Application of In Vitro Digestion Models in the Evaluation of Dietary Supplements
Abstract
1. Introduction
2. Materials and Methods
- The type of method used to study simulated digestion;
- The research material used (food, dietary supplement).
3. Digestive Mechanisms
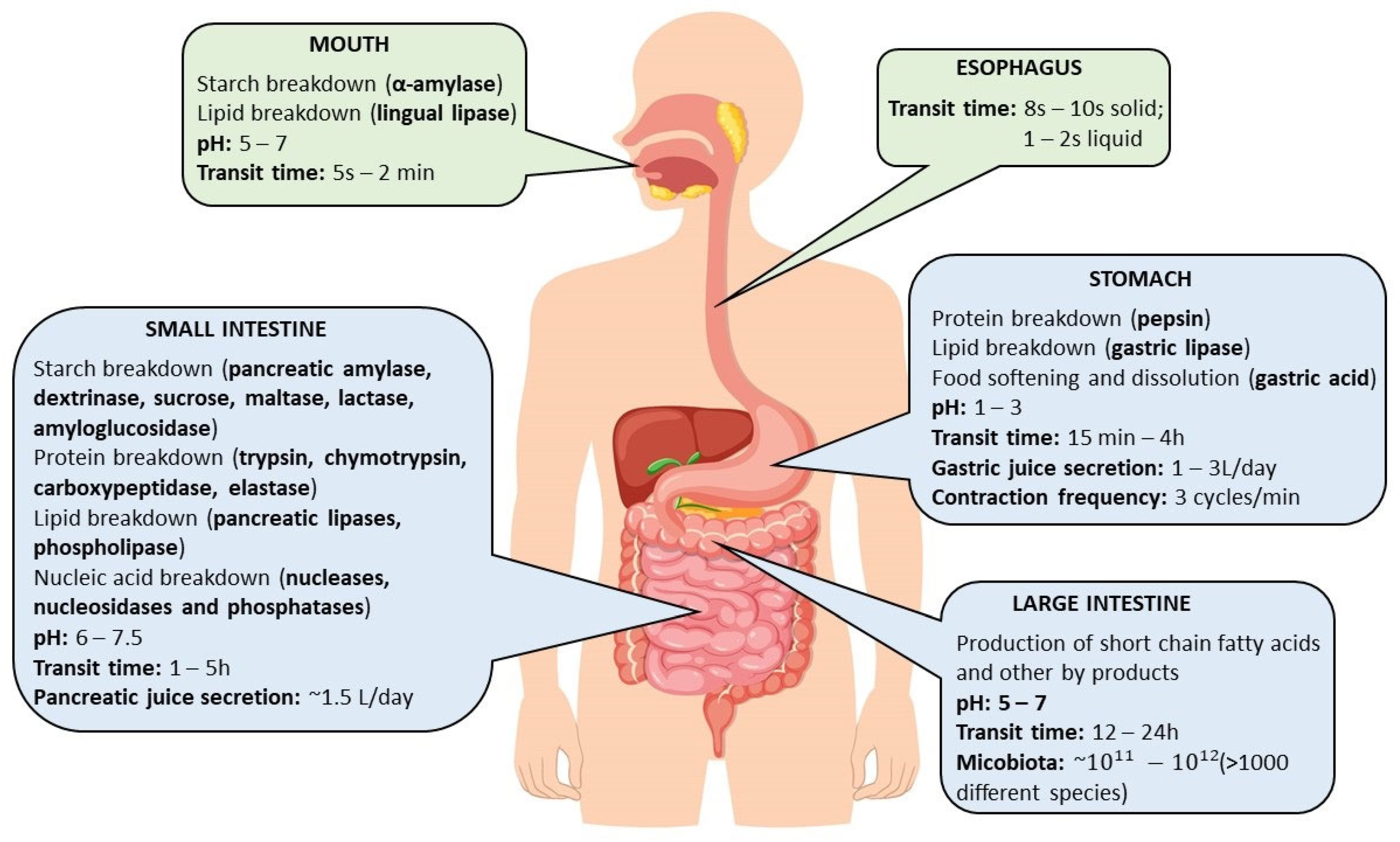
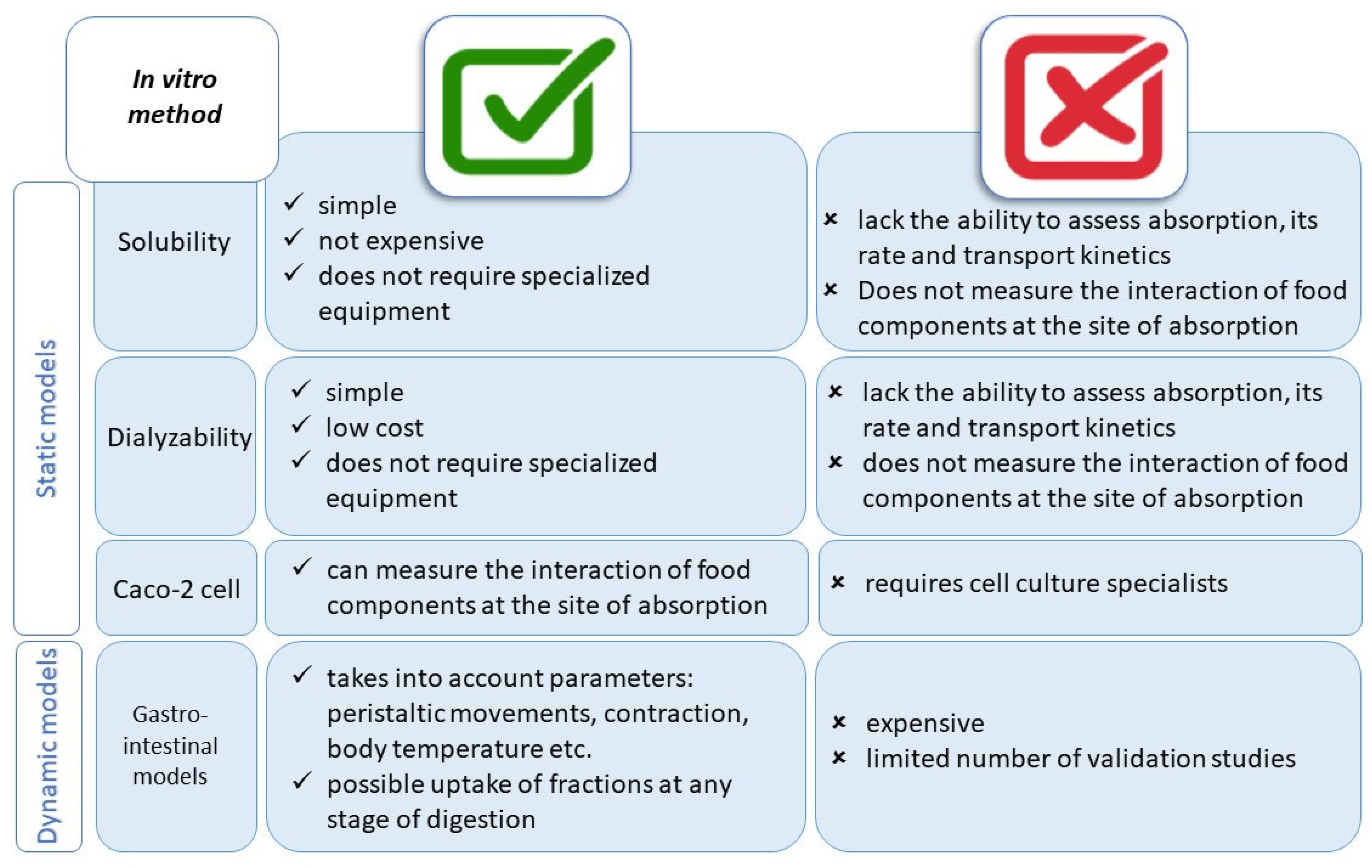
4. In Vitro Methods
4.1. From Simple to Advanced Methods of Simulated Digestion
4.2. Development of In Vitro Methods
5. Application of In Vitro-Simulated Digestion Models to Assess the Bioavailability of Ingredients from Foods, Including Dietary Supplements
5.1. The Effect of a Meal on the Bioaccessibility of a Dietary Supplement Ingredient
5.2. The Effect of Chemical/Pharmaceutical Form on the Bioaccessibility of Dietary Supplement Ingredients
5.3. The Effect of the Carrier Used on the Bioaccessibility of a Dietary Supplement Ingredient
5.4. Other Purposes of Studying the Impact of the Bioaccessibility of Dietary Supplement Ingredients
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust Your Gut: Bioavailability and Bioaccessibility of Dietary Compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Benito, P.; Miller, D. Iron Absorption and Bioavailability: An Updated Review. Nutr. Res. 1998, 18, 581–603. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Lourenço, H.; Costa, S.; Nunes, M.L. Bioaccessibility Assessment Methodologies and Their Consequences for the Risk–Benefit Evaluation of Food. Trends Food Sci. Technol. 2015, 41, 5–23. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In Vitro Bioaccessibility Assessment as a Prediction Tool of Nutritional Efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The Influence of the Gut Microbiota on the Bioavailability of Oral Drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, F.; Li, L.; Guo, L.; Zhang, B.; Yu, S.; Zhao, W. Bioavailability Based on the Gut Microbiota: A New Perspective. Microbiol. Mol. Biol. Rev. 2020, 84, e00072-19. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E.; Spanogiannopoulos, P.; Pieper, L.M.; Bustion, A.E.; Turnbaugh, P.J. How to Determine the Role of the Microbiome in Drug Disposition. Drug Metab. Dispos. 