Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure
2.3. Identification and Quantification of Polyphenols
2.4. Organic Acids and Sugar Analysis
2.5. Total Phenolic Content
2.6. Determination of Antioxidant Activity
2.6.1. DPPH Radical Scavenging Activity
2.6.2. Ferric-Reducing Antioxidant Power (FRAP)
2.7. Statistical Analysis
2.7.1. Chemometric Analysis
2.7.2. ANN Modeling
2.7.3. Sensitivity Analysis
3. Results and Discussion
3.1. Chemometric Analysis of the Results of Phenolic Content
3.2. Chemometric Analysis of the Results of Fruit Acid and Sugar Content
3.3. ANN Modeling of Antioxidant Activity
3.4. Global Sensitivity Analysis—Yoon’s Interpretation Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. Rome. 2022. Available online: http://faostat.fao.org (accessed on 1 May 2020).
- Mushtaq, R.; Wani, A.W.; Nayik, G.A. Apple. In Antioxidants in Fruits: Properties and Health Benefits; Gulzar, A.N., Amir, G., Eds.; Springer: Singapore, 2020; pp. 507–521. [Google Scholar]
- Kljajić, N.; Paraušić, V.; Vuković, P. Economic aspects of fruit production in Serbia on the example of apple orchards. Ekonomika 2023, 69, 41–53. [Google Scholar] [CrossRef]
- Mignard, P.; Beguería, S.; Giménez, R.; Font i Forcada, C.; Reig, G.; Moreno, M.Á. Effect of genetics and climate on apple sugars and organic acids profiles. Agronomy 2022, 12, 827. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.; Tuohy, K.M. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef]
- Vallée Marcotte, B.; Verheyde, M.; Pomerleau, S.; Doyen, A.; Couillard, C. Health benefits of apple juice consumption: A review of interventional trials on humans. Nutrients 2022, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycka, E.; Amarowicz, R. Diet and health: Apple polyphenols as antioxidants. Food Rev. Int. 2008, 24, 235–251. [Google Scholar] [CrossRef]
- Bohn, T.; Bouayed, J. Apples: An apple a day, still keeping the doctor away? In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 595–612. [Google Scholar]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and apple by-products: Antioxidant properties and food applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2023, 69, 243–256. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of chlorogenic acid against diabetes mellitus and its complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Song, L.; Yang, H.; Liang, D.; Chu, D.; Yang, L.; Li, M.; Guo, J. A chlorogenic acid-loaded hyaluronic acid-based hydrogel facilitates anti-inflammatory and pro-healing effects for diabetic wounds. J. Drug Deliv. Sci. Technol. 2022, 70, 103232. [Google Scholar] [CrossRef]
