Nitric Oxide (NO) Level and Myeloperoxidase (MPO) Activity Assay
2.5. Inflammatory Cytokine Determination
2.6. Quantitative Real-Time PCR Analysis
2.7. Western Blot Analysis
2.8. Immunofluorescence Assay
2.9. Microbial Analysis
2.10. Statistical Analysis
3. Results
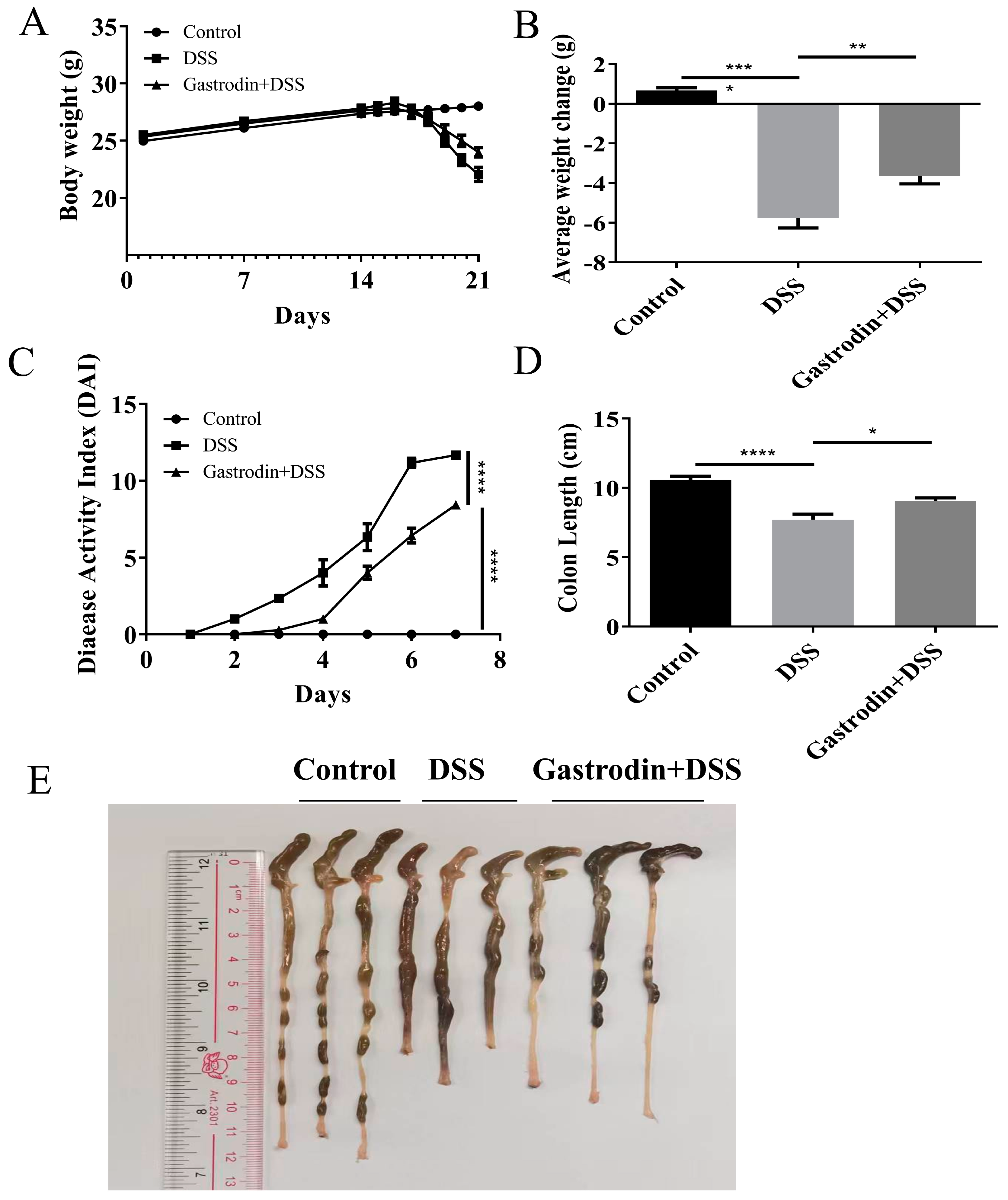
3.1. Gastrodin Relieved Clinical Symptoms Induced by DSS in Mice
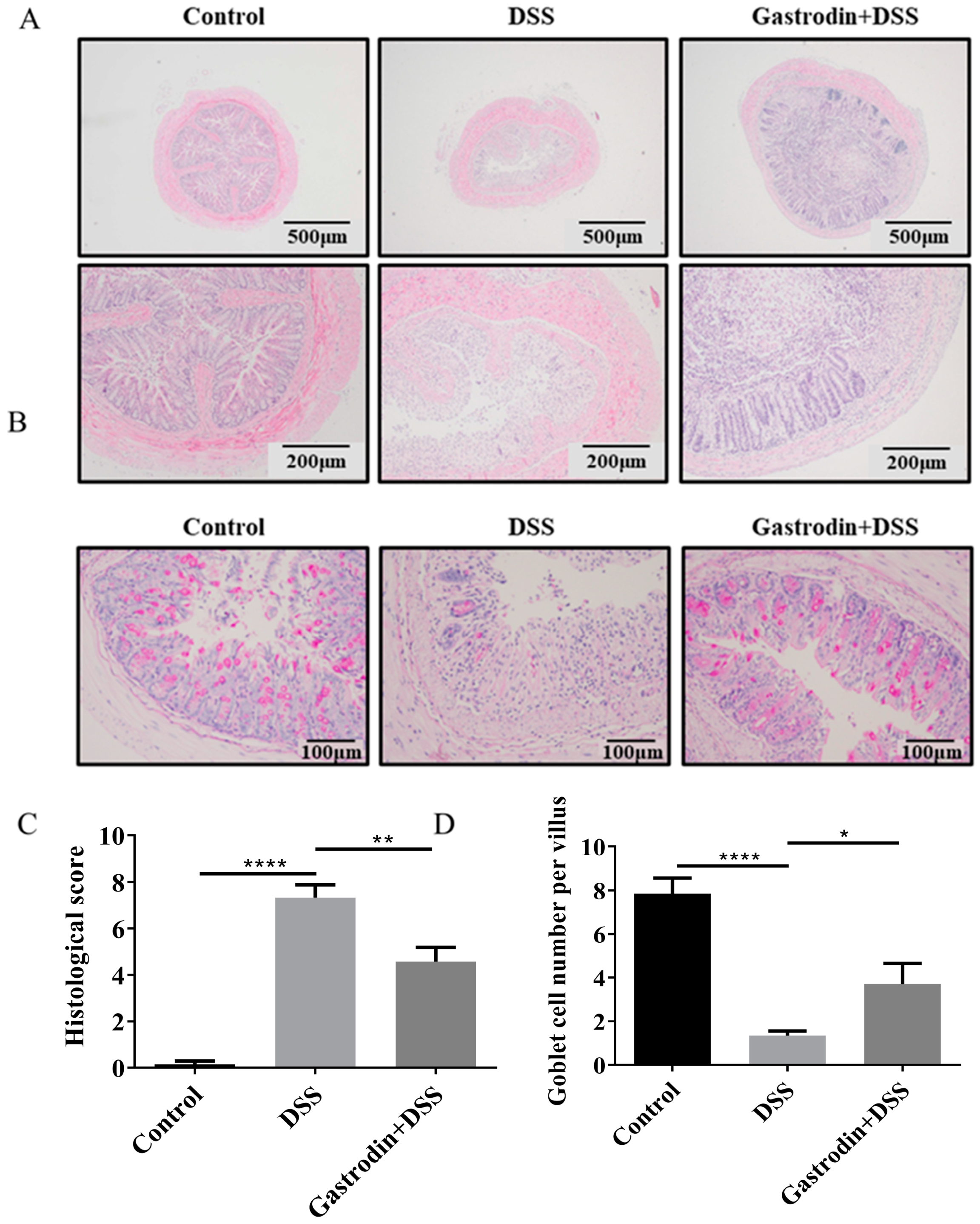
3.2. Gastrodin Ameliorates Histopathological Changes in the Colon of Mice
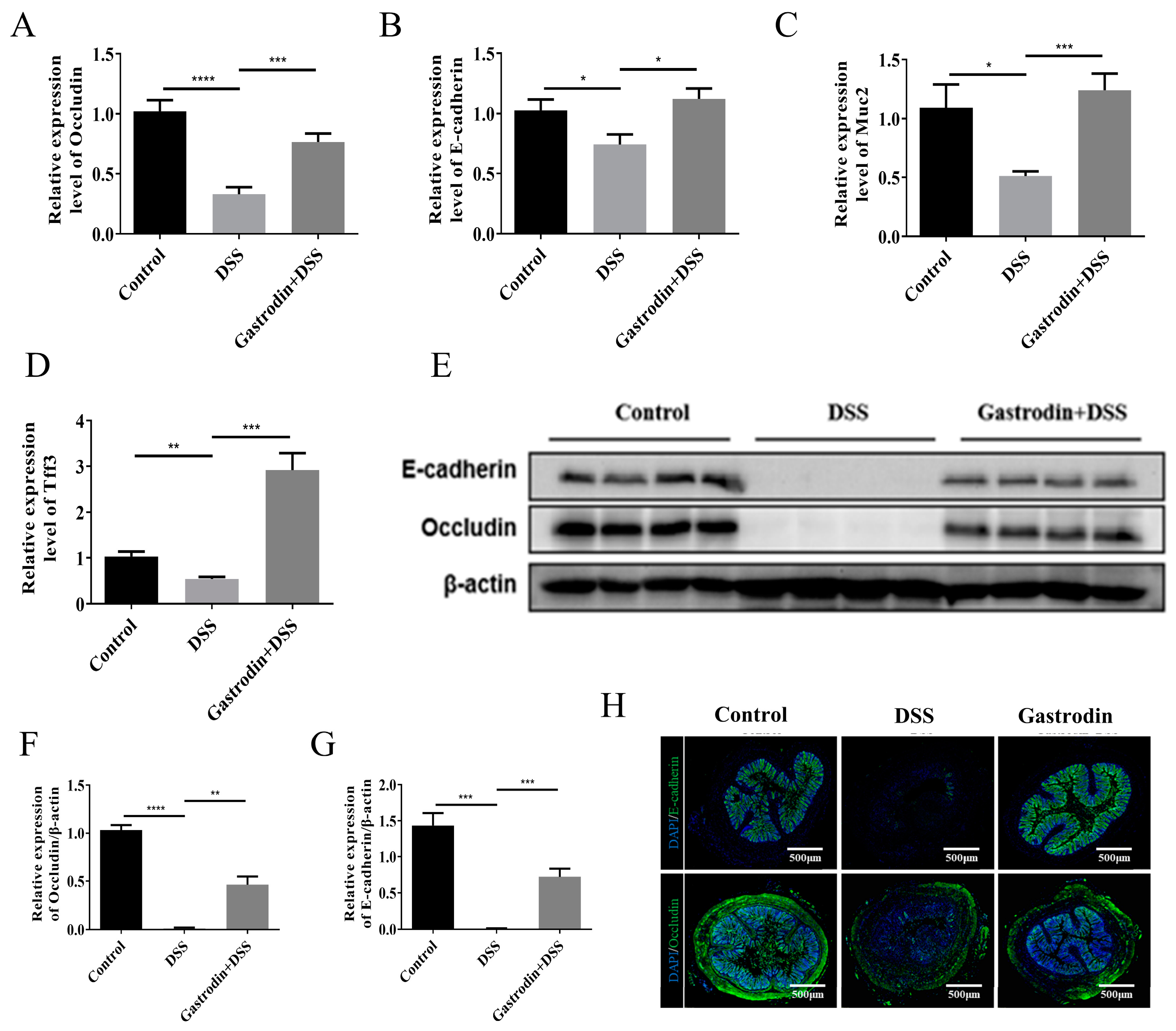
3.3. Gastrodin Increased the Expression of Epithelial Tissue Protein in Mice
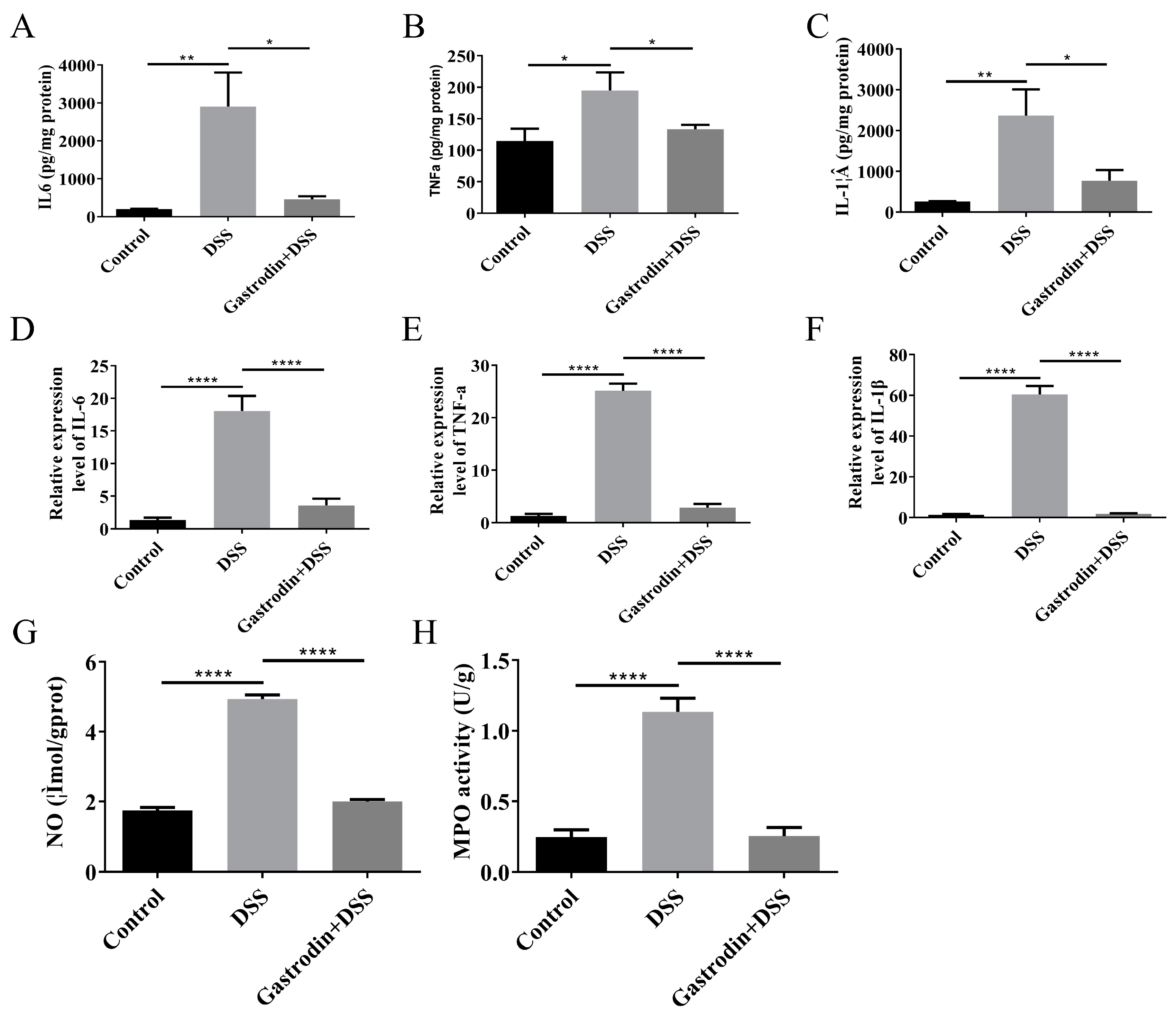
3.4. Gastrodin-Modulated Inflammatory Cytokines and Oxidative Stress in Mice
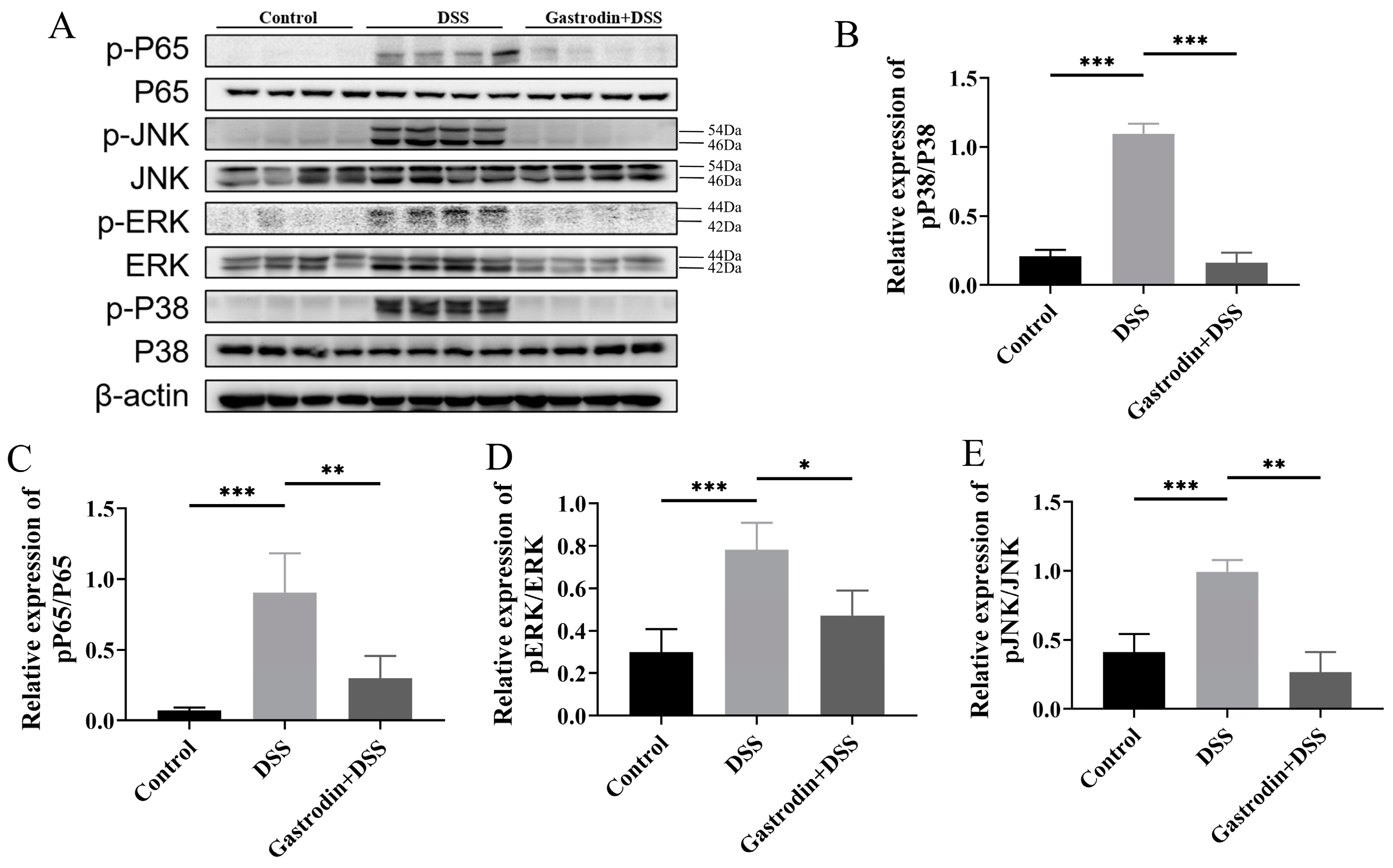
3.5. Gastrodin Suppresses Inflammatory Response through Modulating NF-κB and MAPK Pathways
3.6. Gastrodin-Modulated Intestinal Microbiota in DSS-Induced Colitis Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Park, J.; Jeong, G.; Song, M.; Yon, D.; Lee, S.; Koyanagi, A.; Jacob, L.; Kostev, K.; Dragioti, E.; Radua, J.; et al. The global, regional, and national burden of inflammatory bowel diseases, 1990–2019: A systematic analysis for the global burden of disease study 2019. Dig. Liver Dis. 2023, 55, 1352–1359. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 2019, 68, 242–251. