Effects of Osmotic Dehydration on Mass Transfer of Tender Coconut Kernel
Abstract
1. Introduction
2. Materials and Methods
2.1. Tender Coconut Kernel Treatment and Osmotic Dehydration
2.2. Determination of Moisture Content
2.3. Determination of Soluble Solids
2.4. Calculation of Solid Content Increase Rate (SG), Dry Base Solid Content Increase Rate (DSG), Water Loss Rate (WL), and Dehydration Efficiency (DEI)
2.5. Calculation of Water Diffusion Coefficient and Solid Diffusion Coefficient of Tender Coconut Kernel
2.6. Calculation of Mass Transfer Coefficient and Osmotic Equilibrium Point of Coconut Kernel
2.7. Statistical Analysis
3. Results
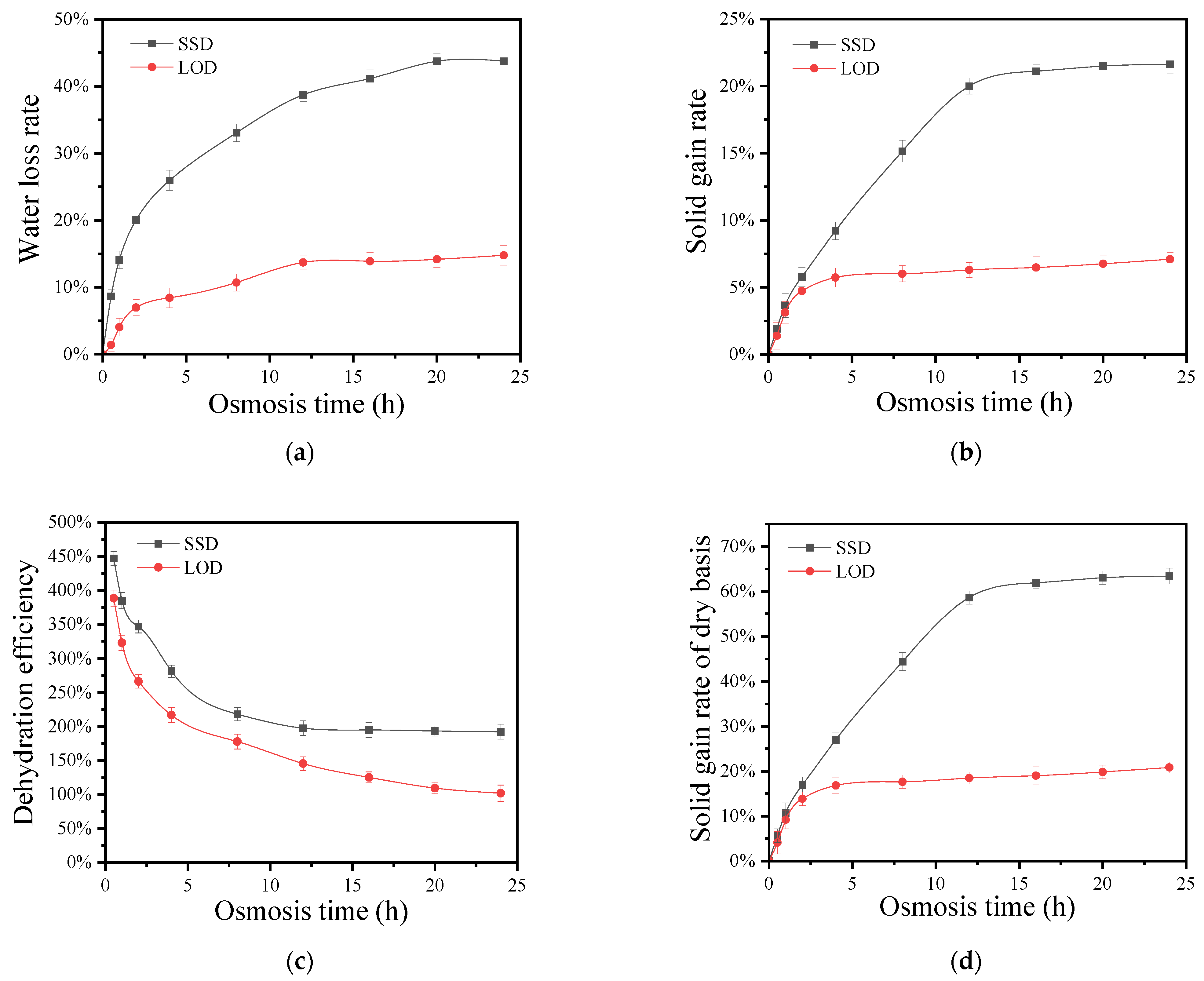
3.1. Effects of SSD and LOD of Sucrose on Dehydration and Mass Transfer of TCK
3.1.1. Effects of SSD and LOD of Sucrose on the Water Loss Rate of TCK
3.1.2. Effects of SSD and LOD of Sucrose on Solid Gain Rate
3.1.3. Effects of SSD and LOD of Sucrose on Dehydration Efficiency of Coconut Kernel
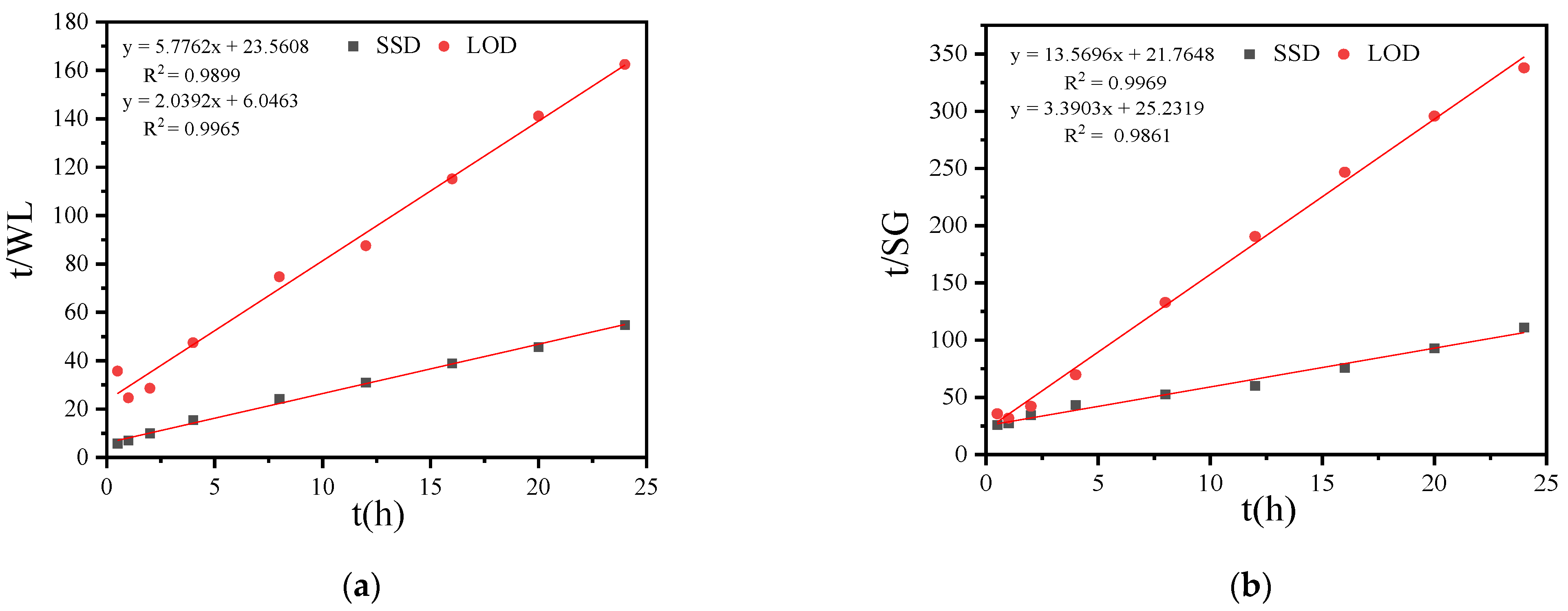
3.1.4. Effects of SSD and LOD of Sucrose on Mass Transfer Coefficient and Osmotic Equilibrium Point
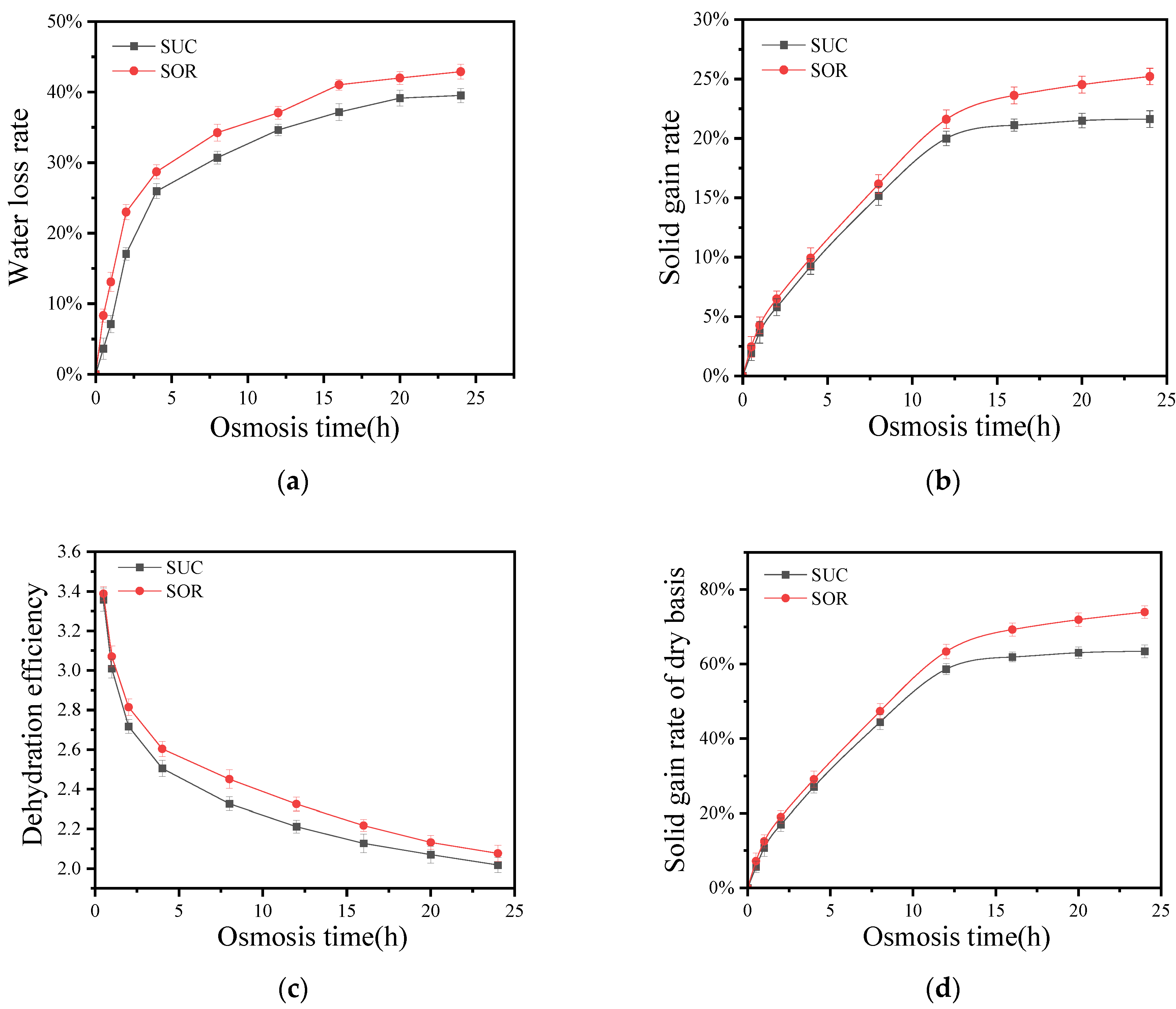
3.2. Effects of Solid Osmosis of Sucrose and Sorbitol on Dehydration and Mass Transfer of Coconut Kernel
3.2.1. Effects of Solid Osmosis of Sucrose and Sorbitol on the Water Loss Rate of Coconut Kernel
3.2.2. Effects of Solid Osmosis of Sucrose and Sorbitol on the Solid Gain Rate of Coconut Kernel
3.2.3. Effects of SSD of Sucrose and Sorbitol on Dehydration Efficiency and Dry Basis Solid Gain Rate