- Starowicz, M.; Achrem-Achremowicz, B.; Piskuła, M.K.; Zieliński, H. Phenolic compounds from apples: Reviewing their occurrence, absorption, bioavailability, processing, and antioxidant activity—A review. Pol. J. Food Nutr. Sci. 2020, 70, 321–336. [Google Scholar] [CrossRef]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol. 2016, 53, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Petriccione, M.; Rego, P.; Scortichini, M.; Napolitano, A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. 2013, 140, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Liu, Y.; Shi, X.; Wang, Y.; Zhang, C.; Zhao, Z. The effect of fruit bagging on the color, phenolic compounds and expression of the anthocyanin biosynthetic and regulatory genes on the ‘Granny Smith’ apples. Eur. Food Res. Technol. 2013, 237, 875–885. [Google Scholar] [CrossRef]
- Bulatović, M.L.; Rajić, Z.; Đoković, J. Development of fruit production and processing in the Republic of Serbia. Екoнoмика пoљoпривреде 2013, 60, 141–151. [Google Scholar]
- Horvacki, N.; Andrić, F.; Gašić, U.; Đurović, D.; Tešić, Ž.; Fotirić Akšić, M.; Milojković-Opsenica, D. Phenolic compounds as phytochemical tracers of varietal origin of some autochthonous apple cultivars grown in Serbia. Molecules 2022, 27, 7651. [Google Scholar] [CrossRef] [PubMed]
- Mitić, S.S.; Stojanović, B.T.; Stojković, M.B.; Mitić, M.N.; Pavlović, J.L. Total phenolics, flavonoids and antioxidant activity of different apple cultivars. Bulg. Chem. Commun. 2013, 45, 326–331. [Google Scholar]
- Mureşan, E.A.; Muste, S.; Borşa, A.; Vlaic, R.; Mureşan, V. Evaluation of physical-chemical indexes, sugars, pigments and phenolic compounds of fruits from three apple varieties at the end of storage period. Bull. UASVM Food Sci. Technol. 2014, 71, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mureșan, A.E.; Sestras, A.F.; Militaru, M.; Păucean, A.; Tanislav, A.E.; Pușcaș, A.; Mateescu, M.; Mureșan, V.; Marc, R.A.; Sestras, R.E. Chemometric comparison and classification of 22 apple genotypes based on texture analysis and physico-chemical quality attributes. Horticulturae 2022, 8, 64. [Google Scholar] [CrossRef]