2018, 46, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex Interactions among Diet, Gastrointestinal Transit, and Gut Microbiota in Humanized Mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Patricia, J.J.; Dhamoon, A.S. Physiology, Digestion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Boland, M. Human Digestion—A Processing Perspective. J. Sci. Food Agric. 2016, 96, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; O’Moráin, C. Digestive Function of the Stomach. Dig. Dis. 2014, 32, 186–191. [Google Scholar] [CrossRef]
- Camilleri, M.; Colemont, L.J.; Phillips, S.F.; Brown, M.L.; Thomforde, G.M.; Chapman, N.; Zinsmeister, A.R. Human Gastric Emptying and Colonic Filling of Solids Characterized by a New Method. Am. J. Physiol. Gastrointest. Liver Physiol. 1989, 257, G284–G290. [Google Scholar] [CrossRef] [PubMed]
- Proano, M.; Camilleri, M.; Phillips, S.F.; Brown, M.L.; Thomforde, G.M. Transit of Solids through the Human Colon: Regional Quantification in the Unprepared Bowel. Am. J. Physiol. Gastrointest. Liver Physiol. 1990, 258, G856–G862. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. A Physiological Approach for Preparing and Conducting Intestinal Bioavailability Studies Using Experimental Systems. Food Chem. 2002, 76, 225–230. [Google Scholar] [CrossRef]
- Metcalf, A.M.; Phillips, S.F.; Zinsmeister, A.R.; MacCarty, R.L.; Beart, R.W.; Wolff, B.G. Simplified Assessment of Segmental Colonic Transit. Gastroenterology 1987, 92, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Safadi, A.O.; Lui, F. Physiology, Stomach. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sensoy, I. A Review on the Food Digestion in the Digestive Tract and the Used in Vitro Models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Elia, I.; Schmieder, R.; Christen, S.; Fendt, S.-M. Organ-Specific Cancer Metabolism and Its Potential for Therapy. In Metabolic Control; Herzig, S., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2016; pp. 321–353. ISBN 978-3-319-29806-1. [Google Scholar]
- Azzouz, L.L.; Sharma, S. Physiology, Large Intestine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Your Digestive System & How It Works—NIDDK. Available online: https://www.niddk.nih.gov/health-information/digestive-diseases/digestive-system-how-it-works (accessed on 27 October 2023).