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Cheng, P.; Qian, C.; Xu, F.; Yang, Y.; Wang, A.; Chen, W.; Sun, Z.; Lu, Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [CrossRef] [PubMed]
- Plichta, D.R.; Graham, D.B.; Subramanian, S.; Xavier, R.J. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell 2019, 178, 1048–1056. [Google Scholar] [CrossRef]
- Hoffmann, P.; Lamerz, D.; Hill, P.; Kirchner, M.; Gauss, A. Gene Polymorphisms of NOD2, IL23R, PTPN2 and ATG16L1 in Patients with Crohn’s Disease: On the Way to Personalized Medicine? Genes 2021, 12, 866. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am. J. Transl. Res. 2016, 8, 2490. [Google Scholar]
- Ma, H.; Zhou, M.; Duan, W.; Chen, L.; Wang, L.; Liu, P. Anemoside B4 prevents acute ulcerative colitis through inhibiting of TLR4/NF-κB/MAPK signaling pathway. Int. Immunopharmacol. 2020, 87, 106794. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; Mciver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2018, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gkouskou, K.K.; Deligianni, C.; Tsatsanis, C.; Eliopoulos, A.G. The gut microbiota in mouse models of inflammatory bowel disease. Front. Cell. Infect. Microbiol. 2014, 4, 28. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; De Lima Romão, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, H.