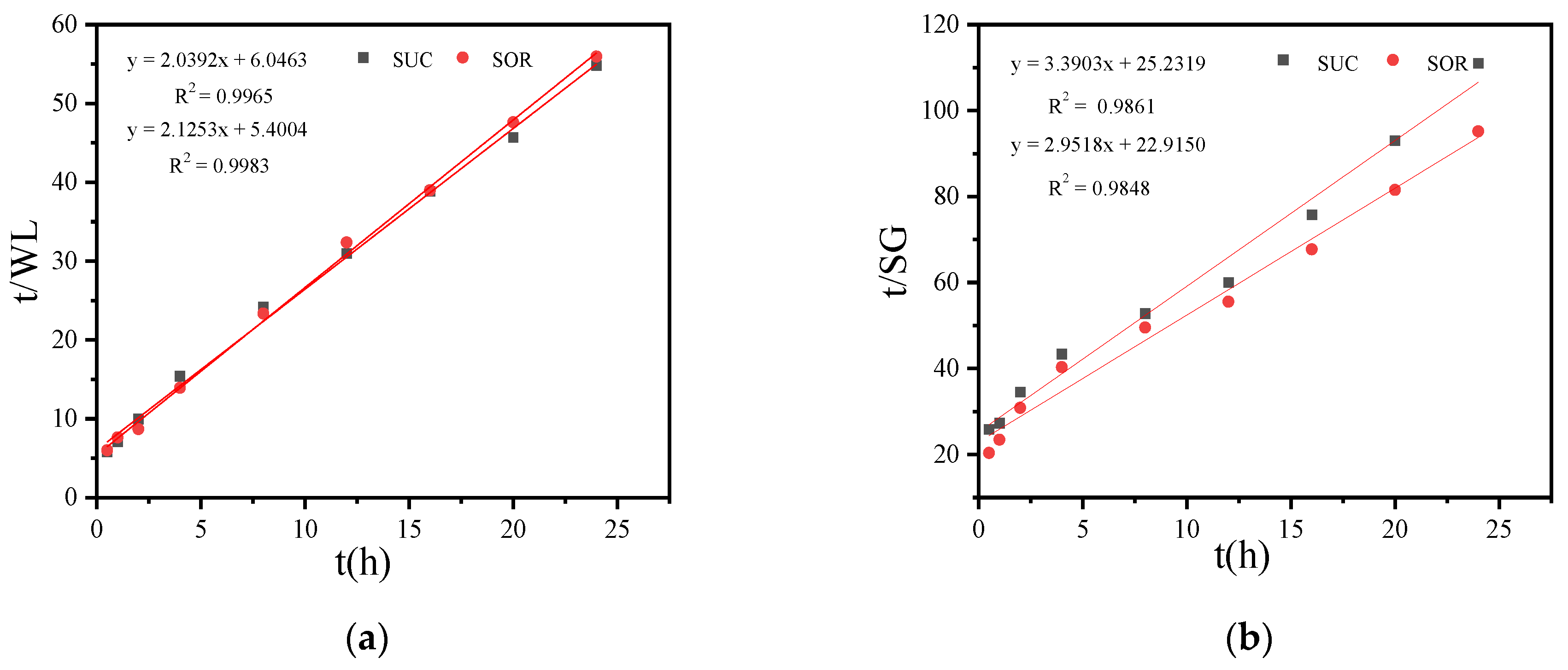
3.2.4. Effects of SSD of Sucrose and Sorbitol on Mass Transfer Coefficient and Osmotic Equilibrium Point
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Xi, X. Bio-screening and quantification of methyl paraben in vinegar and coconut juice separated by HPTLC. Food Sci. Hum. Wellness 2024, 13, 1602–1607. [Google Scholar] [CrossRef]
- Takamatsu, S.; Kuwahara, T.; Kochi, R.; Suzuki, S. Percolation of Primary Crystals in Cell Walls of Aluminum Alloy Foam via Semi-Solid Route. Metals 2020, 10, 847. [Google Scholar] [CrossRef]
- Ganjloo, A.; Bimakr, M. Influence of sucrose solution concentration and temperature on mass exchange during osmotic dehydration of eggplant (Solanum melongena L.) cubes. Int. Food Res. J. 2015, 22, 807–811. [Google Scholar]
- Kaur, D.; Singh, M.; Zalpouri, R.; Singh, I. Osmotic dehydration of fruits using unconventional natural sweeteners and non-thermal-assisted technologies: A review. J. Food Process. Preserv. 2022, 46, e16890. [Google Scholar] [CrossRef]
- Manca, L.M.; Mir-Palomo, S.; Caddeo, C.; Nacher, A.; Díez-Sales, O.; Peris, J.E.; Pedraz, J.L.; Fadda, A.M.; Manconi, M. Sorbitol-penetration enhancer containing vesicles loaded with baicalin for the protection and regeneration of skin injured by oxidative stress and UV radiation. Int. J. Pharm. 2019, 555, 175–183. [Google Scholar] [CrossRef]
- Wiktor, A.; Chadzynska, M.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules 2022, 27, 1376. [Google Scholar] [CrossRef] [PubMed]