- Minekus, M. The TNO Gastro-Intestinal Model (TIM). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of In Vitro Bioaccessibility and Bioavailability Methods for Calcium, Carotenoids, Folate, Iron, Magnesium, Polyphenols, Zinc, and Vitamins B(6), B(12), D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An In Vitro Digestion Method Adapted for Carotenoids and Carotenoid Esters: Moving Forward towards Standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lazarte, A.; Montilla, A.; Mulet-Cabero, A.-I.; Rigby, N.; Olano, A.; Mackie, A.; Villamiel, M. Study on the Digestion of Milk with Prebiotic Carbohydrates in a Simulated Gastrointestinal Model. J. Funct. Foods 2017, 33, 149–154. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S.; Simsek, S.; Dupont, D.; Menfaatli, E.; Eker, A.T. In Vitro Digestibility of Goat Milk and Kefir with a New Standardised Static Digestion Method (INFOGEST Cost Action) and Bioactivities of the Resultant Peptides. Food Funct. 2015, 6, 2322–2330. [Google Scholar] [CrossRef]
- Floury, J.; Bianchi, T.; Thévenot, J.; Dupont, D.; Jamme, F.; Lutton, E.; Panouillé, M.; Boué, F.; Le Feunteun, S. Exploring the Breakdown of Dairy Protein Gels during In Vitro Gastric Digestion Using Time-Lapse Synchrotron Deep-UV Fluorescence Microscopy. Food Chem. 2018, 239, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Mamone, G.; Nitride, C.; Picariello, G.; Addeo, F.; Ferranti, P.; Mackie, A. Tracking the Fate of Pasta (T. durum semolina) Immunogenic Proteins by In Vitro Simulated Digestion. J. Agric. Food Chem. 2015, 63, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Mat, D.J.L.; Le Feunteun, S.; Michon, C.; Souchon, I. In Vitro Digestion of Foods Using pH-Stat and the INFOGEST Protocol: Impact of Matrix Structure on Digestion Kinetics of Macronutrients, Proteins and Lipids. Food Res. Int. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Sarkar, A.; Murray, B.; Holmes, M.; Ettelaie, R.; Abdalla, A.; Yang, X. In Vitro Digestion of Pickering Emulsions Stabilized by Soft Whey Protein Microgel Particles: Influence of Thermal Treatment. Soft Matter. 2016, 12, 3558–3569. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Primo, C.; Elbaz-Younes, I.; Hirschi, K.D. Bioavailability of Transgenic microRNAs in Genetically Modified Plants. Genes Nutr. 2017, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Bonzi, P.; Vangsøe, C.T.; Nielsen, K.L.; Lærke, H.N.; Hedemann, M.S.; Knudsen, K.E.B. The Relationship between In Vitro and In Vivo Starch Digestion Kinetics of Breads Varying in Dietary Fibre. Foods 2020, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Schlegel, P.; Baumann, C.; Stoffers, H.; Guggisberg, D.; Brügger, C.; Dürr, D.; Stoll, P.; Vergères, G.; Portmann, R. Physiological Comparability of the Harmonized INFOGEST In Vitro Digestion Method to In Vivo Pig Digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.K.; Rolke, C.L.; Breusing, N.; Bosy-Westphal, A.; Högel, J.; Carle, R.; Schweiggert, R.M. Bioavailability of β-Cryptoxanthin Is Greater from Pasteurized Orange Juice than from Fresh Oranges—A Randomized Cross-over Study. Mol. Nutr. Food Res. 2015, 59, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Schricker, B.R.; Rasmussen, R.R.; Van Campen, D. An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 1981, 34, 2248–2256. [Google Scholar] [CrossRef]
- Costa, J.; Ahluwalia, A. Advances and Current Challenges in Intestinal In Vitro Model Engineering: A Digest. Front. Bioeng. Biotechnol. 2019, 7, 144. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef] [PubMed]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Xiao, J.-J.; Liu, Y.-Y.; Deng, Y.-J.; Feng, W.-Z.; Wei, D.; Liao, M.; Cao, H.-Q. Gut Microbiota Influence on Oral Bioaccessibility and Intestinal Transport of Pesticides in Chaenomeles speciosa. Food Chem. 2021, 339, 127985. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wang, P.; Li, Y.; Du, H.; Chen, X.; Sun, G.; Cui, Y. Arsenic in Rice Bran Products: In Vitro Oral Bioaccessibility, Arsenic Transformation by Human Gut Microbiota, and Human Health Risk Assessment. J. Agric. Food Chem. 2019, 67, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.; De Carvalho, N.M.; De Oliveira, D.L.; Madureira, A.R. A Critical Review on In Vitro and Ex Vivo Models of the Intestinal Epithelium of Humans and Monogastric Animals. Gastrointest. Disord. 2024, 6, 337–358. [Google Scholar] [CrossRef]
- Lee, E.H.; Cha, K.H.; Vuong, T.T.; Kim, S.M.; Pan, C.-H. Comparison of Static and Dynamic In Vitro Digestion Models to Estimate the Bioaccessibility of Lutein in Lutein-Rich Foods. Appl. Biol. Chem. 2018, 61, 441–447. [Google Scholar] [CrossRef]
- Ferrua, M.J.; Singh, R.P. Human Gastric Simulator (Riddet Model). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–71. ISBN 978-3-319-16104-4. [Google Scholar]
- Thuenemann, E.C.; Mandalari, G.; Rich, G.T.; Faulks, R.M. Dynamic Gastric Model (DGM). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Marzorati, M.; Verhelst, A.; Luta, G.; Sinnott, R.; Verstraete, W.; de Wiele, T.V.; Possemiers, S. In Vitro Modulation of the Human Gastrointestinal Microbial Community by Plant-Derived Polysaccharide-Rich Dietary Supplements. Int. J. Food Microbiol. 2010, 139, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escalera, S.; Wellejus, A. Evaluation of Caco-2 and Human Intestinal Epithelial Cells as In Vitro Models of Colonic and Small Intestinal Integrity. Biochem. Biophys. 2022, 31, 101314. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 Cell Culture Practices. Toxicol. Vitro 2012, 26, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Doucet, M.; Grassel, C.L.; Delaine-Elias, B.; Zachos, N.C.; Barry, E.M. Evaluating Shigella Flexneri Pathogenesis in the Human Enteroid Model. Infect. Immun. 2019, 87, e00740-18. [Google Scholar] [CrossRef] [PubMed]
