Amazon Fruits as Healthy Ingredients in Muscle Food Products: A Review
Abstract
1. Introduction
2. Health Issues Related to Muscle Food Products: Lipid Profile and Use of Synthetic Antioxidants
3. Potential Health Benefits of Amazon Fruits
4. Amazon Fruit Ingredients in Muscle Food Products
4.1. Antioxidants
| Amazon Resource | Ingredient Preparation Method | Characteristics of the Ingredient | Main Findings | Reference |
|---|---|---|---|---|
| Açai extract powder |
|
| 250 mg/kg of açai extract did not affect the color of pork burgers. Furthermore, it was comparable to sodium erythorbate (500 mg/kg) in reducing lipid oxidation. | [49] |
| Açai extract powder | Commercial açai extract powder |
| Reduction of the oxidation of polyunsaturated fatty acids and lower changes in volatile compounds of reduced-fat beef burgers. | [45] |
| Açai extract |
|
| Açai extract in whey protein isolate-based edible coatings suppressed microbial growth (total viable aerobic count and coliforms) in meatballs stored at 4 °C and −18 °C. | [48] |
| Guarana seeds |
|
| Guarana seed extract did not affect the color of pork burgers at the lower dose (250 mg/kg). The antioxidant power in lipids and proteins was more effective in the extracts than the synthetic antioxidant (BHT). However, the natural extract was not effective as an antimicrobial. | [50] |
| Guarana seeds |
| Not determined. | 250 mg/kg guarana extract delayed discoloration, retarded lipid and protein oxidation and did not affect the sensory characteristics of lamb burgers. | [51] |
| Annatto seeds | Grinding and sieving (100 mesh). | Bixin content: 14 ± 2 mg/g. | Annatto (0.05%) was effective in reducing the formation of thiobarbituric acid reactive substances and cholesterol oxidation in pork patties after thermal treatment. Sodium erythorbate combined with annatto protected bixin from degradation. | [52] |
| Norbixin from annatto | Commercial norbixin | Norbixin represented 10% of the annatto composition. | Norbixin was efficient in preventing lipid oxidation of sausages. | [46] |
4.2. Fat Replacers
| Amazon Resource | Ingredient Preparation Method | Characteristics of the Ingredient | Main Findings | Reference |
|---|---|---|---|---|
| Gelled emulsion containing cocoa bean shell flour |
|
| At two levels of animal fat replacement in beef burgers (50 and 100%), there was an increase in moisture and ash, a reduction in fat and proteins, an increase in linolenic and linolenic fatty acids, and in the polyunsaturated/saturated fatty acid ratio, reduction of the atherogenicity index and thrombogenicity index, and increased cooking yield. At 50% fat replacement, the samples were sensorially acceptable. However, at both levels of fat replacement, there was an increase in lipid oxidation. | [57] |
| Hydrogel emulsion containing encapsulated açai oil |