- Pravitha, M.; Manikantan, M.; Kumar, V.A.; Beegum, P.S.; Pandiselvam, R. Comparison of drying behavior and product quality of coconut chips treated with different osmotic agents. LWT 2022, 162, 113432. [Google Scholar] [CrossRef]
- Ashtiani, S.H.M.; Aghkhani, M.H.; Feizy, J.; Martynenko, A. Effect of Cold Plasma Pretreatment Coupled with Osmotic Dehydration on Drying Kinetics and Quality of Mushroom (Agaricus bisporus). Food Bioprocess Technol. 2023, 16, 2854–2876. [Google Scholar] [CrossRef]
- Sulistyawati, I.; Verkerk, R.; Fogliano, V.; Dekker, M. Modelling the kinetics of osmotic dehydration of mango: Optimizing process conditions and pre-treatment for health aspects. J. Food Eng. 2020, 280, 109985. [Google Scholar] [CrossRef]
- Zhao, H.W.; Cao, B.B.; Zhang, X.T.; Zhang, Z.Y.; Li, D.J.; Nie, M.M.; Gu, Q.H.; Wang, Y.H.; Wei, B.Q.; Niu, L.Y.; et al. The influence of different infiltration methods on the dehydration efficiency and quality of mangoes. Food Ind. Technol. 2022, 43, 98–105. [Google Scholar]
- Prithani, R.; Dash, K.K. Mass transfer modelling in ultrasound assisted osmotic dehydration of kiwi fruit. Innov. Food Sci. Emerg. Technol. 2020, 64, 102407. [Google Scholar] [CrossRef]
- Souraki, A.B.; Tondro, H.; Ghavami, M. Simulation of mass transfer during osmotic dehydration of apple: A power law approximation method. Heat Mass Transf. 2014, 50, 1443–1453. [Google Scholar] [CrossRef]
