Mass Spectrometric Study of the Most Common Potential Migrants Extractible from the Inner Coatings of Metallic Beverage Cans
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. HPLC-MS Screening Test and MRM Analysis
2.3. HPLC-QTOF-MS Analysis
3. Results and Discussion
3.1. Detection of BADGE-BuOEtOH Adducts, BAMGE + BuOEtOH and Cyclo-di-BADGE as the Most Common Potential Migrants
3.2. The Fragmentation Pathways of the Detected Migrants as Studied by HPLC-QTOF-MS/MS Analysis
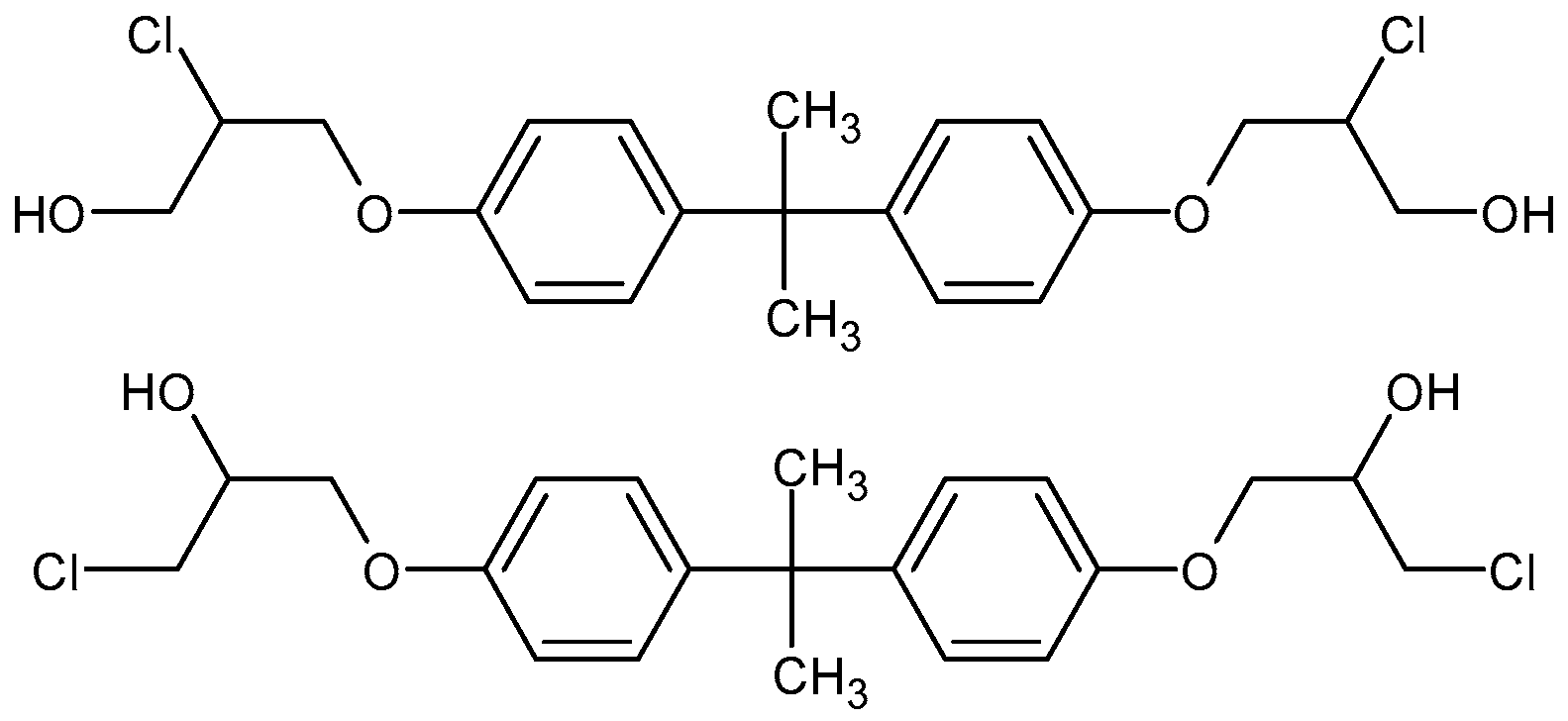
3.3. Migrant Isomers Detected upon HPLC-QTOF-MS/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, M.M.; Falbe, J.; Schillinger, D.; Basu, S.; McCulloch, C.E.; Madsen, K.A. Sugar-sweetened beverage consumption 3 years after the Berkeley, California, sugar-sweetened beverage tax. Am. J. Public Health 2019, 109, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.P.V.; Pimenta, A.M.; Sobrinho, P.D.S.C.; Hermsdorff, H.H.M.; Bressan, J.; Nobre, L.N. Consumption of sweetened beverages is associated with the incidence of type 2 diabetes in Brazilian adults (CUME project). Nutr. Metab. Cardiovasc. Dis. 2023, 33, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Barbato, F.; Mita, D.G.; Grumetto, L. Occurrence of Bisphenol A and its analogues in some foodstuff marketed in Europe. Food Chem. Toxicol. 2019, 131, 110575. [Google Scholar] [CrossRef] [PubMed]
- Hartle, J.C.; Navas-Acien, A.; Lawrence, R.S. The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ. Res. 2016, 150, 375–382. [Google Scholar] [CrossRef]