- Mignard, P.; Beguería, S.; Reig, G.; Forcada, C.F.; Moreno, M.A. Genetic origin and climate determine fruit quality and antioxidant traits on apple (Malus x domestica Borkh). Sci. Hortic. 2021, 285, 110142. [Google Scholar] [CrossRef]
- Villanueva-Carvajal, A.; Bernal-Martínez, L.R.; García-Gasca, M.T.; Dominguez-Lopez, A. In vitro gastrointestinal digestion of Hibiscus sabdariffa L.: The use of its natural matrix to improve the concentration of phenolic compounds in gut. LWT-Food Sci. Technol. 2013, 51, 260–265. [Google Scholar] [CrossRef]
- Bhuyan, U.; Handique, J.G. Plant polyphenols as potent antioxidants: Highlighting the mechanism of antioxidant activity and synthesis/development of some polyphenol conjugates. Stud. Nat. Prod. Chem. 2022, 75, 243–266. [Google Scholar]
- He, F.; Chen, J.; Dong, K.; Leng, Y.; Xu, J.; Hu, P.; Yao, Y.; Xiong, J.; Pei, X. Multi-technical analysis on the antioxidative capacity and total phenol contents of 94 traditional Chinese dietary medicinal herbs. Food Sci. Nutr. 2018, 6, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Scalzo, R.L.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Turemko, M. Electroactive phenolic contributors and antioxidant capacity of flesh and peel of 11 apple cultivars measured by cyclic voltammetry and HPLC–DAD–MS/MS. Antioxidants 2020, 9, 1054. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Wendelin, S.; Eder, R. Quantitative Bestimmung von Hydroxyzimtsäuren und Hydroxyzimtsäurenderivaten in Weißweinen mittels HPLC. Mitteilungen Klosterneubg. 1997, 47, 164–172. [Google Scholar]
- Wendelin, S.; Korntheuer, K.; Baumann, R.; Brandes, W. Vergleich von Wildheidelbeeren (Vaccinium myrtillus) und Kulturblaubeeren (Vaccinium corymbosum) hinsichtlich ausgewählter Inhaltsstoffe. Mitteilungen Klosterneubg. 2018, 68, 221–240. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2, 2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2020, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Mohammed, N.A.; Ismail, Z.Z. Prediction of pollutants removal from cheese industry wastewater in constructed wetland by artificial neural network. Int. J. Sci. Environ. Technol. 2021, 19, 9775–9790. [Google Scholar] [CrossRef]
- Rajković, D.; Marjanović Jeromela, A.; Pezo, L.; Lončar, B.; Zanetti, F.; Monti, A.; Kondić Špika, A. Yield and quality prediction of winter rapeseed—Artificial neural network and random forest models. Agronomy 2021, 12, 58. [Google Scholar] [CrossRef]