- Assis, R.F.; Morais, M.R.; Morais, M.A. Mathematical modelling of osmotic dehydration kinetics of apple cubes. J. Food Process. Preserv. 2017, 41, e12895. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K. Effect of ultrasonic vacuum pretreatment on mass transfer kinetics during osmotic dehydration of black jamun fruit. Ultrason. Sonochemistry 2019, 58, 104693. [Google Scholar] [CrossRef] [PubMed]
- Daszkiewicz, T.; Michalak, M.; Śmiecińska, K. A comparison of the quality of plain yogurt and its analog made from coconut flesh extract. J. Dairy Sci. 2024, 107, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Mayor, L.; Moreira, R.; Chenlo, F.; Sereno, A. Kinetics of osmotic dehydration of pumpkin with sodium chloride solutions. J. Food Eng. 2006, 74, 253–262. [Google Scholar] [CrossRef]
- Wang, R.; Niu, L.Y.; Hu, L.L.; Li, D.J.; Zhang, Z.Y.; Nie, M.M.; Xiao, Y.D.; Liu, C.J.; Xiao, L.X. Moisture distribution and migration law of strawberry during liquid and solid osmotic dehydration. Food Ferment. Ind. 2023, 49, 134–139. [Google Scholar]
- Luo, Y.Y.; Yang, L.; Zhang, J.B. Photoelectrochemical Polymerization for Solid-State Dye Sensitized Solar Cells. Macromol. Rapid Commun. 2021, 43, e2100762. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.3-2016; National Food Safety Standard—Determination of Moisture in Food. China Standards Publishing House: Beijing, China, 2016.
- Dai, R.; Xu, Y.; Chen, P.; Wang, X.; Zheng, T.; Wang, Y.; Ruan, D.; Qiao, Z. 3D sponge-like coconut-meat carbon/molybdenum disulfide nanosheet composite as high-performance anodes for lithium-ion batteries. Int. J. Electrochem. Sci. 2024, 19, 100480. [Google Scholar] [CrossRef]