- Braunrath, R.; Podlipna, D.; Padlesak, S.; Cichna-Markl, M. Determination of bisphenol A in canned foods by immunoaffinity chromatography, HPLC, and fluorescence detection. J. Agric. Food Chem. 2005, 53, 8911. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Corriveau, J.; Popovic, S. Levels of bisphenol A in canned soft drink products in Canadian markets. J. Agric. Food Chem. 2009, 57, 1307. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Apelbaum, T.Z.; Goeyens, L.; Neels, H.; Covaci, A. Intake of bisphenol A from canned beverages and foods on the Belgian market. Food Addit. Contam. Part A 2010, 27, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical determination of bisphenol A in plastic bottled drinking water and canned beverages using a molecularly imprinted chitosan-graphene composite film modified electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, J.; Yin, J.; Shao, B.; Zhang, J. Molecularly imprinted solid-phase extraction for selective extraction of bisphenol analogues in beverages and canned food. J. Agric. Food Chem. 2014, 62, 11130–11137. [Google Scholar] [CrossRef]
- Fasano, E.; Esposito, F.; Scognamiglio, G.; Di Francesco, F.; Montuori, P.; Amodio Cocchieri, R.; Cirillo, T. Bisphenol A contamination in soft drinks as a risk for children’s health in Italy. Food Addit. Contam. Part A 2015, 32, 1207–1214. [Google Scholar] [CrossRef]
- Kovačič, A.; Gys, C.; Gulin, M.R.; Kosjek, T.; Heath, D.; Covaci, A.; Heath, E. The migration of bisphenols from beverage cans and reusable sports bottles. Food Chem. 2020, 331, 127326. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhong, H.N.; Qiu, K.; Li, D.; Wu, G.; Sui, H.X.; Song, Y. Exposure to bisphenol A and its substitutes, bisphenol F and bisphenol S from canned foods and beverages on Chinese market. Food Control 2021, 120, 107502. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, D.; Bhandari, R.; Malik, A.K.; Kaur, S.; Singh, B. Bisphenol A in canned soft drinks, plastic-bottled water, and household water tank from Punjab, India. J. Hazard. Mater. Adv. 2023, 9, 100205. [Google Scholar] [CrossRef]
- Liang, M.; Hou, X.; Xian, Y.; Wu, Y.; Hu, J.; Chen, R.; Wang, L.; Huang, Y.; Zhang, X. Banana-peel-derived magnetic porous carbon as effective adsorbent for the enrichment of six bisphenols from beverage and water samples. Food Chem. 2022, 376, 131948. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Fast liquid chromatography-tandem mass spectrometry for the analysis of bisphenol A-diglycidyl ether, bisphenol F-diglycidyl ether and their derivatives in canned food and beverages. J. Chromatogr. A 2011, 1218, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Toptancı, İ. Risk assessment of bisphenol related compounds in canned convenience foods, olives, olive oil, and canned soft drinks in Turkey. Environ. Sci. Pollut. Res. 2023, 30, 54177–54192. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Ayala, H.; Núñez, O.; Moyano, E.; Galceran, M.T. Field-amplified sample injection-micellar electrokinetic capillary chromatography for the analysis of bisphenol A, bisphenol F, and their diglycidyl ethers and derivatives in canned soft drinks. Electrophoresis 2010, 31, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hu, Y.; Chen, Y.; Wu, G.; Li, G.; Zhong, Q. Effective enrichment and detection of bisphenol diglycidyl ether, novolac glycerol ether and their derivatives in canned food using a novel magnetic sulfonatocalix[6]arene covalent cross-linked polymer as the adsorbent. Food Chem. 2023, 399, 133918. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Pisciottano, I.D.M.; Esposito, F.; Fasano, E.; Scognamiglio, G.; Mita, G.D.; Cirillo, T. Determination of BPA, BPB, BPF, BADGE and BFDGE in canned energy drinks by molecularly imprinted polymer cleaning up and UPLC with fluorescence detection. Food Chem. 2017, 220, 406–412. [Google Scholar] [CrossRef]
- Russo, G.; Varriale, F.; Barbato, F.; Grumetto, L. Are canned beverages industries progressively switching to bisphenol AF? J. Food Sci. 2019, 84, 3303–3311. [Google Scholar] [CrossRef]
- Biles, J.E.; White, K.D.; McNeal, T.P.; Begley, T.H. Determination of the diglycidyl ether of bisphenol A and its derivatives in canned foods. J. Agric. Food Chem. 1999, 47, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Loureiro, P.V.; Sendón, R.; Losada, P.P.; de Quirós, A.R.B. Application of chromatographic analysis for detecting components from polymeric can coatings and further determination in beverage samples. J. Chromatogr. A 2021, 1638, 461886. [Google Scholar] [CrossRef]
- Oldring, P.K.T.; Castle, L.; Hart, A.; Holmes, M.J. Migrants from food cans revisited-application of a stochastic model for a more realistic assessment of exposure to bisphenol A diglycidyl ether (BADGE). Packag. Technol. Sci. 2006, 19, 121–137. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Nieto, M.T.; Paseiro-Losada, P.; de Quirós, A.R.B. Food and beverage can coatings: A review on chemical analysis, migration, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3558–3611. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, G.; Oldring, P.K.T. Estimates of per capita exposure to substances migrating from canned foods and beverages. Food Addit. Contam. 2002, 19, 891–903. [Google Scholar] [CrossRef]