| Not determined. | Higher content of polyunsaturated fatty acids of about 32%, reduction of saturated fatty acids by 22%, lower atherogenicity index, and thrombogenicity index, increase in hypocholesterolemic/hypercholesterolemic ratio, reduction in cooking losses, and no significant differences in sensory attributes were observed between control and 25 and 50% beef fat reduction in burgers. However, lipid oxidation tended to increase. | [58] |
| Sacha inchi oil | Commercial sacha inchi oil | Not determined. | By replacing chicken fat with sacha inchi oil (0.5–1.5 g/100 g of ground chicken) in chicken sausages, there was a reduction in saturated fat, omega-6/omega-3 ratio, and the atherogenic and thrombogenic index, increase in omega 3 fatty acids; at 0.5 g sacha inchi, there was an improvement of emulsion stability, no effects in cooking losses texture properties, and sensory acceptability. However, lipid and protein oxidation increased at 1.5 g/100 g sacha inchi oil. | [59] |
| Pulp and peel flour of peach palm |
| Not determined. | At 25 and 50% fat replacement in beef-based burgers, pulp and peel flours decreased hardness, springiness, cohesiveness, chewiness, fat, cooking losses, and diameter reduction. Lower lipid oxidation was obtained with peel flour. | [61] |
4.3. Colorants and Extenders
| Role/Amazon Resource | Ingredient Preparation Method | Characteristics of the Ingredient | Main Findings | Reference |
|---|---|---|---|---|
| Colorant/colorifico containing annatto seeds | Commercial colorifico | Bixin content: 173 ± 24 mg/100 g | Stable and intense red and yellow color in both raw and grilled chicken patties containing 0.4 g/100 g colorifico; lipid oxidation delayed in grilled patties; vitamin E higher in raw chicken; bixin was not stable after grilling. | [63] |
| Colorant/annatto powder | Commercial annatto powder | Norbixin content: 1% | Sausages containing 60%:40% annatto powder:nitrite had higher redness and lower yellowness without presenting significant differences with the control sample (100% nitrite) in microbial counts and sensory properties. | [64] |
| Colorant/oil extract of peach palm fruit residues |
| Total carotenoids varying from 2.61 to 28.13 mg/kg extract, containing 9 to 97 mL/kg extract added to the product. | In Frankfurter sausages, there was an increase in lightness, yellowness, chroma, and hue. On the contrary, redness decreased. | [65] |
| Extender/pulp flour of peach palm |
| Not determined. | 3% of peach palm in tilapia sausages decreased instrumental consistency, adhesiveness, cohesiveness, cutting work, and yield, increased hardness, gumminess, springiness, shear force, and sensory texture, flavor, and odor. | [66] |
| Extender/cocoa shell powder |