- Ochoa-Martínez, C.I.; Ayala-Aponte, A.A. Prediction of mass transfer kinetics during osmotic dehydration of apples using neural networks. LWT-Food Sci. Technol. 2007, 40, 638–645. [Google Scholar] [CrossRef]
- Yoon, G.H.; Donoso, A.; Carlos Bellido, J.; Ruiz, D. Highly efficient general method for sensitivity analysis of eigenvectors with repeated eigenvalues without passing through adjacent eigenvectors. Int. J. Numer. Methods Eng. 2020, 121, 4473–4492. [Google Scholar] [CrossRef]
- Geană, E.I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of phenolic compounds and triterpene acids of twelve apple (Malus domestica Borkh.) cultivars. Foods 2021, 10, 267. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A comparative study of phenolic content in apple fruits. Int. J. Food Prop. 2015, 18, 945–953. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic review of phenolic compounds in apple fruits: Compositions, distribution, absorption, metabolism, and processing stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Zupan, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Sugar and phenol content in apple with or without watercore. J. Sci. Food Agric. 2016, 96, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Piagentini, A.M.; Pirovani, M.E. Total phenolics content, antioxidant capacity, physicochemical attributes, and browning susceptibility of different apple cultivars for minimal processing. Int. J. Fruit Sci. 2017, 17, 102–116. [Google Scholar] [CrossRef]
- Lamperi, L.; Chiuminatto, U.; Cincinelli, A.; Galvan, P.; Giordani, E.; Lepri, L.; Del Bubba, M. Polyphenol levels and free radical scavenging activities of four apple cultivars from integrated and organic farming in different Italian areas. J. Agric. Food Chem. 2008, 56, 6536–6546. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of predominant organic acid components in Malus species: Correlation with apple domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Galindo, E.; Piagentini, A.M. Sequential ultrasound-assisted extraction of pectin and phenolic compounds for the valorisation of ‘Granny Smith’apple peel. Food Biosci. 2022, 49, 101958. [Google Scholar] [CrossRef]
- Çetin, N.; Sağlam, C. Rapid detection of total phenolics, antioxidant activity and ascorbic acid of dried apples by chemometric algorithms. Food Biosci. 2022, 47, 101670. [Google Scholar] [CrossRef]