- Déat, E.; Blanquet-Diot, S.; Jarrige, J.-F.; Denis, S.; Beyssac, E.; Alric, M. Combining the Dynamic TNO-Gastrointestinal Tract System with a Caco-2 Cell Culture Model: Application to the Assessment of Lycopene and α-Tocopherol Bioavailability from a Whole Food. J. Agric. Food Chem. 2009, 57, 11314–11320. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhai, Q. In Vitro Models to Study Human Gut-Microbiota Interactions: Applications, Advances, and Limitations. Microbiol. Res. 2023, 270, 127336. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Liu, Y.; Cui, M.; Wan, Q.; Chu, X. The In Vitro Intestinal Cell Model: Different Co-Cultured Cells Create Different Applications. J. Drug Target. 2024, 32, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 Monolayers in Experimental and Theoretical Predictions of Drug Transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Li, J. Carrier-Mediated Transport and Efflux Mechanisms in Caco-2 Cells. Adv. Drug Deliv. Rev. 1996, 22, 53–66. [Google Scholar] [CrossRef]
- Li, X.-G.; Chen, M.; Zhao, S.; Wang, X. Intestinal Models for Personalized Medicine: From Conventional Models to Microfluidic Primary Intestine-on-a-Chip. Stem. Cell Rev. Rep. 2022, 18, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Hautefort, I.; Poletti, M.; Papp, D.; Korcsmaros, T. Everything You Always Wanted to Know About Organoid-Based Models (and Never Dared to Ask). Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Elbrecht, D.H.; Long, C.J.; Hickman, J.J. Transepithelial/Endothelial Electrical Resistance (TEER) Theory and Applications for Microfluidic Body-on-a-Chip Devices. J. Rare Dis. Res. Treat. 2016, 1, 46–52. [Google Scholar]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Ménard, O.; Baumann, C.; Duerr, D.; Schlegel, P.; Stoll, P.; Vergères, G.; Dupont, D.; Portmann, R. Digestion of Milk Proteins: Comparing Static and Dynamic In Vitro Digestion Systems with In Vivo Data. Food Res. Int. 2019, 118, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The Need to Study Human Milk as a Biological System. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Santos-Hernández, M.; Miralles, B.; Amigo, L.; Recio, I. Peptidomic Data of Egg White Gastrointestinal Digests Prepared Using the Infogest Harmonized Protocol. Data Brief. 2020, 31, 105932. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comparative Peptidomic Profile and Bioactivities of Cooked Beef, Pork, Chicken and Turkey Meat after In Vitro Gastro-Intestinal Digestion. J. Proteom. 2019, 208, 103500. [Google Scholar] [CrossRef] [PubMed]
- Rebellato, A.P.; Grazielle Siqueira Silva, J.; Probio de Moraes, P.; Trajano, B.; Azevedo Lima Pallone, J. Static In Vitro Digestion Methods for Assessing Essential Minerals in Processed Meat Products. Food Res. Int. 2022, 155, 111121. [Google Scholar] [CrossRef] [PubMed]
- Atallah, N.; Deracinois, B.; Boulier, A.; Baniel, A.; Jouan-Rimbaud Bouveresse, D.; Ravallec, R.; Flahaut, C.; Cudennec, B. In Vitro Assessment of the Impact of Industrial Processes on the Gastrointestinal Digestion of Milk Protein Matrices Using the INFOGEST Protocol. Foods 2020, 9, 1580. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.C.; Perina, N.P.; Mosquera, E.M.B.; Tomé, T.M.; Lazarini, T.; Mariutti, L.R.B. DHA Bioaccessibility in Infant Formulas and Preschool Children Milks. Food Res. Int. 2021, 149, 110698. [Google Scholar] [CrossRef] [PubMed]
- Uğur, H.; Çatak, J.; Mızrak, Ö.F.; Çebi, N.; Yaman, M. Determination and Evaluation of In Vitro Bioaccessibility of Added Vitamin C in Commercially Available Fruit-, Vegetable-, and Cereal-Based Baby Foods. Food Chem. 2020, 330, 127166. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and Bioactives in Citrus Fruits: Different Citrus Varieties, Fruit Parts, and Growth Stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef]
- Bustos, M.C.; Vignola, M.B.; Pérez, G.T.; León, A.E. In Vitro Digestion Kinetics and Bioaccessibility of Starch in Cereal Food Products. J. Cereal Sci. 2017, 77, 243–250. [Google Scholar] [CrossRef]
- Gouseti, O.; Lovegrove, A.; Kosik, O.; Fryer, P.J.; Mills, C.; Gates, F.; Tucker, G.; Latty, C.; Shewry, P.; Bakalis, S. Exploring the Role of Cereal Dietary Fiber in Digestion. J. Agric. Food Chem. 2019, 67, 8419–8424. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Glahn, R.P.; Laparra, J.M.; Welch, R.M.; Lei, X.; Kelly, J.D.; Rutzke, M.A.; Miller, D.D. Iron and Zinc Bioavailabilities to Pigs from Red and White Beans (Phaseolus vulgaris L.) Are Similar. J. Agric. Food Chem. 2009, 57, 3134–3140. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Han, B.-Z.; Nout, M.J.R.; Hamer, R.J. In Vitro Solubility of Calcium, Iron and Zinc in Relation to Phytic Acid Levels in Rice-Based Consumer Products in China. Int. J. Food Sci. Nutr. 2010, 61, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Frontela, C.; Scarino, M.L.; Ferruzza, S.; Ros, G.; Martínez, C. Effect of Dephytinization on Bioavailability of Iron, Calcium and Zinc from Infant Cereals Assessed in the Caco-2 Cell Model. World J. Gastroenterol. 2009, 15, 1977. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Riedl, K.; García-Cano, I.; Chaves, F.; Walsh, K.R.; Jimenez-Flores, R.; Failla, M.L. Efficient In Vitro Digestion of Lipids and Proteins in Bovine Milk Fat Globule Membrane Ingredient (MFGMi) and Whey-Casein Infant Formula with Added MFGMi. J. Dairy Sci. 2023, 106, 3086–3097. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zhou, X.; Li, C.; Liu, Y. Changes in the Extent and Products of In Vitro Protein Digestion during the Ripening Periods of Chinese Dry-Cured Hams. Meat Sci. 2021, 171, 108290. [Google Scholar] [CrossRef]