| 15.2% protein, 15.1% lipid, 48.1% total dietary fiber, 14.7% carbohydrate, 1.9% moisture, 7% ash, 1.10 mg/g epicatechin, 1.04 isoquercetin. | 1.5 to 3% cocoa shell powder in burgers increased fiber, lipids, and hardness, decreased weight and volume loss during cooking, no effects in sensory traits, and a slight reduction in Pseudomonas was observed. | [67] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomchinsky, B.; Gonçalves, G.G.; Ferreira, A.B. Food Composition Data: Edible Plants from the Amazon BT. In Local Food Plants of Brazil; Jacob, M.C.M., Albuquerque, U.P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 271–295. ISBN 978-3-030-69139-4. [Google Scholar]
- Avila-Sosa, R.; Montero-Rodríguez, A.F.; Aguilar-Alonso, P.; Vera-López, O.; Lazcano-Hernández, M.; Morales-Medina, J.C.; Navarro-Cruz, A.R. Antioxidant Properties of Amazonian Fruits: A Mini Review of In Vivo and In Vitro Studies. Oxid. Med. Cell. Longev. 2019, 2019, 8204129. [Google Scholar] [CrossRef]
- Peixoto Araujo, N.M.; Arruda, H.S.; Marques, D.R.P.; de Oliveira, W.Q.; Pereira, G.A.; Pastore, G.M. Functional and Nutritional Properties of Selected Amazon Fruits: A Review. Food Res. Int. 2021, 147, 110520. [Google Scholar] [CrossRef]
- Amorim, I.S.; Amorim, D.S.; Godoy, H.T.; Mariutti, L.R.B.; Chisté, R.C.; da Silva Pena, R.; Bogusz Junior, S.; Chim, J.F. Amazonian Palm Tree Fruits: From Nutritional Value to Diversity of New Food Products. Heliyon 2024, 10, e24054. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Tu, J.; Xu, B. Muscle Food and Human Health: A Systematic Review from the Perspective of External and Internal Oxidation. Trends Food Sci. Technol. 2023, 138, 85–99. [Google Scholar] [CrossRef]
- Kumari, P.V.K.; Akhila, S.; Rao, Y.S.; Devi, B.R. Alternative to Artificial Preservatives. Syst. Rev. Pharm. 2019, 10, 99–102. [Google Scholar] [CrossRef]
- Lofgren, P.A. Meat, Poultry, and Meat Products: Nutritional Value. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 160–167. ISBN 978-0-12-384885-7. [Google Scholar]
- Mori, T.A.; Hodgson, J.M. Fatty Acids: Health Effects of Omega-6 Polyunsaturated Fatty Acids, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 209–214. ISBN 978-0-12-384885-7. [Google Scholar]
- Simopoulos, A.P. Omega-3 Polyunsaturated Fatty Acids: Nutrigenetic and Nutrigenomic Aspects in the Determination of Dietary Requirements, Development, and Chronic Diseases. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 405–412. ISBN 978-0-12-384885-7. [Google Scholar]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of Consumption of Red and Processed Meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1700047. [Google Scholar] [CrossRef]
- Broncano, J.M.; Petrón, M.J.; Parra, V.; Timón, M.L. Effect of Different Cooking Methods on Lipid Oxidation and Formation of Free Cholesterol Oxidation Products (COPs) in Latissimus Dorsi Muscle of Iberian Pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef]