- Peter, A.; Žlabur Šic, J.; Šurić, J.; Voća, S.; Purgar, D.D.; Pezo, L.; Voća, N. Invasive plant species biomass—Evaluation of functional value. Molecules 2021, 26, 3814. [Google Scholar] [CrossRef]
- Đorđević, N.O.; Todorović, N.; Novaković, I.T.; Pezo, L.L.; Pejin, B.; Maraš, V.; Tešević, V.V.; Pajović, S.B. Antioxidant activity of selected polyphenolics in yeast cells: The case study of Montenegrin Merlot wine. Molecules 2018, 23, 1971. [Google Scholar] [CrossRef] [PubMed]
- Stajčić, S.M.; Pezo, L.L.; Ćetković, G.S.; Čanadanović-Brunet, J.M.; Mandić, A.I.; Tumbas-Šaponjac, V.T.; Vulić, J.J.; Travičić, V.N.; Belović, M.M. Antioxidant activity according to bioactive compounds content in dried pumpkin waste. J. Serb. Chem. Soc. 2024, 89, 13–27. [Google Scholar] [CrossRef]
- Nićetin, M.; Pezo, L.; Pergal, M.; Lončar, B.; Filipović, V.; Knežević, V.; Demir, H.; Filipović, J.; Manojlović, D. Celery root phenols content, antioxidant capacities and their correlations after osmotic dehydration in molasses. Foods 2022, 11, 1945. [Google Scholar] [CrossRef]
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Kviesis, J.; Salar, F.J.; Moreno, D.A.; Drudze, I. Wild apple (Malus spp.) by-products as a source of phenolic compounds and vitamin C for food applications. Food Biosci. 2022, 38, 100744. [Google Scholar] [CrossRef]
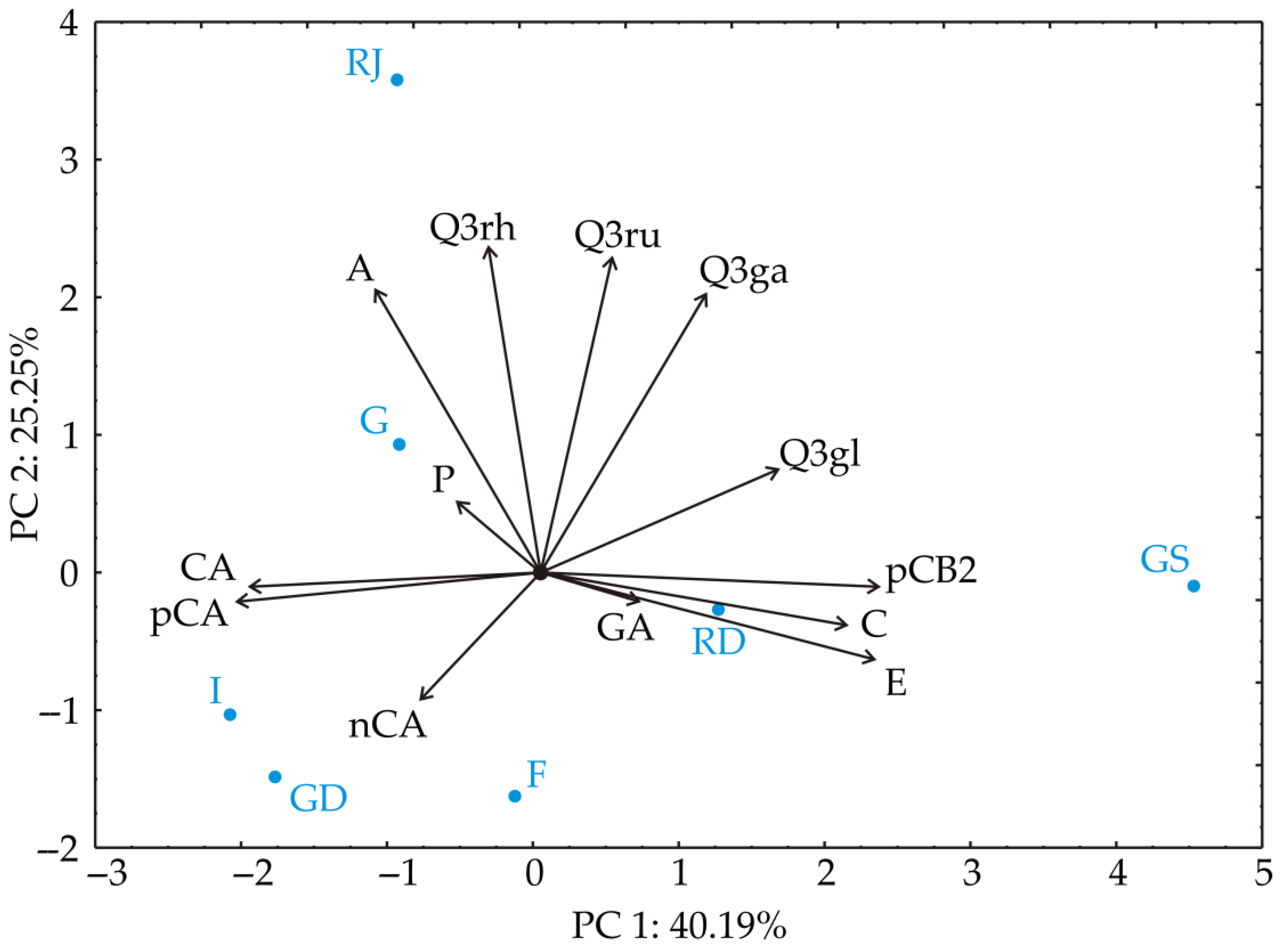
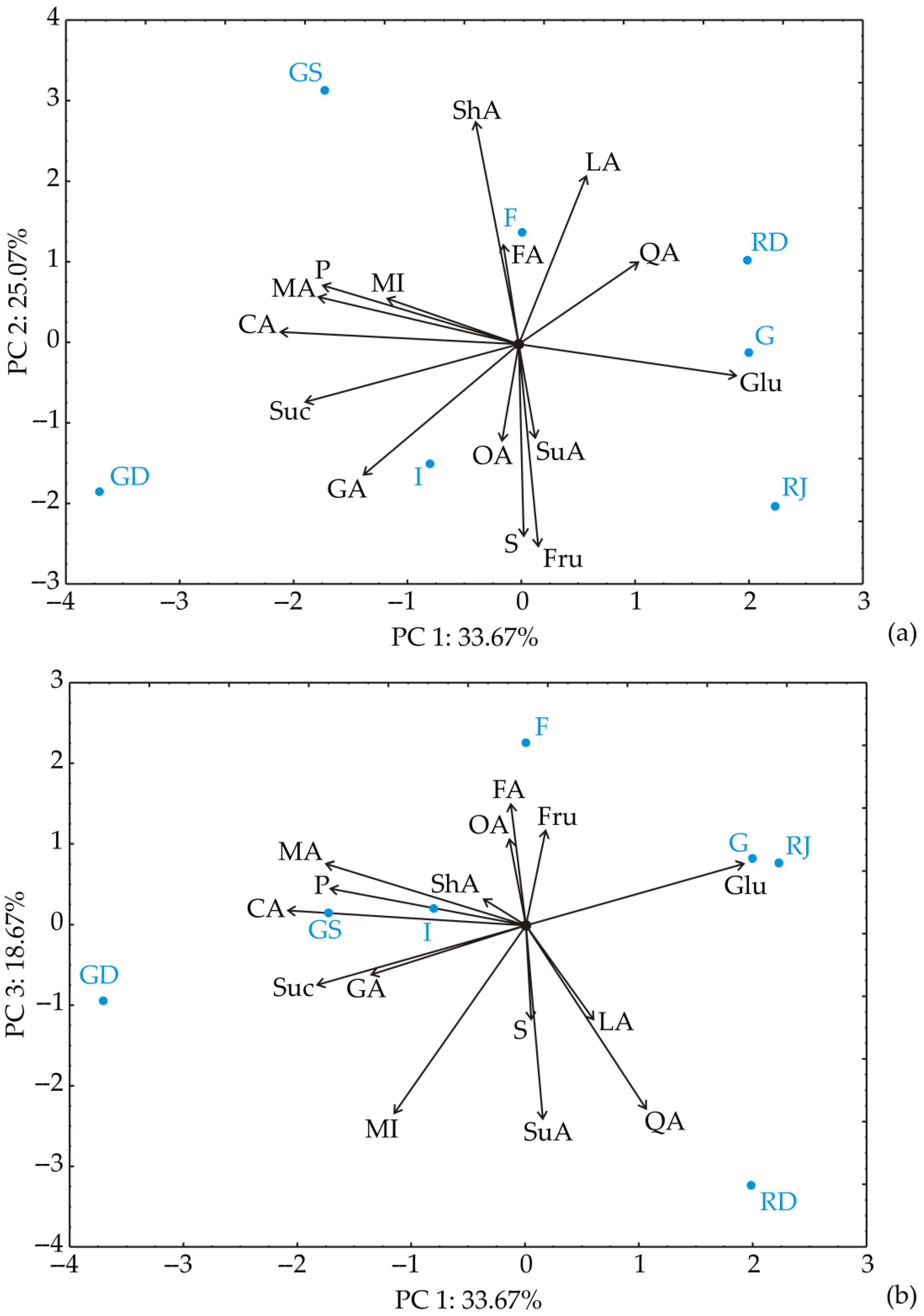


| Conc. mg/kg FW | Gala | Red Jonaprince | Red Delicious | Fuji | Granny Smith | Idared | Golden Delicious | |
|---|---|---|---|---|---|---|---|---|
| A | 0.24 ± 0.41 ab | 0.86 ± 0.58 b | n.d. | n.d. | n.d. | 0.21 ± 0.36 ab | 0.29 ± 0.49 ab | |
| Phenolic acids * | HBA | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| GA | 0.17 ± 0.07 a | 0.14 ± 0.04 a | 0.56 ± 0.13 b | 0.16 ± 0.12 a | 0.18 ± 0.03 a | 0.22 ± 0.05 a | 0.10 ± 0.09 a | |
| pCA | 2.57 ± 0.53 cd | 1.72 ± 0.53 bc | 2.15 ± 0.42 c | 1.33 ± 0.07 b | n.d. | 3.14 ± 0.16 d | 2.36 ± 0.18 cd | |
| CA | 156.37 ± 46.01 cd | 94.93 ± 30.04 b | 75.90 ± 15.06 ab | 104.52 ± 5.22 bc | 30.24 ± 8.71 a | 162.72 ± 10.27 d | 97.49 ± 14.88 bc | |
| nCA | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.17 ± 0.03 | |
| Flavan-3-ols | C | 9.46 ± 4.10 bc | 3.02 ± 0.63 a | 13.02 ± 1.67 c | 5.57 ± 0.90 ab | 21.19 ± 1.48 d | 8.89 ± 1.64 bc | 4.02 ± 0.16 a |
| E | 31.04 ± 4.79 a | 26.40 ± 4.13 a | 48.92 ± 6.21 b | 36.79 ± 4.46 a | 59.53 ± 7.65 b | 27.58 ± 2.35 a | 32.88 ± 2.82 a | |