- Oldring, P.K.T.; Castle, L.; O’Mahony, C.; Dixon, J. Estimates of dietary exposure to bisphenol A (BPA) from light metal packaging using food consumption and packaging usage data: A refined deterministic approach and a fully probabilistic (FACET) approach. Food Addit. Contam. Part A 2014, 31, 466–489. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.; Derek Yach, D.; Mensah, G.A. Major multinational food and beverage companies and informal sector contributions to global food consumption: Implications for nutrition policy. Glob. Health 2011, 7, 26. [Google Scholar] [CrossRef]
- Berger, U.; Oehme, M. Identification of derivatives of bisphenol A diglycidyl ether and novolac glycidyl ether in can coatings by liquid chromatography/ion trap mass spectrometry. J. AOAC Int. 2000, 83, 1367–1376. [Google Scholar] [CrossRef]
- Bradley, E.L.; Driffield, M.; Harmer, N.; Oldring, P.K.T.; Castle, L. Identification of potential migrants in epoxy phenolic can coatings. Int. J. Polym. Anal. Charact. 2008, 13, 200–223. [Google Scholar] [CrossRef]
- Biedermann, S.; Zurfluh, M.; Grob, K.; Vedani, A.; Brüschweiler, B.J. Migration of cyclo-diBA from coatings into canned food: Method of analysis, concentration determined in a survey and in silico hazard profiling. Food Chem. Toxicol. 2013, 58, 107–115. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; DeVries, J.; Begley, T.H. Evaluation of short-term and long-term migration testing from can coatings into food simulants: Epoxy and acrylic-phenolic coatings. J. Agric. Food Chem. 2017, 65, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Bustos, J.; Lestido-Cardama, A.; Santillana, M.I.; Sendón, R.; Paseiro Losada, P.; de Quirós, A.R.B. Quantification of cyclo-di-BADGE and identification of several BADGE derivatives in canned food samples. Coatings 2023, 13, 792. [Google Scholar] [CrossRef]
- Salami, F.; Lin, J.; Chen, B.; Zhang, B.H.; Zhao, Y. Capture and characterization of elusive cyclo-di-BADGE. New J. Chem. 2023, 47, 18302–18314. [Google Scholar] [CrossRef]
- Schaefer, A.; Simat, T.J. Migration from can coatings: Part 3. Synthesis, identification and quantification of migrating epoxy-based substances below 1000 Da. Food Addit. Contam. Part A 2004, 21, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Beszterda, M.; Kasperkowiak, M.; Frańska, M.; Jęziołowska, S.; Frański, R. Ethoxylated butoxyethanol-BADGE adducts—New potential migrants from epoxy resin can coating material. Materials 2021, 14, 3682. [Google Scholar] [CrossRef] [PubMed]
- Beszterda, M.; Tądrowska, M.; Frański, R. Multi-detection method for the fast screening of bisphenol A diglycidyl ether conjugates in the can-coating material. J. Coat. Technol. Res. 2022, 19, 1901–1907. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; de Quirós, A.R.B. Tentative identification of BADGE derivatives in epoxy type coatings in a model sample: A beverage can. J. Coat. Technol. Res. 2022, 19, 1893–1900. [Google Scholar] [CrossRef]