- Angeli, J.P.F.; Garcia, C.C.M.; Sena, F.; Freitas, F.P.; Miyamoto, S.; Medeiros, M.H.G.; Di Mascio, P. Lipid Hydroperoxide-Induced and Hemoglobin-Enhanced Oxidative Damage to Colon Cancer Cells. Free Radic. Biol. Med. 2011, 51, 503–515. [Google Scholar] [CrossRef]
- Codex Alimentarius GSFA Food Additive. Available online: https://www.fao.org/gsfaonline/additives/results.html?techFunction=4&searchBy=tf (accessed on 28 June 2024).
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic Phenolic Antioxidants: Metabolism, Hazards and Mechanism of Action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A Dietary Mixture of Oxysterols Induces In Vitro Intestinal Inflammation through TLR2/4 Activation: The Protective Effect of Cocoa Bean Shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef]
- Laslo, M.; Sun, X.; Hsiao, C.-T.; Wu, W.W.; Shen, R.-F.; Zou, S. A Botanical Containing Freeze Dried Açai Pulp Promotes Healthy Aging and Reduces Oxidative Damage in Sod1 Knockdown Flies. Age 2013, 35, 1117–1132. [Google Scholar] [CrossRef]
- Diotallevi, C.; Fava, F.; Gobbetti, M.; Tuohy, K. Healthy Dietary Patterns to Reduce Obesity-Related Metabolic Disease: Polyphenol-Microbiome Interactions Unifying Health Effects across Geography. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Genova, V.M.; Gambero, A.; de Souza Freitas Campos, P.; Macedo, G.A. Polyphenolic Compounds Mechanisms as Inhibitors of Advanced Glycation End Products and Their Relationship to Health and Disease. In Molecular Mechanisms of Functional Food; Wiley: Hoboken, NJ, USA, 2022; pp. 1–27. ISBN 9781119804055. [Google Scholar]
- Jaramillo-Vivanco, T.; Balslev, H.; Montúfar, R.; Cámara, R.M.; Giampieri, F.; Battino, M.; Cámara, M.; Alvarez-Suarez, J.M. Three Amazonian Palms as Underestimated and Little-Known Sources of Nutrients, Bioactive Compounds and Edible Insects. Food Chem. 2022, 372, 131273. [Google Scholar] [CrossRef]
- Teixeira, N.; Melo, J.C.S.; Batista, L.F.; Paula-Souza, J.; Fronza, P.; Brandão, M.G.L. Edible Fruits from Brazilian Biodiversity: A Review on Their Sensorial Characteristics versus Bioactivity as Tool to Select Research. Food Res. Int. 2019, 119, 325–348. [Google Scholar] [CrossRef]
- Barbosa, P.O.; Pala, D.; Silva, C.T.; de Souza, M.O.; do Amaral, J.F.; Vieira, R.A.L.; Folly, G.A.d.F.; Volp, A.C.P.; de Freitas, R.N. Açai (Euterpe oleracea Mart.) Pulp Dietary Intake Improves Cellular Antioxidant Enzymes and Biomarkers of Serum in Healthy Women. Nutrition 2016, 32, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Udani, J.K.; Singh, B.B.; Singh, V.J.; Barrett, M.L. Effects of Açai (Euterpe oleracea Mart.) Berry Preparation on Metabolic Parameters in a Healthy Overweight Population: A Pilot Study. Nutr. J. 2011, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, G.R.; Fragoso, M.F.; Borguini, R.G.; de Araújo Santiago, M.C.P.; Fernandes, A.A.H.; Barbisan, L.F. Protective Effects of Spray-Dried Açaí (Euterpe oleracea Mart) Fruit Pulp against Initiation Step of Colon Carcinogenesis. Food Res. Int. 2015, 77, 432–440. [Google Scholar] [CrossRef]