| pCB1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| pCB2 | 64.17 ± 9.27 ab | 56.47 ± 9.91 a | 73.11 ± 5.93 ab | 58.38 ± 11.66 a | 92.96 ± 24.12 b | 54.92 ± 3.96 a | 58.92 ± 4.51 a | |
| Flavonol | Q3ga | 6.96 ± 2.91 a | 9.14 ± 7.73 a | 8.25 ± 4.18 a | 3.17 ± 2.01 a | 8.06 ± 3.58 a | 4.68 ± 2.92 a | 3.87 ± 5.21 a |
| Q3gl | 1.72 ± 0.50 a | 2.21 ± 1.48 a | 1.34 ± 0.49 a | 2.33 ± 2.78 a | 2.78 ± 0.92 a | 1.00 ± 0.10 a | 1.37 ± 1.05 a | |
| Q3ru | 7.52 ± 2.33 a | 7.93 ± 4.48 a | 5.90 ± 1.82 a | 3.13 ± 1.33 a | 5.88 ± 2.03 a | 4.27 ± 0.68 a | 2.85 ± 2.03 a | |
| Q3rh | 10.41 ± 5.91 ab | 22.90 ± 11.46 b | 5.69 ± 1.57 a | 4.40 ± 1.66 a | 8.19 ± 1.95 a | 4.96 ± 1.17 a | 8.79 ± 3.32 a | |
| Dihydrochalcone | P | 18.97 ± 4.32 bc | 12.85 ± 2.42 ab | 23.92 ± 8.34 c | 8.25 ± 1.21 a | 5.58 ± 3.37 a | 13.00 ± 1.61 ab | 10.78 ± 2.67 ab |
| Cultivar | TPC (mg GAE/kg FW) | DPPH IC50 (μg/mL) | FRAP (mg AAE/kg FW) |
|---|---|---|---|
| Gala * | 620.67 ± 57.55 ab | 360.94 ± 88.65 a | 210.41 ± 42.19 a |
| Red Jonaprince | 972.74 ± 182.74 d | 399.35 ± 90.15 a | 347.29 ± 95.26 a |
| Red Delicious | 844.98 ± 13.26 cd | 302.67 ± 9.66 a | 267.22 ± 26.81 a |
| Fuji | 696.61 ± 24.74 abc | 435.26 ± 40.25 a | 311.18 ± 44.11 a |
| Granny Smith | 579.39 ± 24.28 a | 1182.17 ± 275.04 b | 300.82 ± 52.30 a |
| Idared | 807.63 ± 6.56 bcd | 349.43 ± 9.68 a | 318.10 ± 40.76 a |
| Golden Delicious | 692.12 ± 7.75 abc | 439.95 ± 50.76 a | 214.14 ± 29.56 a |
| Conc. mg/kg FW | Gala | Red Jonaprince | Red Delicious | Fuji | Granny Smith | Idared | Golden Delicious |
|---|---|---|---|---|---|---|---|
| QA * | 178.78 ± 79.73 b | 132.01 ± 27.29 ab | 526.77 ± 29.59 c | 127.30 ± 16.94 ab | 151.01 ± 25.27 ab | 76.33 ± 16.13 a | 88.31 ± 20.20 ab |
| LA | 69.39 ± 27.04 ab | n.d. | 165.25 ± 89.55 b | 93.05 ± 30.25 ab | 101.74 ± 40.03 ab | 86.22 ± 55.78 ab | n.d. |
| ShA | 69.35 ± 14.06 a | 74.05 ± 11.73 a | 208.96 ± 120.63 a | 312.73 ± 132.77 a | 411.66 ± 405.67 a | 114.51 ± 7.08 a | 68.07 ± 17.10 a |
| GA | n.d. | 37.03 ± 49.62 a | n.d. | n.d. | n.d. | n.d. | 181.46 ± 36.12 b |
| SuA | 126.82 ± 64.23 a | 146.36 ± 110.31 a | 193.57 ± 28.07 a | 127.64 ± 53.78 a | 119.04 ± 17.80 a | 169.18 ± 10.56 a | 161.66 ± 7.72 a |
| MA | 4023.98 ± 631.65 ab | 3138.72 ± 687.10 a | 2744.00 ± 656.17 a | 4771.05 ± 453.67 bc | 6958.48 ± 484.10 d | 6359.65 ± 752.74 d | 5860.42 ± 435.24 cd |