- Rizwana, H.; Aljowaie, R.M.; Al Otibi, F.; Alwahibi, M.S.; Alharbi, S.A.; Al Asmari, S.A.; Aldosari, N.S.; Aldehaish, H.A. Antimicrobial and antioxidant potential of the silver nanoparticles synthesized using aqueous extracts of coconut meat (Cocos nucifera L.). Sci. Rep. 2023, 13, 16270. [Google Scholar] [CrossRef]
- Carlos, V.M.; Mario, M.V. Concentration-dependent moisture diffusion coefficient estimation in peas drying considering shrinkage: An observer approach. Biosyst. Eng. 2022, 218, 256–273. [Google Scholar]
- Rina, Y.; Novelina; Perdana, D.P. The Effect of Citric Acid Addition on Physicochemical and Organoleptic Characteristics of Young Coconut Meat (Cocos nucifera L.) and Butterfly Pea (Clitoria ternatea) Sheet Jam. IOP Conf. Ser. Earth Environ. Sci. 2023, 1177, 012033. [Google Scholar]
- Rehman, S.U.; Song, H.S.; Kim, H.S.; Hassan, M.H.; Joh, D.W.; Song, R.-H.; Lim, T.-H.; Hong, J.-E.; Park, S.-J.; Lee, S.-B. A dynamic infiltration technique to synthesize nanolayered cathodes for high performance and robust solid oxide fuel cells. J. Energy Chem. 2022, 70, 201–210. [Google Scholar] [CrossRef]
- Li, Z.H.; Bi, J.F.; Yi, J.Y.; Guo, Y.X.; Li, J.; Zhu, F.M. Mass transfer kinetics of different small molecule sugars penetrating strawberries and their impact on the quality of vacuum freeze-dried strawberries. Food Sci. 2022, 43, 95–104. [Google Scholar]