- Muhtadi, M.; Haryoto, H.; Sujono, T.A.; Suhendi, A. Antidiabetic and Antihypercholesterolemia Activities of Rambutan (Nephelium Lappaceum L.) and Durian (Durio zibethinus Murr.) Fruit Peel Extracts. J. Appl. Pharm. Sci. 2016, 6, 2231–3354. [Google Scholar] [CrossRef]
- Chung, A.P.Y.S.; Ton, S.H.; Gurtu, S.; Palanisamy, U.D. Ellagitannin Geraniin Supplementation Ameliorates Metabolic Risks in High-Fat Diet-Induced Obese Sprague Dawley Rats. J. Funct. Foods 2014, 9, 173–182. [Google Scholar] [CrossRef]
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid Isolation of Geraniin from Nephelium Lappaceum Rind Waste and Its Anti-Hyperglycemic Activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J.A. Rambutan (Nephelium Lappaceum L.): Nutritional and Functional Properties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- de Souza Schmidt Gonçalves, A.E.; Lellis-Santos, C.; Curi, R.; Lajolo, F.M.; Genovese, M.I. Frozen Pulp Extracts of Camu-Camu (Myrciaria dubia McVaugh) Attenuate the Hyperlipidemia and Lipid Peroxidation of Type 1 Diabetic Rats. Food Res. Int. 2014, 64, 1–8. [Google Scholar] [CrossRef]
- Inoue, T.; Komoda, H.; Uchida, T.; Node, K. Tropical Fruit Camu-Camu (Myrciaria dubia) Has Anti-Oxidative and Anti-Inflammatory Properties. J. Cardiol. 2008, 52, 127–132. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.B.; Genovese, M.I. Chemical Composition of Cupuassu (Theobroma grandiflorum) and Cocoa (Theobroma cacao) Liquors and Their Effects on Streptozotocin-Induced Diabetic Rats. Food Res. Int. 2013, 51, 929–935. [Google Scholar] [CrossRef]
- Carvalho, R.P.; Lemos, J.R.G.; de Aquino Sales, R.S.; Martins, M.G.; Nascimento, C.H.; Bayona, M.; Marcon, J.L.; Monteiro, J.B. The consumption of red pupunha (Bactris gasipaes Kunth) increases HDL cholesterol and reduces weight gain of lactating and post-lactating wistar rats. J. Aging Res. Clin. Pract. 2013, 2, 257–260. [Google Scholar]
- Curimbaba, T.F.S.; Almeida-Junior, L.D.; Chagas, A.S.; Quaglio, A.E.V.; Herculano, A.M.; Di Stasi, L.C. Prebiotic, Antioxidant and Anti-Inflammatory Properties of Edible Amazon Fruits. Food Biosci. 2020, 36, 100599. [Google Scholar] [CrossRef]
- Garmendia, F.; Pando, R.; Ronceros, G. Efecto Del Aceite de Sacha Inchi (Plukenetia Volúbilis l) Sobre El Perfil Lipídico En Pacientes Con Hiperlipoproteinemia. Rev. Peru. Med. Exp. Salud Publica 2011, 28, 628–632. [Google Scholar] [CrossRef]
- Patrick, M.; Kim, H.A.; Oketch-Rabah, H.; Marles, R.J.; Roe, A.L.; Calderón, A.I. Safety of Guarana Seed as a Dietary Ingredient: A Review. J. Agric. Food Chem. 2019, 67, 11281–11287. [Google Scholar] [CrossRef]