- Sousa, R.; Recio, I.; Heimo, D.; Dubois, S.; Moughan, P.J.; Hodgkinson, S.M.; Portmann, R.; Egger, L. In Vitro Digestibility of Dietary Proteins and In Vitro DIAAS Analytical Workflow Based on the INFOGEST Static Protocol and Its Validation with In Vivo Data. Food Chem. 2023, 404, 134720. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Lobillo, P.; Ferreira-Lazarte, A.; Hernández-Hernández, O.; Villamiel, M. In Vitro Digestion of Polysaccharides: InfoGest Protocol and Use of Small Intestinal Extract from Rat. Food Res. Int. 2021, 140, 110054. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xie, W.; Cui, Q. Effects of Phytases and Dehulling Treatments on In Vitro Iron and Zinc Bioavailability in Faba Bean (Vicia faba L.) Flour and Legume Fractions. J. Food Sci. 2010, 75, C191–C198. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Platel, K. Finger Millet (Eleucine coracana) Flour as a Vehicle for Fortification with Zinc. J. Trace Elem. Med. Biol. 2010, 24, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, L.; Liu, H.; Hu, J. In Vitro Gastrointestinal Digestion of Whole Grain Noodles Supplemented with Soluble Dietary Fiber and Their Effects on Children Fecal Microbiota. Food Biosci. 2023, 53, 102600. [Google Scholar] [CrossRef]
- Wróbel, K.; Wróbel, K.; Valtierra Márquez, G.R.; Rodríguez Almanza, M.L. Studies on Bioavailability of Some Bulk and Trace Elements in Mexican Tortilla Using an In Vitro Model. Biol. Trace Elem. Res. 1999, 68, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento da Silva, E.; de Farias, L.O.; Cadore, S. The Total Concentration and Bioaccessible Fraction of Nutrients in Purées, Instant Cereals and Infant Formulas by ICP OES: A Study of Dietary Recommended Intakes and the Importance of Using a Standardized In Vitro Digestion Method. J. Food Compos. Anal. 2018, 68, 65–72. [Google Scholar] [CrossRef]
- Kong, D.; Li, X.; Yao, J.; He, Y.; Luo, J.; Yang, M. Health Risk Assessment and Bioaccessibility of Toxic Elements in Edible and Medicinal Plants under Different Consumption Methods. Microchem. J. 2020, 159, 105577. [Google Scholar] [CrossRef]
- Hu, L.; Fan, H.; Wu, D.; Wan, J.; Wang, X.; Huang, R.; Liu, W.; Shen, F. Assessing Bioaccessibility of Se and I in Dual Biofortified Radish Seedlings Using Simulated In Vitro Digestion. Food Res. Int. 2019, 119, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Pizarro, F.; Olivares, M. Iron Bioavailability in Corn-Masa Tortillas Is Improved by the Addition of Disodium EDTA. J. Nutr. 2003, 133, 3158–3161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yun, S.; Habicht, J.-P.; Miller, D.D.; Glahn, R.P. An In Vitro Digestion/Caco-2 Cell Culture System Accurately Predicts the Effects of Ascorbic Acid and Polyphenolic Compounds on Iron Bioavailability in Humans. J. Nutr. 2004, 134, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, V.; Witthöft, C. Orange Juice Is a Good Folate Source in Respect to Folate Content and Stability during Storage and Simulated Digestion. Eur. J. Nutr. 2008, 47, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, V.; Öhrvik, H.; Tallkvist, J.; Witthöft, C. Folates in Bread: Retention during Bread-Making and In Vitro Bioaccessibility. Eur. J. Nutr. 2010, 49, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Biehler, E.; Hoffmann, L.; Krause, E.; Bohn, T. Divalent Minerals Decrease Micellarization and Uptake of Carotenoids and Digestion Products into Caco-2 Cells12. J. Nutr. 2011, 141, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, L.; Colle, I.J.P.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Quantifying the Influence of Thermal Process Parameters on In Vitro β-Carotene Bioaccessibility: A Case Study on Carrots. J. Agric. Food Chem. 2011, 59, 3162–3167. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of Fatty Acyl Composition and Quantity of Triglycerides on Bioaccessibility of Dietary Carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, N.; Bonetti, G.; Brandolini, V.; Cavazzini, A.; Maietti, A.; Meca, G.; Mañes, J. Stinging Nettle (Urtica dioica L.) as a Functional Food Additive in Egg Pasta: Enrichment and Bioaccessibility of Lutein and β-Carotene. J. Funct. Foods 2018, 47, 547–553. [Google Scholar] [CrossRef]
- Viera, I.; Herrera, M.; Roca, M. Influence of Food Composition on Chlorophyll Bioaccessibility. Food Chem. 2022, 386, 132805. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Reguant, J.; Romero, M.-P.; Macià, A.; Motilva, M.-J. Effect of Fat Content on the Digestibility and Bioaccessibility of Cocoa Polyphenol by an In Vitro Digestion Model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Chukwumah, Y.; Walker, L.; Vogler, B.; Verghese, M. In Vitro Absorption of Dietary Trans-Resveratrol from Boiled and Roasted Peanuts in Caco-2 Cells. J. Agric. Food Chem. 2011, 59, 12323–12329. [Google Scholar] [CrossRef] [PubMed]
- Fazzari, M.; Fukumoto, L.; Mazza, G.; Livrea, M.A.; Tesoriere, L.; Marco, L.D. In Vitro Bioavailability of Phenolic Compounds from Five Cultivars of Frozen Sweet Cherries (Prunus avium L.). J. Agric. Food Chem. 2008, 56, 3561–3568. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Acién-Fernández, G.; Molina-Grima, E.; Fernández-Sevilla, J.M.; Pérez-Sacristán, B.; Blanco-Navarro, I. In Vitro Bioaccesibility of Lutein and Zeaxanthin from the Microalgae Scenedesmus Almeriensis. Food Chem. 2009, 114, 747–752. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Zhou, C.; Wang, Y.; Zalán, Z.; Cai, T. Inhibitory Effects of Chlorophyll Pigments on the Bioaccessibility of β-Carotene: Influence of Chlorophyll Structure and Oil Matrix. Food Chem. 2024, 451, 139457. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Seiquer, I.; Navarro, M.P. Influence of Diets Rich in Maillard Reaction Products on Calcium Bioavailability. Assays in Male Adolescents and in Caco-2 Cells. J. Agric. Food Chem. 2009, 57, 9532–9538. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.A.; Mariutti, L.R. Static and Semi-Dynamic In Vitro Digestion Methods: State of the Art and Recent Achievements towards Standardization. Curr. Opin. Food Sci. 2021, 41, 260–273. [Google Scholar] [CrossRef]