| FA | 34.35 ± 19.07 a | 32.71 ± 3.01 a | 62.72 ± 37.33 a | 304.87 ± 219.17 b | 71.97 ± 33.62 a | 48.58 ± 34.91 a | 51.49 ± 12.78 a |
| OA | 16.54 ± 4.08 a | 24.40 ± 10.08 a | 14.36 ± 10.40 a | 25.82 ± 3.98 a | 16.25 ± 2.01 a | 44.40 ± 42.71 a | 16.91 ± 4.60 a |
| CA | 47.65 ± 5.84 a | 47.12 ± 10.35 a | 52.46 ± 43.57 a | 91.55 ± 29.44 ab | 106.01 ± 29.60 ab | 94.80 ± 11.84 ab | 131.87 ± 10.45 b |
| P | 118.66 ± 25.08 ab | 102.34 ± 38.61 a | 136.98 ± 38.49 ab | 238.18 ± 56.27 b | 195.22 ± 106.25 ab | 151.25 ± 6.00 ab | 248.49 ± 27.61 b |
| Conc. g/kg FW | Gala | Red Jonaprince | Red Delicious | Fuji | Granny Smith | Idared | Golden Delicious | |
|---|---|---|---|---|---|---|---|---|
| Sugars * | Glu | 20.95 ± 0.70 abc | 24.42 ± 4.92 c | 21.11 ± 1.68 bc | 21.89 ± 1.77 bc | 16.29 ± 0.87 ab | 18.81 ± 1.26 abc | 14.64 ± 1.85 a |
| Fru | 54.43 ± 3.84 c | 52.87 ± 9.41 c | 40.52 ± 2.92 ab | 49.89 ± 3.37 bc | 34.60 ± 1.30 c | 55.61 ± 1.51 c | 52.62 ± 4.47 c | |
| Suc | 15.84 ± 0.53 a | 20.04 ± 4.59 ab | 22.20 ± 2.20 ab | 22.77 ± 1.52 ab | 24.77 ± 1.17 bc | 28.45 ± 4.70 bc | 32.92 ± 2.41 c | |
| Gal | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Sugar alcohols | S | 2.61 ± 0.52 a | 3.62 ± 1.56 a | 3.47 ± 0.20 a | 2.87 ± 0.64 a | 2.31 ± 1.55 a | 3.57 ± 0.43 a | 3.58 ± 0.09 a |
| MI | 0.59 ± 0.12 ab | 0.48 ± 0.06 a | 0.82 ± 0.05 c | 0.47 ± 0.10 a | 0.81 ± 0.04 c | 0.69 ± 0.10 bc | 0.85 ± 0.11 c |
| Phenolics | DPPH | FRAP |
|---|---|---|
| Arbutin | −0.288 | 0.292 |
| Gallic acid | −0.236 | −0.025 |
| Neo-chlorogenic acid | −0.08 | −0.561 |
| Catechin | 0.745 | −0.009 |
| Chlorogenic acid | −0.709 | −0.189 |
| p-coumaric acid | −0.853 * | −0.317 |
| Procyanidin B2 | 0.834 * | −0.054 |
| Epicatechin | 0.737 | −0.021 |
| Qu-3-galactoside | 0.236 | 0.209 |
| Qu-3-glucoside | 0.716 | 0.363 |
| Phloridzin | −0.656 | −0.364 |
| Qu-3-rutinoside | 0.039 | 0.126 |
| Qu-3-rhamnoside | −0.048 | 0.286 |
| Total polyphenols content | −0.462 * | 0.543 * |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A * | 0.055 | 0.184 | −0.187 | 0.839 | 0.337 | 0.062 | 0.114 | 0.217 | 0.214 | 0.178 | 0.335 |
| GA | 0.663 | −0.232 | 0.355 | 0.748 | 0.278 | 0.230 | −0.118 | 0.494 | −0.062 | −0.835 | −0.301 |
| C | 0.021 | −0.277 | 0.330 | −0.222 | −0.196 | 0.169 | −0.083 | 0.242 | 0.163 | −0.224 | −0.148 |
| CA | 0.124 | −0.260 | 0.006 | 0.003 | −0.214 | 0.101 | −0.221 | −0.272 | −0.378 | −0.116 | −0.490 |