- Stringheta, P.C.; Silva, P.I.; Costa, A.G. V Annatto/Urucum—Bixa Orellana. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 23–30. ISBN 978-0-12-803138-4. [Google Scholar]
- Martínez, J.; Nieto, G.; Castillo, J.; Ros, G. Influence of in Vitro Gastrointestinal Digestion and/or Grape Seed Extract Addition on Antioxidant Capacity of Meat Emulsions. LWT-Food Sci. Technol. 2014, 59, 834–840. [Google Scholar] [CrossRef]
- Santos, Y.J.S.; Facchinatto, W.M.; Rochetti, A.L.; Carvalho, R.A.; Le Feunteun, S.; Fukumasu, H.; Morzel, M.; Colnago, L.A.; Vanin, F.M. Systemic Characterization of Pupunha (Bactris gasipaes) Flour with Views of Polyphenol Content on Cytotoxicity and Protein in Vitro Digestion. Food Chem. 2023, 405, 134888. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant By-Product Antioxidants: Control of Protein-Lipid Oxidation in Meat and Meat Products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-Based Natural Antioxidants in Meat and Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.-K.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent Developments of Natural Antimicrobials and Antioxidants on Fish and Fishery Food Products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef] [PubMed]
- Hanula, M.; Szpicer, A.; Górska-Horczyczak, E.; Khachatryan, G.; Pogorzelski, G.; Pogorzelska-Nowicka, E.; Poltorak, A. Hydrogel Emulsion with Encapsulated Safflower Oil Enriched with Acai Extract as a Novel Fat Substitute in Beef Burgers Subjected to Storage in Cold Conditions. Molecules 2022, 27, 2397. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Capitani, C.D.; Decker, E.A.; Castro, I.A. Effect of Natural Pigments on the Oxidative Stability of Sausages Stored under Refrigeration. Meat Sci. 2010, 84, 718–726. [Google Scholar] [CrossRef]
- Packer, V.G.; Melo, P.S.; Bergamaschi, K.B.; Selani, M.M.; Villanueva, N.D.M.; de Alencar, S.M.; Contreras-Castillo, C.J. Chemical Characterization, Antioxidant Activity and Application of Beetroot and Guava Residue Extracts on the Preservation of Cooked Chicken Meat. J. Food Sci. Technol. 2015, 52, 7409–7416. [Google Scholar] [CrossRef]
- Şen, D.B.; Kılıç, B. Effects of Edible Coatings Containing Acai Powder and Matcha Extracts on Shelf Life and Quality Parameters of Cooked Meatballs. Meat Sci. 2021, 179, 108547. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, E.R.B.; dos Santos, J.M.; Carvalho, L.T.; Borgonovi, T.F.; Lorenzo, J.M.; da Silva-Barretto, A.C. Açaí Extract Powder as Natural Antioxidant on Pork Patties during the Refrigerated Storage. Meat Sci. 2022, 184, 108667. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana Seed Extracts as a Useful Strategy to Extend the Shelf Life of Pork Patties: UHPLC-ESI/QTOF Phenolic Profile and Impact on Microbial Inactivation, Lipid and Protein Oxidation and Antioxidant Capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of Guarana (Paullinia Cupana) Seed and Pitanga (Eugenia Uniflora L.) Leaf Extracts on Lamb Burgers with Fat Replacement by Chia Oil Emulsion during Shelf Life Storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef] [PubMed]