-G.; Zhang, M.-N.; Zhang, M.-H.; Wang, H.; Yang, X.-Z. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J. Gastroenterol. 2021, 27, 2834–2849. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Bi, X.; Ai, Q.; Guo, J.; Dong, S.; Xu, J.; Zhong, L.; Lu, D. Effects of gastrodin on apoptotic factors of cerebral cortex neuron in epileptic rats. Chin. J. Neuroanat. 2016, 32, 37–42. [Google Scholar]
- Chen, L.; Liu, X.; Wang, H.; Qu, M. Gastrodin Attenuates Pentylenetetrazole-Induced Seizures by Modulating the Mitogen-Activated Protein Kinase-Associated Inflammatory Responses in Mice. Neurosci. Bull. 2016, 33, 264–272. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lai, Y.-S.; Lin, S.-H.; Lu, K.-H.; Lin, Y.-E.; Panyod, S.; Ho, C.-T.; Sheen, L.-Y. Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J. Ethnopharmacol. 2016, 182, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.J.; Deng, S.X. Pharmacological studies on Gastrodia elata blume. Acta Bot. Yunnanica 1980, 2, 230–234. [Google Scholar]
- Huang, L.; Shao, M.; Zhu, Y. Gastrodin inhibits high glucose-induced inflammation, oxidative stress and apoptosis in podocytes by activating the AMPK/Nrf2 signaling pathway. Exp. Ther. Med. 