- Ośko, J.; Pierlejewska, W.; Grembecka, M. Comparison of the Potential Relative Bioaccessibility of Zinc Supplements—In Vitro Studies. Nutrients 2023, 15, 2813. [Google Scholar] [CrossRef] [PubMed]
- Tuna, B.H.; Gürbüz, M.; Uğur, H.; Çatak, J.; Yaman, M. Vitamin C Bioaccessibility of Commercially Available Dietary Supplements: Quantity vs Efficiency, Does It Matter? J. Food Compos. Anal. 2023, 123, 105558. [Google Scholar] [CrossRef]
- Bawiec, P.; Sawicki, J.; Łasińska-Pracuta, P.; Czop, M.; Sowa, I.; Iłowiecka, K.; Koch, W. In Vitro Evaluation of Bioavailability of Se from Daily Food Rations and Dietary Supplements. Nutrients 2023, 15, 1511. [Google Scholar] [CrossRef] [PubMed]
- Dowley, A.; Long-Smith, C.M.; Demehin, O.; Nolan, Y.; O’Connell, S.; O’Gorman, D.M. The Bioaccessibility and Tolerability of Marine-Derived Sources of Magnesium and Calcium. Methods 2024, 226, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Brandon, E.F.A.; Bakker, M.I.; Kramer, E.; Bouwmeester, H.; Zuidema, T.; Alewijn, M. Bioaccessibility of Vitamin A, Vitamin C and Folic Acid from Dietary Supplements, Fortified Food and Infant Formula. Int. J. Food Sci. Nutr. 2014, 65, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Biltoft-Jensen, A.P.; Jakobsen, J. In Vitro Bioaccessibility of Vitamin K (Phylloquinone and Menaquinones) in Food and Supplements Assessed by INFOGEST 2.0—Vit K. Curr. Res. Food Sci. 2022, 5, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bryszewska, M. Comparison Study of Iron Bioaccessibility from Dietary Supplements and Microencapsulated Preparations. Nutrients 2019, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, X.; McClements, D.J.; Zou, L.; Liu, X.; Liu, F. Co-Encapsulation of Epigallocatechin Gallate (EGCG) and Curcumin by Two Proteins-Based Nanoparticles: Role of EGCG. J. Agric. Food Chem. 2019, 67, 13228–13236. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Guerreiro, F.; Lourenço, J.P.; Lopes, J.A.; Cámara-Martos, F. Microencapsulation of Selenium by Spray-Drying as a Tool to Improve Bioaccessibility in Food Matrix. Food Chem. 2023, 402, 134463. [Google Scholar] [CrossRef] [PubMed]
- Corgneau, M.; Gaiani, C.; Petit, J.; Nikolova, Y.; Banon, S.; Ritié-Pertusa, L.; Le, D.T.L.; Scher, J. Nutritional Quality Evaluation of Commercial Protein Supplements. Int. J. Food Sci. Technol. 2019, 54, 2586–2594. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Cui, Q.; Cheng, J.; Killpartrik, A.; Kemp, A.H.; Guo, M. Characterization, Antioxidant Capacity, and Bioaccessibility of Coenzyme Q10 Loaded Whey Protein Nanoparticles. LWT 2022, 160, 113258. [Google Scholar] [CrossRef]
- Toro-Uribe, S.; López-Giraldo, L.J.; Alvarez-Rivera, G.; Ibáñez, E.; Herrero, M. Insight of Stability of Procyanidins in Free and Liposomal Form under an In Vitro Digestion Model: Study of Bioaccessibility, Kinetic Release Profile, Degradation, and Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Qian, Y.; Rong, X.; Cao, K.; McClements, D.J.; Hu, K. Encapsulation of Quercetin in Biopolymer-Coated Zein Nanoparticles: Formation, Stability, Antioxidant Capacity, and Bioaccessibility. Food Hydrocoll. 2021, 120, 106980. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Liu, Y. Ultrasound-Mediated Host–Guest Self-Assembly between Different Dietary Fatty Acids and Sodium Caseinate and Their Complexes Improving the Water Dispersibility, Stability, and Bioaccessibility of Quercetin. Food Chem. 2024, 448, 139054. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of Resveratrol in Zein/Pectin Core-Shell Nanoparticles: Stability, Bioaccessibility, and Antioxidant Capacity after Simulated Gastrointestinal Digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Song, H.; Jeon, D.; Unno, T. Evaluation of Prebiotics through an In Vitro Gastrointestinal Digestion and Fecal Fermentation Experiment: Further Idea on the Implementation of Machine Learning Technique. Foods 2022, 11, 2490. [Google Scholar] [CrossRef]
- Marinova, V.Y.; Rasheva, I.K.; Kizheva, Y.K.; Dermenzhieva, Y.D.; Hristova, P.K. Microbiological Quality of Probiotic Dietary Supplements. Biotechnol. Biotechnol. Equip. 2019, 33, 834–841. [Google Scholar] [CrossRef]
- Da Silva, M.N.; Tagliapietra, B.L.; do Amaral Flores, V.; dos Santos Richards, N.S.P. In Vitro Test to Evaluate Survival in the Gastrointestinal Tract of Commercial Probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Enhancement of Phytochemical Bioaccessibility from Plant-Based Foods Using Excipient Emulsions: Impact of Lipid Type on Carotenoid Solubilization from Spinach. Food Funct. 2018, 9, 4352–4365. [Google Scholar] [CrossRef] [PubMed]
- Aman Mohammadi, M.; Farshi, P.; Ahmadi, P.; Ahmadi, A.; Yousefi, M.; Ghorbani, M.; Hosseini, S.M. Encapsulation of Vitamins Using Nanoliposome: Recent Advances and Perspectives. Adv. Pharm. Bull. 2023, 13, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Marisa Ribeiro, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of Polyphenols—The Specific Case of the Microencapsulation of Sambucus nigra L. Extracts—A Review. Trends Food Sci. Technol. 2020, 105, 454–467. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Methodologies for Simulation of Gastrointestinal Digestion of Different Controlled Delivery Systems and Further Uptake of Encapsulated Bioactive Compounds. Trends Food Sci. Technol. 2021, 114, 510–520. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, R.; Tian, X.; Zhou, X.; Pan, X.; Wong, A. Assessing the Risk of Probiotic Dietary Supplements in the Context of Antibiotic Resistance. Front. Microbiol. 2017, 8, 908. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Villena, M.; Fernandez-Pacheco, P.; Castillo, N.; Bevilacqua, A.; Briones Pérez, A. Probiotic Capability in Yeasts: Set-up of a Screening Method. LWT—Food Sci. Technol. 2018, 89, 657–665. [Google Scholar] [CrossRef]