- Figueirêdo, B.C.; Trad, I.J.; Mariutti, L.R.B.; Bragagnolo, N. Effect of Annatto Powder and Sodium Erythorbate on Lipid Oxidation in Pork Loin during Frozen Storage. Food Res. Int. 2014, 65, 137–143. [Google Scholar] [CrossRef]
- Estévez, M. Critical Overview of the Use of Plant Antioxidants in the Meat Industry: Opportunities, Innovative Applications and Future Perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Rios-Mera, J.D.; Saldaña, E.; Contreras-Castillo, C.J. Strategies for Obtaining Healthy Meat Products. In The Food Industry: Perceptions, Practices and Future Prospects; Santos, D.T., Carvalho, G.B., Castillo-Torres, R.B., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; pp. 233–250. ISBN 978-168507344-2, 978-168507302-2. [Google Scholar]
- Rios-Mera, J.D.; Selani, M.M.; Patinho, I.; Saldaña, E.; Contreras-Castillo, C.J. Modification of NaCl Structure as a Sodium Reduction Strategy in Meat Products: An Overview. Meat Sci. 2021, 174, 108417. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, K.; Aredo, V.; Rios-Mera, J.D.; Ambrosio, C.M.S.; Siche, R.; Saldaña, E. Chapter 13—Market Needs and Consumer’s Preferences for Healthier Foods. In Developments in Food Quality and Safety; Lorenzo, J.M., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 337–355. ISBN 978-0-443-15346-4. [Google Scholar]
- Botella-Martinez, C.; Lucas-González, R.; Lorenzo, J.M.; Santos, E.M.; Rosmini, M.; Sepúlveda, N.; Teixeira, A.; Sayas-Barberá, E.; Pérez-Alvarez, J.A.; Fernandez-Lopez, J.; et al. Cocoa Coproducts-Based and Walnut Oil Gelled Emulsion as Animal Fat Replacer and Healthy Bioactive Source in Beef Burgers. Foods 2021, 10, 2706. [Google Scholar] [CrossRef] [PubMed]
- Hanula, M.; Szpicer, A.; Górska-Horczyczak, E.; Khachatryan, G.; Pogorzelska-Nowicka, E.; Poltorak, A. Quality of Beef Burgers Formulated with Fat Substitute in a Form of Freeze-Dried Hydrogel Enriched with Açai Oil. Molecules 2022, 27, 3700. [Google Scholar] [CrossRef]
- Wongpattananukul, S.; Nungarlee, U.; Ruangprach, A.; Sulong, S.; Sanporkha, P.; Adisakwattana, S.; Ngamukote, S. Effect of Inca Peanut Oil on Omega-3 Polyunsaturated Fatty Acids, Physicochemical, Texture and Sensory Properties in Chicken Sausage. LWT 2022, 163, 113559. [Google Scholar] [CrossRef]
- Badar, I.H.; Liu, H.; Chen, Q.; Xia, X.; Kong, B. Future Trends of Processed Meat Products Concerning Perceived Healthiness: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4739–4778. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, H.; Llatas, A.Y.; Arteaga, H.; Saldaña, E.; Tello, F.; Rios-Mera, J.D. Pijuayo (Bactris gasipaes) Pulp and Peel Flours as Partial Substitutes for Animal Fat in Burgers: Physicochemical Properties. Biol. Life Sci. Forum 2023, 26, 78. [Google Scholar] [CrossRef]