| pCA | 0.018 | −0.314 | −0.248 | −0.136 | −0.025 | −0.303 | −0.185 | −0.502 | −0.353 | −0.024 | 0.008 |
| pCB2 | −0.488 | 0.027 | −0.009 | −0.886 | −0.339 | −0.144 | 0.114 | −0.100 | 0.103 | 0.399 | 0.351 |
| E | −0.210 | −0.042 | 0.113 | −0.605 | −0.171 | −0.016 | 0.100 | 0.072 | 0.027 | −0.014 | 0.266 |
| Q3ga | 0.009 | 0.110 | −0.063 | 0.520 | 0.060 | 0.162 | 0.127 | 0.369 | 0.301 | −0.013 | 0.189 |
| Q3gl | −0.174 | 0.233 | 0.230 | −0.133 | −0.215 | 0.396 | 0.247 | 0.552 | 0.198 | 0.003 | −0.097 |
| P | −0.235 | 0.040 | −0.384 | −0.537 | −0.234 | −0.399 | −0.193 | −0.770 | −0.482 | 0.223 | 0.258 |
| Q3ru | −0.215 | 0.261 | −0.144 | 0.243 | −0.168 | 0.031 | 0.094 | 0.033 | 0.042 | 0.274 | 0.016 |
| Q3rh | −0.231 | 0.351 | −0.342 | 0.396 | 0.140 | −0.041 | 0.306 | 0.083 | 0.203 | 0.374 | 0.436 |
| Bias | −0.080 | −0.120 | 0.022 | −0.162 | −0.067 | 0.006 | −0.010 | −0.038 | −0.145 | −0.157 | 0.099 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Bias | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | −0.193 | −0.669 | 0.025 | −0.005 | 0.357 | 0.025 | −0.198 | 0.227 | 0.532 | 0.423 | −0.074 | −0.118 |
| FRAP | 0.438 | −0.143 | 0.419 | 0.598 | −0.262 | 0.269 | −0.126 | 0.371 | 0.010 | −0.493 | −0.620 | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvetković, B.; Bajić, A.; Belović, M.; Pezo, L.; Dragojlović, D.; Šimurina, O.; Djordjević, M.; Korntheuer, K.; Philipp, C.; Eder, R. Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach. Foods 2024, 13, 2291. https://doi.org/10.3390/foods13142291
Cvetković B, Bajić A, Belović M, Pezo L, Dragojlović D, Šimurina O, Djordjević M, Korntheuer K, Philipp C, Eder R. Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach. Foods. 2024; 13(14):2291. https://doi.org/10.3390/foods13142291
Chicago/Turabian StyleCvetković, Biljana, Aleksandra Bajić, Miona Belović, Lato Pezo, Danka Dragojlović, Olivera Šimurina, Marijana Djordjević, Karin Korntheuer, Christian Philipp, and Reinhard Eder. 2024. "Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach" Foods 13, no. 14: 2291. https://doi.org/10.3390/foods13142291
APA StyleCvetković, B., Bajić, A., Belović, M., Pezo, L., Dragojlović, D., Šimurina, O., Djordjević, M., Korntheuer, K., Philipp, C., & Eder, R. (2024). Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach. Foods, 13(14), 2291. https://doi.org/10.3390/foods13142291