- Pandiselvam, R.; Khanashyam, A.C.; Manikantan, M.R.; Balasubramanian, D.; Beegum, P.P.S.; Ramesh, S.V.; Kothakota, A.; Niral, V.; Shil, S. Textural Properties of Coconut Meat: Implication on the Design of Fiber Extraction and Coconut Processing Equipment. J. Nat. Fibers 2022, 19, 11092–11104. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, Z.; Yang, X.; Liu, W.; Fan, B.; Yan, Z.; Guo, X. In-situ grown Ni Co bimetal anchored on porous straw-derived biochar composites with boosted microwave absorption properties. Int. J. Miner. Metall. Mater. 2023, 30, 515–524. [Google Scholar] [CrossRef]
- González-Pérez, E.J.; Ramírez-Corona, N.; López-Malo, A. Mass Transfer During Osmotic Dehydration of Fruits and Vegetables: Process Factors and Non-Thermal Methods. Food Eng. Rev. 2021, 13, 344–374. [Google Scholar] [CrossRef]
- Liwu, S.L.; Rindengan, B.; Pradhana, A.Y.; Wungkana, J.; Pasang, P. Effect of application edible coating on the quality of kopyor coconut meat during storage. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012125. [Google Scholar] [CrossRef]
- Chen, L.C.; Xue, S.W.; Dai, B.H.; Wang, Y.F.; Zhao, H.M. Sucrose Osmotic Self-Oscillation Drives Membrane Permeability. J. Agric. Food Chem. 2023, 71, 7557–7565. [Google Scholar] [CrossRef]
- Çam, M.; Kaya, Z.; Güler, S.; Harman, H.; Kırıkçı, K. Influence of egg storage time; position and turning on egg weight loss; embryonic mortality and hatching traits in chukar partridge (Alectoris chukar). Ital. J. Anim. Sci. 2022, 21, 1632–1641. [Google Scholar] [CrossRef]
- Li, Y.D.; Wu, T.; Deng, X.J.; Tian, D.; Ma, C.; Wang, X.; Li, Y.; Zhou, H. Characteristic aroma compounds in naturally withered and combined withered γ-aminobutyric acid white tea revealed by HS-SPME-GC-MS and relative odor activity value. LWT 2023, 176, 114467. [Google Scholar] [CrossRef]