- Talukder, S. Effect of Dietary Fiber on Properties and Acceptance of Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Castro, W.F.; Mariutti, L.R.B.; Bragagnolo, N. The Effects of Colorifico on Lipid Oxidation, Colour and Vitamin E in Raw and Grilled Chicken Patties during Frozen Storage. Food Chem. 2011, 124, 126–131. [Google Scholar] [CrossRef]
- Zarringhalami, S.; Sahari, M.A.; Hamidi-Esfehani, Z. Partial Replacement of Nitrite by Annatto as a Colour Additive in Sausage. Meat Sci. 2009, 81, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-Zárate, L.X.; Hleap-Zapata, J.I.; Ordóñez-Santos, L.E. Análisis de Los Parámetros de Color En Salchichas Frankfurt Adicionadas Con Extracto Oleoso de Residuos de Chontaduro (Bactris Gasipaes). Inf. Tecnol. 2015, 26, 45–54. [Google Scholar] [CrossRef]
- Zapata, J.I.H.; de la Pava, G.C.R. Propiedades Texturales y Sensoriales de Salchichas de Tilapia Roja (Oreochromis sp.) Con Adición de Harina de Chontaduro. Ing. Desarro. 2015, 33, 199–215. [Google Scholar]
- Delgado-Ospina, J.; Lucas-González, R.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Martuscelli, M.; Chaves-López, C. Potential of the Cocoa Shell to Improve the Quality Properties of a Burger-like Meat Product. J. Food Process. Preserv. 2022, 46, e16752. [Google Scholar] [CrossRef]
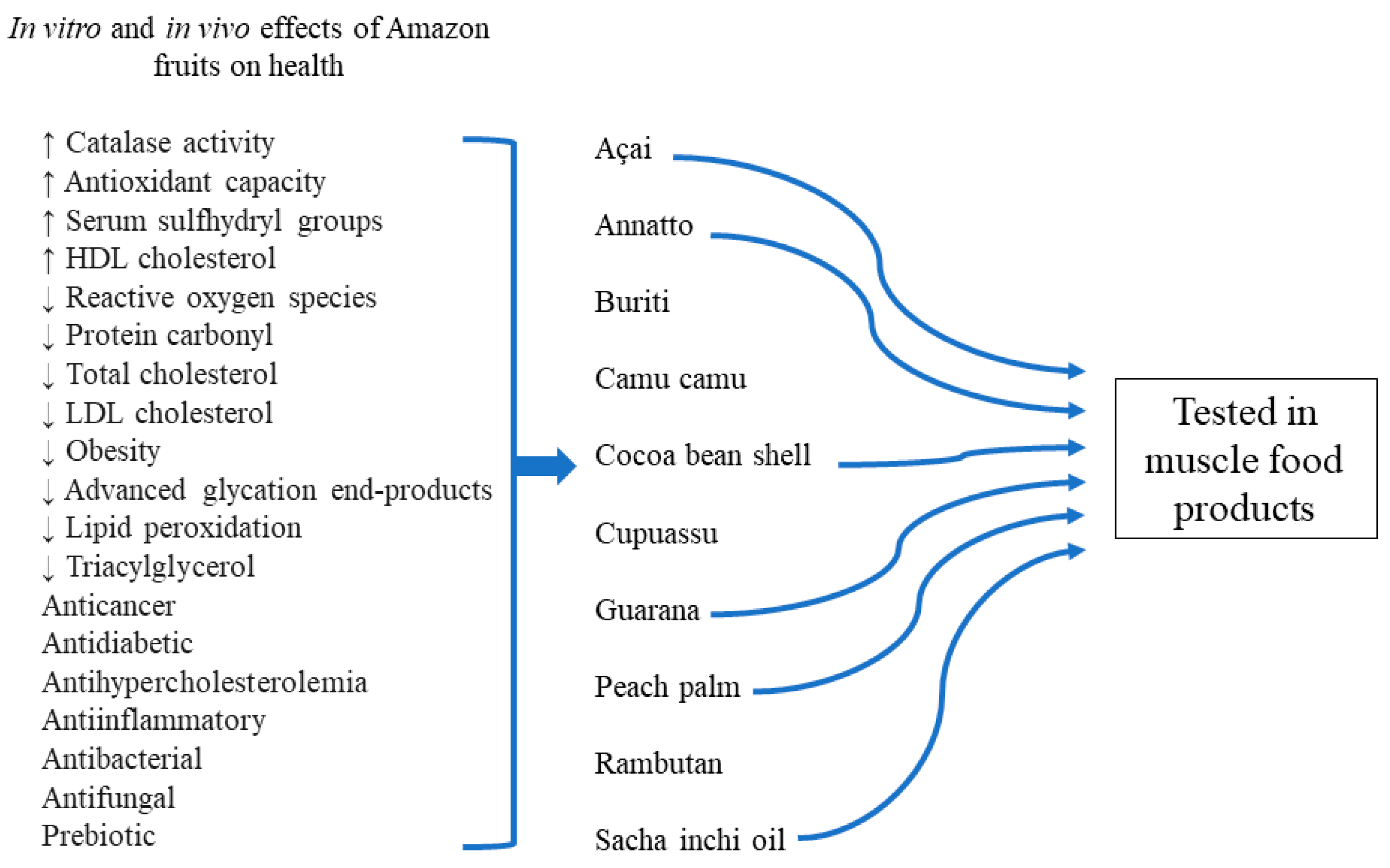
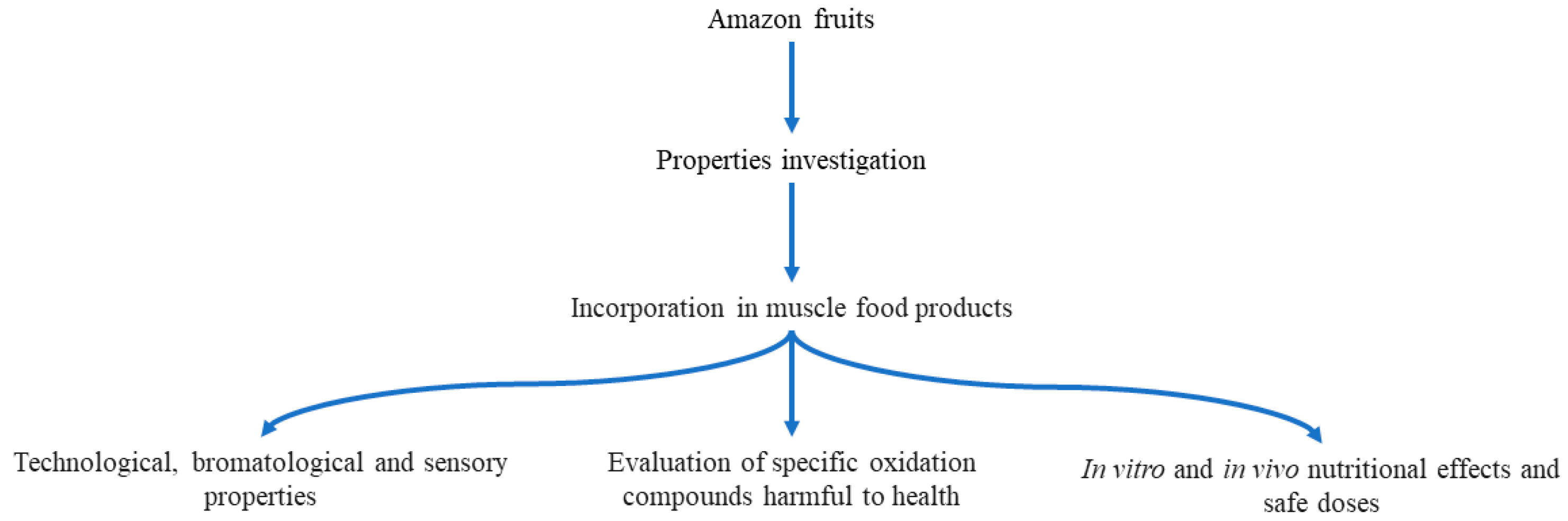
| Antioxidant | Product | Maximum Level (mg/kg) |
|---|---|---|
| BHT | Meat products | 100 |
| Fish and fish products | 200 | |
| BHA | Meat products | 100 |
| Fish and fish products | 200 | |
| Propyl gallate | Meat products | 200 |
| Fish and fish products | 100 | |
| TBHQ | Meat products | 100 |
| Fish oil | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios-Mera, J.D.; Arteaga, H.; Ruiz, R.; Saldaña, E.; Tello, F. Amazon Fruits as Healthy Ingredients in Muscle Food Products: A Review. Foods 2024, 13, 2110. https://doi.org/10.3390/foods13132110
Rios-Mera JD, Arteaga H, Ruiz R, Saldaña E, Tello F. Amazon Fruits as Healthy Ingredients in Muscle Food Products: A Review. Foods. 2024; 13(13):2110. https://doi.org/10.3390/foods13132110
Chicago/Turabian StyleRios-Mera, Juan D., Hubert Arteaga, Roger Ruiz, Erick Saldaña, and Fernando Tello. 2024. "Amazon Fruits as Healthy Ingredients in Muscle Food Products: A Review" Foods 13, no. 13: 2110. https://doi.org/10.3390/foods13132110
APA StyleRios-Mera, J. D., Arteaga, H., Ruiz, R., Saldaña, E., & Tello, F. (2024). Amazon Fruits as Healthy Ingredients in Muscle Food Products: A Review. Foods, 13(13), 2110. https://doi.org/10.3390/foods13132110