2021, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yang, L.; Jiao, J.; Jiang, Y.; Li, H.; Yin, G.; Yang, P.; Sun, L. Aspirin in combination with gastrodin protects cardiac function and mitigates gastric mucosal injury in response to myocardial ischemia/reperfusion. Front. Pharmacol. 2022, 13, 995102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Wang, H.-X.; Li, Q.-X.; Chen, A.-X.; Wang, X.-X.; Zhou, S.; Xie, S.-T.; Li, H.-Z.; Wang, J.-J.; Zhang, Q.; et al. A comparative study of vestibular improvement and gastrointestinal effect of betahistine and gastrodin in mice. Biomed. Pharmacother. 2022, 153, 113344. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, Y.; Zheng, X.; Yang, Y. Gastrodin attenuates perfluorooctanoic acid-induced liver injury by regulating gut microbiota composition in mice. Bioengineered 2021, 12, 11546–11556. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, Y.; Yang, D.; Liang, Y.; Yang, L.; Hu, S.; Liu, Z.; Fang, Q.; Tian, S.; Ding, Y. Gastrodin Improves Nonalcoholic Fatty Liver Disease Through Activation of the Adenosine Monophosphate–Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 3074–3090. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, H.; Fu, Y.; Dai, S. Application of Herbaceous Medications for Inflammatory Bowel Disease as a Complementary and Alternative Therapy. Inflamm. Bowel Dis. 2019, 25, 1886–1895. [Google Scholar] [CrossRef]