- Huang, T.; Li, E.; Tao, Z.; Guo, X. A nonlinear seepage model of gas and water transport in multi-scale shale gas reservoirs based on dynamic permeability. J. Geophys. Eng. 2018, 15, 1255–1268. [Google Scholar] [CrossRef]
- Tian, Y.; Mi, G.; Chaurasiya, B.; Li, Y.; Shi, D.; Zhang, Y.; Webster, T.; Sun, C.; Shen, Y. Correction to Acid-Induced Activated Cell-Penetrating Peptide-Modified Cholesterol-Conjugated Polyoxyethylene Sorbitol Oleate Mixed Micelles for pH-Triggered Drug Release and Efficient Brain Tumor Targeting Based on Charge Reversal Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 47108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wang, J.; Zhang, L.; Liu, S.; Li, C. Effects of Osmotic Dehydration on Mass Transfer of Tender Coconut Kernel. Foods 2024, 13, 2188. https://doi.org/10.3390/foods13142188
Wu S, Wang J, Zhang L, Liu S, Li C. Effects of Osmotic Dehydration on Mass Transfer of Tender Coconut Kernel. Foods. 2024; 13(14):2188. https://doi.org/10.3390/foods13142188
Chicago/Turabian StyleWu, Sihao, Juntao Wang, Lin Zhang, Sixin Liu, and Congfa Li. 2024. "Effects of Osmotic Dehydration on Mass Transfer of Tender Coconut Kernel" Foods 13, no. 14: 2188. https://doi.org/10.3390/foods13142188
APA StyleWu, S., Wang, J., Zhang, L., Liu, S., & Li, C. (2024). Effects of Osmotic Dehydration on Mass Transfer of Tender Coconut Kernel. Foods, 13(14), 2188. https://doi.org/10.3390/foods13142188