- Ayyash, M.; Liu, S.-Q.; Al Mheiri, A.; Aldhaheri, M.; Raeisi, B.; Al-Nabulsi, A.; Osaili, T.; Olaimat, A. In Vitro Investigation of Health-Promoting Benefits of Fermented Camel Sausage by Novel Probiotic Lactobacillus plantarum: A Comparative Study with Beef Sausages. LWT—Food Sci. Technol. 2019, 99, 346–354. [Google Scholar] [CrossRef]
- Gil-Rodríguez, A.M.; Carrascosa, A.V.; Requena, T. Yeasts in Foods and Beverages: In Vitro Characterisation of Probiotic Traits. LWT—Food Sci. Technol. 2015, 64, 1156–1162. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of Yeasts as Potential Probiotics: A Review of Gastrointestinal Tract Conditions and Investigation Methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef]
| Compound | Product | In Vitro Model | Reference |
|---|---|---|---|
| Dairy products | |||
| Lipids and proteins | Bovine milk fat globule membrane ingredient (MFGMi) and whey–casein infant formula with added MFGMi | Solubility (INFOGEST 2.0) | [75] |
| Meat products | |||
| Proteins | Chinese dry-cured hams | Solubility | [76] |
| Plant products | |||
| Proteins, amino acids | Peanuts, All-Bran® wheat bran cereal, pigeon peas, black beans, zein, whey protein isolate (WPI), and collagen | Solubility (INFOGEST 2.0) | [77] |
| Polysaccharides | 3% solutions of starch, dextran, pectin, and modified citrus pectin | Solubility (INFOGEST 2.0) | [78] |
| Zinc | Faba bean (Vicia Faba L.) flour and legume fractions, finger millet (Eleucine coracana) flour | Dializalibity | [79,80] |
| Soluble dietary fiber | Whole grain noodles | Solubility | [81] |
| Magnesium | Mexican tortilla | Solubility | [82] |
| Cooper, iron, magnesium, manganese, and zinc | Purées, instant cereals, and infant formulas | Solubility (INFOGEST 2.0) | [83] |
| Cooper, mercury, arsenic, cadmium, lead, chromium, nickel, and zinc | Edible and medicinal plants | Solubility | [84] |
| Selenium and iodine | Radish Raphanus sativus | Solubility | [85] |
| Iron | Corn-masa tortillas, semisynthetic (SS) meals | Solubility, dialyzability, uptake by Caco-2 cells and/or transport assays | [86,87] |
| Folates | Orange juice, bread | TIM (dynamic model) | [88,89] |
| Carotenoids | Spinach and condensed milk (4% fat), carrots, salad meal | Solubility, uptake by Caco-2 cells | [90,91,92] |
| Carotenoids | Stinging nettle (Urtica dioica L.) in egg pasta | Solubility (INFOGEST 2.0) | [93] |
| Chlorophylls | Guacamole, virgin olive oil, tortellini, basil hummus, creamed spinach, vegetable pasta, green tea chocolate, avocado and kiwi juices, and pesto sauce | Solubility (INFOGEST 2.0) | [94] |
| Polyphenols | Cocoa, boiled and roasted peanuts; frozen sweet cherries (Prunus avium L.) | Solubility, uptake by Caco-2 cells | [95,96,97] |
| Lutein and zeaxanthin | Microalgae Scenedesmus almeriensis | Solubility | [98] |
| Lycopene and α-Tocopherol | Whole food | TIM (dynamic model) with Caco-2 cell | [51] |
| Oil products | |||
| Carotenoids and chlorophylls | Oil matrix | Solubility | [99] |
| Other | |||
| Calcium | Diets rich in Maillard reaction products | Solubility, dialyzability, Caco-2 cell uptake, and transport | [100] |
| Dietary Supplements (Compounds) | Type of In Vitro Model | Model Construction | Reference |
|---|---|---|---|
| Macrocompounds | |||
| Mixed-polysaccharide | Dynamic model (SHIME) (stomach–small intestine–ascending–transverse and descending colon) | Stomach–small intestine–ascending–transverse and descending colon (pH regulator 5.6–5.9, 6.2–6.5, and 6.6–6.9 in the ascending colon, transverse colon, and descending colon, respectively; 72 h; 37 °C). Growth medium for the microbial inoculum consisted of a carbohydrate-based medium containing arabinogalactan (1 g/L), pectin (2 g/L), xylan (1 g/L), starch (4.2 g/L), glucose (0.4 g/L), yeast extract (3 g/L), peptone (1 g/L), mucin (4 g/L), and cysteine (0.5 g/L). The pH of the medium was 5.5. | [47] |
| Proteins (amino acids) | Static (INFOGEST 2.0) (stomach–small intestine) | Stomach: 11.25 mL of simulated gastric fluid (6.9 mM of KCl, 0.9 mM of KH2PO4, 25 mM of NaHCO3, 47.2 mM of NaCl, 0.1 mM of MgCl2(H2O)6, and 0.5 mM of (NH4)2CO3) with 2.4 mL of porcine pepsin (25,000 UI/mL and 7.5 μL of 0.3 M CaCl2 (pH of 3.0, 2 h, 37 °C) Small intestine: 14.3 mL of simulated intestinal fluid (6.8 mM of KCl, 0.8 mM of KH2PO4, 85 mM of NaHCO3, 38.4 mM of NaCl, 0.33 mM of MgCl2(H2O)6) with 6.5 mL of pancreatin (800 UI/mL), 2.6 mL of bile acids (160 mmol/L and 52 μL of 0.3 M CaCl2) (pH of 7.0, 2 h, 37 °C) | [111] |
| Microcompounds | |||
| Magnesium | Static (INFOGEST 2.0) (mouth–stomach–small intestine) | Mouth: 15.1 mM of KCl, 3.7 mM of KH2PO4, 13.6 mM of NaHCO3, 0.15 mM of MgCl2(H2O)6, 0.5 mM of (NH4)2CO3, 1.1 mM of HCl, and 1.5 mM of CaCl2(H2O)2 Stomach: 6.9 mM of KCl, 0.9 mM of KH2PO4, 25 mM of NaHCO3, 47.2 mM of NaCl, 0.1 mM of MgCl2(H2O)6, and 0.5 mM of (NH4)2CO3 with pepsin and CaCl2 (2000 U/mL and 0.15 mM) (pH of 3.0, 2 h, 37 °C, 200 rpm). Small intestine: 6.8 mM of KCl, 0.8 mM of KH2PO4, 85 mM of NaHCO3, 38.4 mM of NaCl, and 0.33 mM of MgCl2(H2O)6 with pancreatin (100 U/mL and bile alts 10 mM) and CaCl2 (0.6 mM) (pH of 7.0, 2 h, 37 °C, 200 rpm) | [105] |
| Iron | Static (stomach–small intestine) | Stomach: 0.5 mL of pepsin solution (2 mg of pepsin in 0.1 M of sodium hydrogen carbonate and 0.01 M of HCl) (pH of 2.0 ± 0.1, 2 h, 37 °C) Small intestine: 0.5 mL of digestive enzyme and bile salts (0.5 mg of pancretin and 3 mg of bile salts per 1 mL) (pH of 6.8–7.0, 2 h, 37 °C) | [108] |
| Zinc | Static—dialyzability (stomach–small intestine) | Stomach: 0.5 mL of pepsin (pH of 2.0, 2 h, 37 °C, 140 rpm) Small intestine: 2.5 mL of intestinal solution (pH of 7.0, 2 h, 37 °C, 140 rpm) + dialysis membrane with 10 mL of PIPES | [102] |