- Xiao, G.; Tang, R.; Yang, N.; Chen, Y. Review on pharmacological effects of gastrodin. Arch. Pharmacal Res. 2023, 46, 744–770. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2012, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Z.; Lin, Q.; Bei, W.; Guo, J. Pathological and therapeutic roles of bioactive peptide trefoil factor 3 in diverse diseases: Recent progress and perspective. Cell Death Dis. 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Li, F.; Xu, M.; Chen, B.; Zhao, Y.; Wang, M.; Li, L. Gastrodin ameliorates atherosclerosis by inhibiting foam cells formation and inflammation through down-regulating NF-κB pathway. Nutr. Metab. 2023, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-C.; Yu, H.-P.; Chou, A.-H.; Lee, H.-C.; Hu, L.-M.; Liu, F.-C. Gastrodin Alleviates Acetaminophen-Induced Liver Injury in a Mouse Model Through Inhibiting MAPK and Enhancing Nrf2 Pathways. Inflammation 2022, 45, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, J.; Zhang, Z.; Li, J.; Zou, H.; Tan, X.; Wang, Y.; Yao, Y.; Xiong, W. Propionic Acid Driven by the Lactobacillus johnsonii Culture Supernatant Alleviates Colitis by Inhibiting M1 Macrophage Polarization by Modulating the MAPK Pathway in Mice. J. Agric. Food Chem. 2023, 71, 14951–14966. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-X.; Liang, J.-L.; Yang, C.-H.; Li, K.; Niu, M.-T.; Fan, J.-X.; Zhang, X.-Z. Stimulation-responsive mucoadhesive probiotics for inflammatory bowel disease treatment by scavenging reactive oxygen species and regulating gut microbiota. Biomaterials 2023, 301, 122274. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Xia, C.; Liu, N.; Chen, Z.; Zhou, Q.; Li, P. Lactobacillus plantarum ZJ316 alleviates ulcerative colitis by inhibiting inflammation and regulating short-chain fatty acid levels and the gut microbiota in a mouse model. Food Funct. 2023, 14, 3982–3993. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Hu, X.-L.; Yuan, Z.-W.; Xiao, Y.; Ji, P.; Wei, Y.-M.; Hua, Y.-L. Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity. J. Ethnopharmacol. 2022, 300, 115741. [Google Scholar] [CrossRef] [PubMed]
- Min, S.J.; Kim, H.; Yambe, N.; Shin, M.-S. Ameliorative Effects of Korean-Red-Ginseng-Derived Polysaccharide on Antibiotic-Associated Diarrhea. Polymers 2024, 16, 231. [Google Scholar] [CrossRef]
- Ma, S.; Qian, C.; Li, N.; Fang, Z.; Zhao, J.; Zhang, H.; Chen, W.; Liu, Z.; Lu, W. Protein diets with the role of immune and gut microbial regulation alleviate DSS-induced chronic ulcerative colitis. Food Sci. Nutr. 2021, 9, 1259–1270. [Google Scholar] [CrossRef]
- Cao, C.; Wang, L.; Ai, C.; Gong, G.; Wang, Z.; Huang, L.; Song, S.; Zhu, B. Impact of Lycium barbarum arabinogalactan on the fecal metabolome in a DSS-induced chronic colitis mouse model. Food Funct. 2022, 13, 8703–8716. [Google Scholar] [CrossRef]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jia, J.; Teng, Y.; Xie, C.; Li, C.; Zhu, B.; Xia, X. Gastrodin Alleviates DSS-Induced Colitis in Mice through Strengthening Intestinal Barrier and Modulating Gut Microbiota. Foods 2024, 13, 2460. https://doi.org/10.3390/foods13152460
Li J, Jia J, Teng Y, Xie C, Li C, Zhu B, Xia X. Gastrodin Alleviates DSS-Induced Colitis in Mice through Strengthening Intestinal Barrier and Modulating Gut Microbiota. Foods. 2024; 13(15):2460. https://doi.org/10.3390/foods13152460
Chicago/Turabian StyleLi, Jiahui, Jinhui Jia, Yue Teng, Chunyuan Xie, Chunwei Li, Beiwei Zhu, and Xiaodong Xia. 2024. "Gastrodin Alleviates DSS-Induced Colitis in Mice through Strengthening Intestinal Barrier and Modulating Gut Microbiota" Foods 13, no. 15: 2460. https://doi.org/10.3390/foods13152460
APA StyleLi, J., Jia, J., Teng, Y., Xie, C., Li, C., Zhu, B., & Xia, X. (2024). Gastrodin Alleviates DSS-Induced Colitis in Mice through Strengthening Intestinal Barrier and Modulating Gut Microbiota. Foods, 13(15), 2460. https://doi.org/10.3390/foods13152460