| Selenium | Static—dialyzability (stomach–small intestine) | Stomach: 2 mL of 10% pepsin (pH of 2.0, 2 h, 37 °C) Small intestine: 5 mL of 0.4% pancreatin (pH of 6.5, 2 h, 37 °C) + cellulose dialysis tube | [104] |
| Selenium | Static (stomach–small intestine) | Stomach: 0.166 g of pepsin (pH of 2.0, 2 h, 37 °C) Small intestine: 0.034 g of pancreatin and 0.213 bile salts (pH of 5, 2 h, 37 °C) + dialysis membrane | [110] |
| Vitamin C | Static (mouth–stomach–small intestine) | Mouth: 5 mL of oral medium (1,7 mL of NaCl, 8 mL of urea, 15 mg of uric acid, 580 mg of α-amylase, and 50 mg of mucin in 500 mL) (pH of 7.0 ± 0.1, 5 min, 37 °C) Stomach: gastric juice (6.5 HCl, 18 mL of CaCl2•2H2O, 1 g of bovine serum albumin, 5 g of pepsin, and 3 g of mucin in 500 mL (pH of 1.5 ± 0.1, 2 h, 37 °C) Small intestine: 10 mL of duodenal juice (6.4 mL of KCl, 9 mL of CaCl2•2H2O, 1 g of bovine serum albumin, 18 g of pancreatin, and 3 g of lipase in 500 mL) (pH of 7.0 ± 0.1, 2 h) and 5 mL of bile juice (68 mL of NaHCO3, 10 mL of CaCl2•2H2O, 18 g of bovine serum albumin, and 60 g of bile) (pH of 7.0 ± 0.1, 2 h) | [103] |
| Vitamin K | Static (INFOGEST 2.0) (mouth–stomach–small intestine) | Mouth: 1 mL of saliva (2 min, 37 °C) Stomach: 2 mL of simulated gastric fluid with pepsin (2000 U/mL) and gastric lipase (60 U/mL) (pH of 3.0, 2 h, 37 °C) Small intestine: 4 mL of simulated intestinal fluid with bile (10 mM of bile salt) and pancreatin (trypsin activity of 100 U/mL) (pH of 7.0, 2 h, 37 °C) | [107] |
| Vitamin C, A, folic acid | Static (mouth–stomach–small intestine) | Mouth: 6 mL of saliva (pH of 6.5 ± 0.2, 5 min, 55 rpm, 37 ± 2 °C Stomach: 12 mL of gastric juice (pH of 1.5 ± 0.5, 2 h) Small intestine: 12 mL of duodenal juice and 6 mL of bile (pH of 6.0 ± 0.5, 2 h) | [106] |
| Other compounds | |||
| Coenzyme Q10 | Static (stomach–small intestine) | Stomach: 10 mg of pepsin (of pH 2.0, 1 h) Small intestine: 20 mg of pancreatic enzyme and 250 mg of bile salt (pH of 7.0, 2 h) | [112] |
| Curcumin and Epigallocatechin Gallate (EGCG) | Static (stomach–small intestine) | Stomach: 10 mg of pepsin (pH of 1.5, 37 °C, 0–1 h) Small intestine: 20 mg of trypsin enzyme and 250 mg of bile salt extract (pH of 7.0, 37 °C, 1–2 h) | [109] |
| Procyanidin | Static (INFOGEST 2.0) (stomach–small intestine) | Stomach: 1.6 mL ca.2500 U of porcine pepsin stock solution and 5.0 µL of 0.3 M CaCl2 mixed with 7.5 mL of 1.25-fold concentrated buffer solution: 6.9 mM of KCl, 0.9 mM of KH2PO4, 25 mM of NaHCO3, 47.2 mM of NaCl, 0.1 mM of MgCl2(H2O)6, 0.5 mM of (NH4)2CO3, and 15.6 mM of HCl; 1.6 mL of ca. 2500 U/mL porcine pepsin (pH of 3.0, 2 h at 37 °C, 100 rpm) Small intestine: 3.75 mL 800 U/mL of pancreatin solution, 1.87 mL of fresh bile, and 30 µL of 0.3 M of CaCl2 mixed mixed with 8.25 mL of 6.8 mM of KCl, 0.8 mM of KH2PO4, 85 mM of NaHCO3, 38.4 mM of NaCl, and 0.33 mM of MgCl2(H2O)6 and 8.4 mM of HCl (pH of 7.0, 6 h at 37 °C, 100 rpm) | [113] |
| Quercetin | Static (stomach–small intestine) | Stomach: 20 mL of simulated gastric fluids (2 g of NaCl and 7 mL of HCl/1 L distilled water and adjusted to pH of 1.2; then NaOH added to pH 2.5; after that, 0.064 g of pepsine added (2 h, min, 37 °C, 50 rpm) Small intestine: 187.5 mg of bile salt and 0.144 g of pancreatin (pH of 7.0, 4 h at 37 °C, 50 rpm) | [114,115] |
| Resveratrol | Static (stomach–small intestine) | Stomach: 15 mL of simulated gastric fluid (double-distilled water adjusted to pH of 1.2 with HCl, containing 50 mM of NaCl, and 3.2 mg/mL of pepsin) (pH of 2.5, 37 °C, 80 rpm) Small intestine: 4 mL of 12.5 mg/mL bile salt in phosphate-buffered solution (pH of 7.0, 5 mM) and 2.5 mL of 8 mg/mL pancreatin solution (pH of 7.0, 5 mM of phosphate buffer) (pH of 7.0, 4 h, 37 °C, 100 rpm) | [116] |
| Prebiotics | Static (INFOGEST 2.0) (mouth–stomach–small intestine) | Mouth: 5 mL of phosphate-buffered saline (PBS) mixed with 3.5 mL of simulated salivary fluid, 0.5 mL of 1500 U/mL of α-amylase, 25 μL of 0.3 M CaCl2, and 975 μL of distilled water (2 min, 37 °C, 150 rpm) Stomach: 7.5 mL of simulated gastric fluid, 1.6 mL of 25,000 U/mL porcine pepsin, and 5 μL of 0.3 M of CaCl2 and 695 μL of distilled water (pH of 2.5–3, 2 h, 100 rpm) Small intestine: 11 mL of simulated intestinal fluid, 5 mL of 800 U/mL pancreatin, 2.5 mL of 160 mM of bile salt, and 40 μL of 0.3 M of CaCl2 and 1.10 mL of distilled water (pH of 7, 2 h, 100 rpm) | [117] |
| Probiotics | Static (stomach–small intestine) | Stomach: pepsin (3 mg/mL) in sterile saline (0.5% w/v) (pH of 2.0, 3 h) Small intestine: pancreatin USP (1 mg/mL) in sterile saline (0.5% w/v) (pH of 8.0, 4 h) | [118] |
| Probiotics | Static (mouth–stomach–small intestine) | Mouth: 9.0 mL of peptone water (pH of 6.9, 2 min, 37 °C, 200 rpm) Stomach: 0.05 mL of pepsin (pH of 2.0, 1.5 h, 37 °C, 130 rpm) Duodenum: 0.125 mL of pancreatin, 0.125 mL of bovine bile (pH of 5.0, 20 min, 37 °C, 45 rpm) Ileum: 0.125 mL of pancreatin, 0.125 mL of bovine bile (pH of 6.5, 90 min, 37 °C, 45 rpm) | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ośko, J.; Nasierowska, K.; Grembecka, M. Application of In Vitro Digestion Models in the Evaluation of Dietary Supplements. Foods 2024, 13, 2135. https://doi.org/10.3390/foods13132135
Ośko J, Nasierowska K, Grembecka M. Application of In Vitro Digestion Models in the Evaluation of Dietary Supplements. Foods. 2024; 13(13):2135. https://doi.org/10.3390/foods13132135
Chicago/Turabian StyleOśko, Justyna, Katarzyna Nasierowska, and Małgorzata Grembecka. 2024. "Application of In Vitro Digestion Models in the Evaluation of Dietary Supplements" Foods 13, no. 13: 2135. https://doi.org/10.3390/foods13132135
APA StyleOśko, J., Nasierowska, K., & Grembecka, M. (2024). Application of In Vitro Digestion Models in the Evaluation of Dietary Supplements. Foods, 13(13), 2135. https://doi.org/10.3390/foods13132135