- Simal-Gándara, J.; Paz-Abuín, S.; Ahrné, L. A critical review of the quality and safety of BADGE-based epoxy coatings for cans: Implications for legislation on epoxy coatings for food contact. Crit. Rev. Food Sci. Nutr. 1998, 38, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Frańska, M.; Stężycka, O.; Jankowski, W.; Hoffmann, M. Gas-phase internal ribose residue loss from Mg-ATP and Mg-ADP complexes: Experimental and theoretical evidence for phosphate-Mg-adenine interaction. J. Am. Soc. Mass Spectrom. 2022, 33, 1474–1479. [Google Scholar] [CrossRef]
- Xue, J.; Venkatesan, A.K.; Wu, Q.; Halden, R.U.; Kannan, K. Occurrence of bisphenol a diglycidyl ethers (BADGEs) and novolac glycidyl ethers (NOGEs) in archived biosolids from the US EPA’s targeted national sewage sludge survey. Environ. Sci. Technol. 2015, 49, 6538–6544. [Google Scholar] [CrossRef]
- Abdallah, S.; Jampy, A.; Moison, D.; Wieckowski, M.; Messiaen, S.; Martini, E.; Campalans, A.; Radicella, J.P.; Rouiller-Fabre, V.; Livera, G.; et al. Foetal exposure to the bisphenols BADGE and BPAF impairs meiosis through DNA oxidation in mouse ovaries. Environ. Pollut. 2023, 317, 120791. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, A.; Andersson, A.M.; Skakkebæk, N.E. Bisphenol A diglycidyl ether (BADGE) and bisphenol analogs, but not bisphenol A (BPA), activate the CatSper Ca2+ channel in human sperm. Front. Endocrinol. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, H.; Fei, X.; Synder, S.A.; Fang, M.; Liu, M. A comprehensive review on the analytical method, occurrence, transformation and toxicity of a reactive pollutant: BADGE. Environ. Int. 2021, 155, 106701. [Google Scholar] [CrossRef]
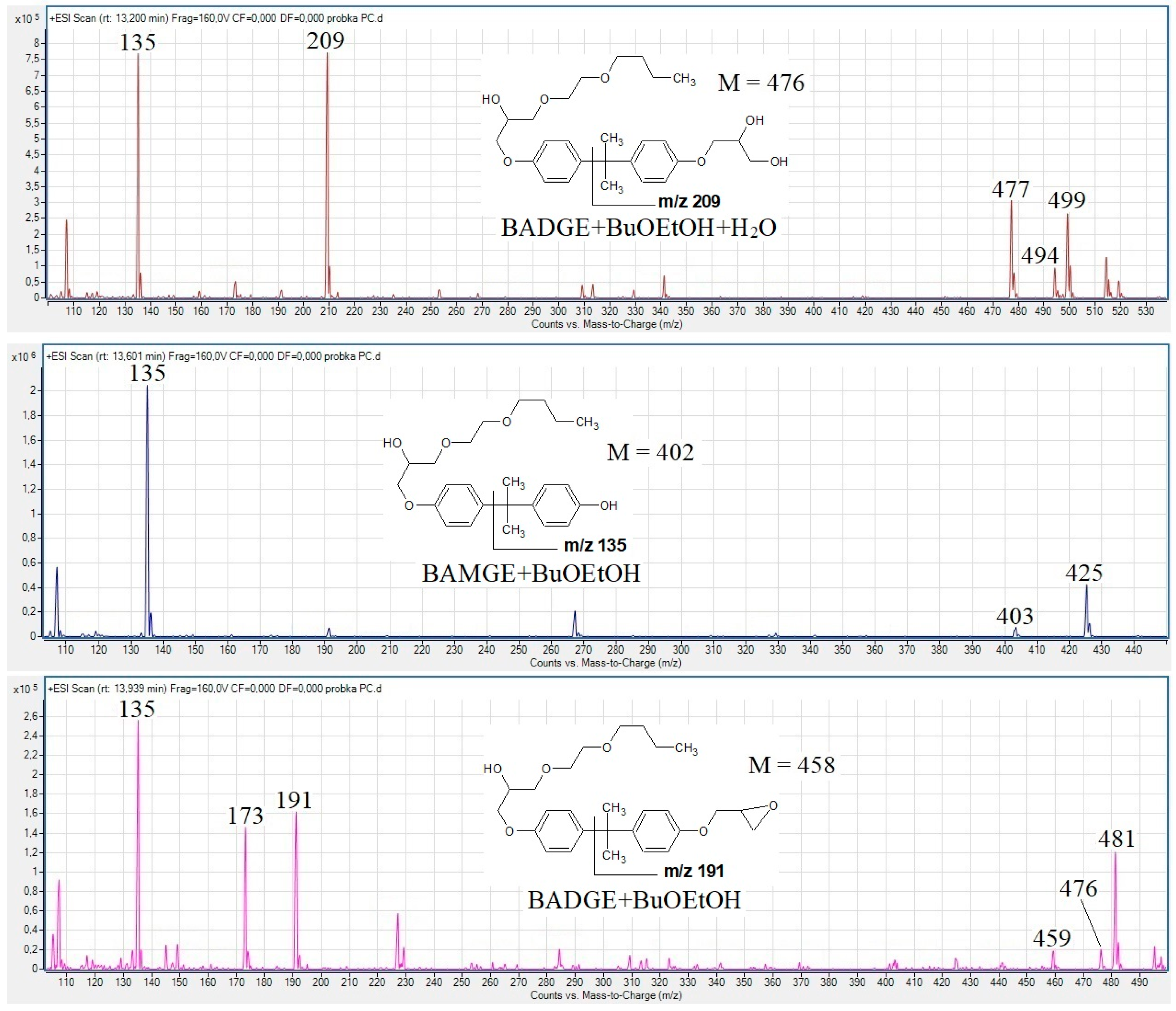
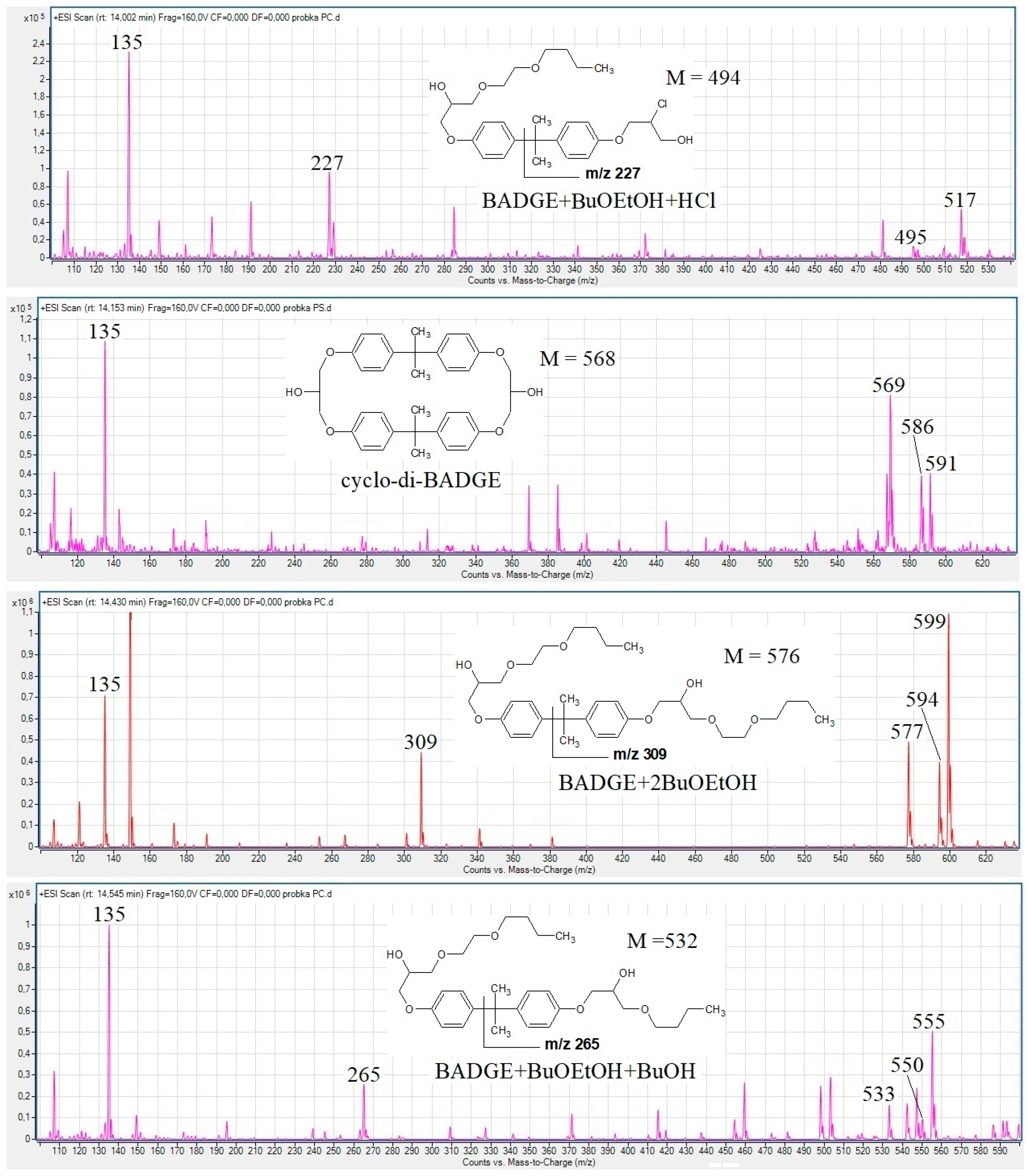
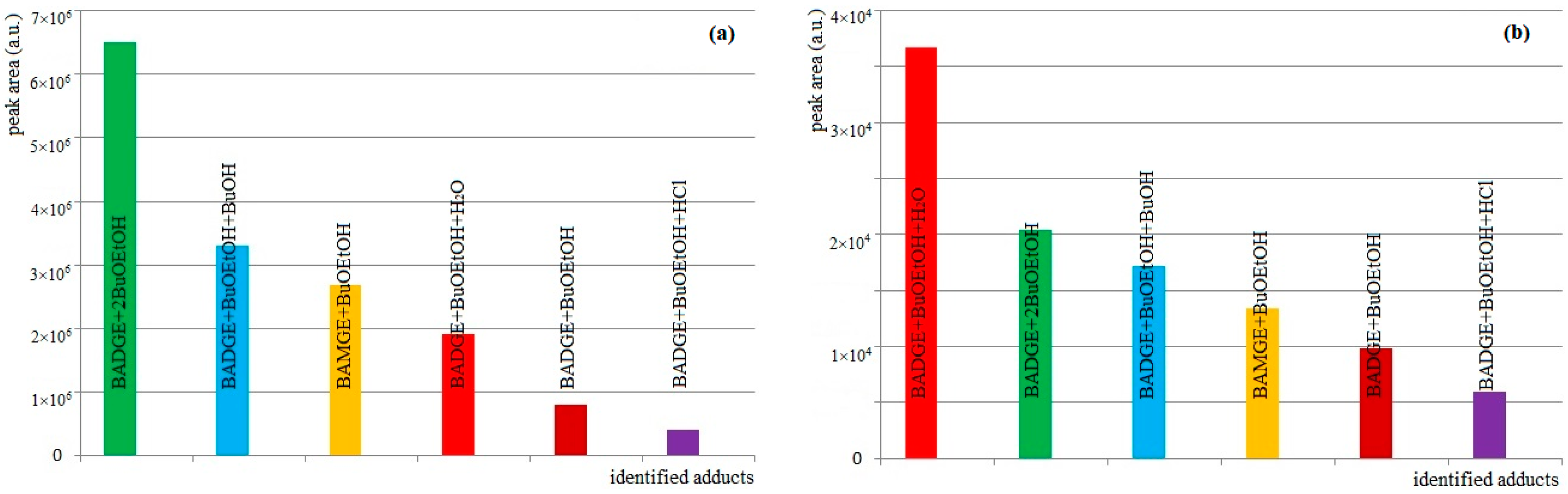
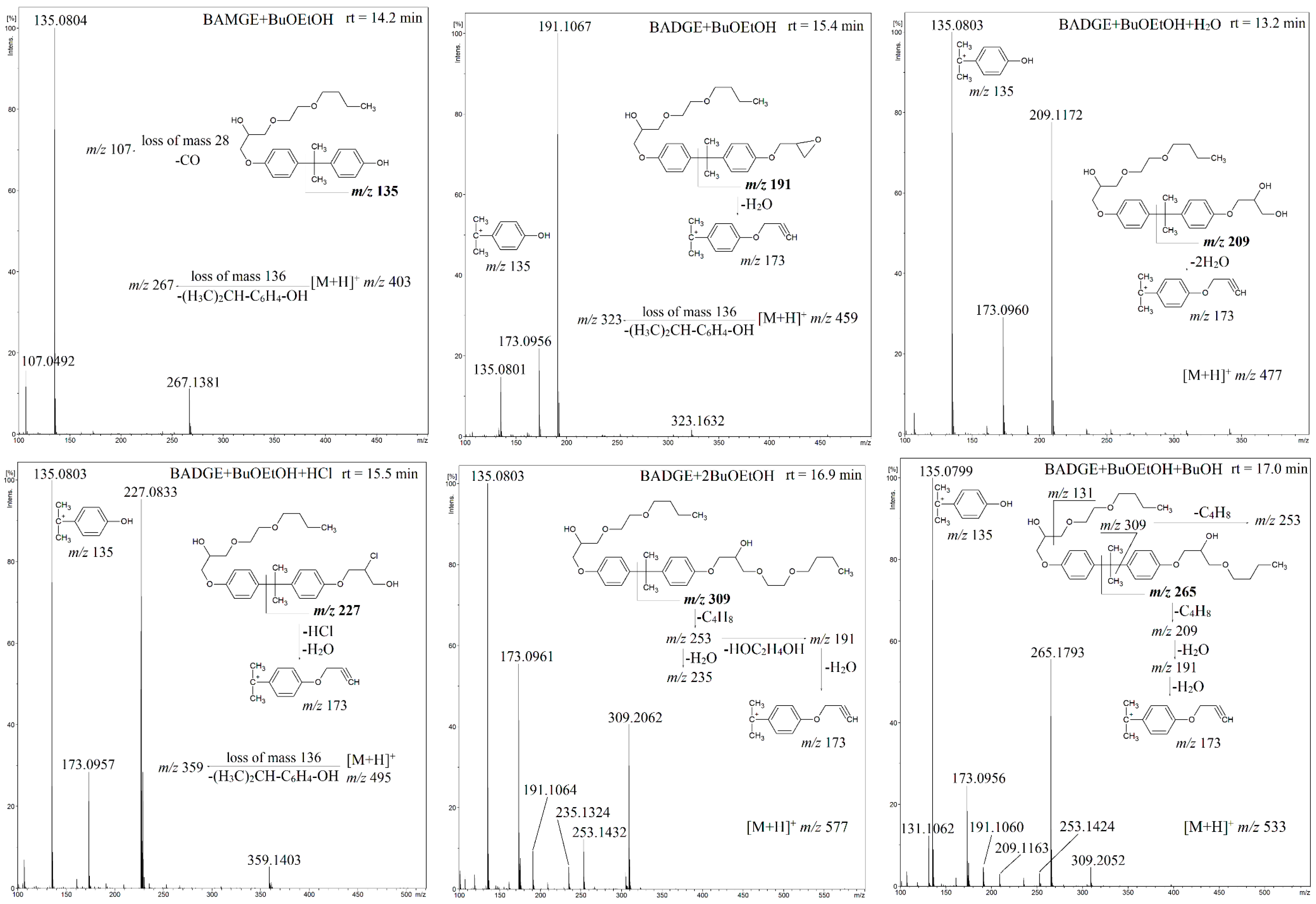
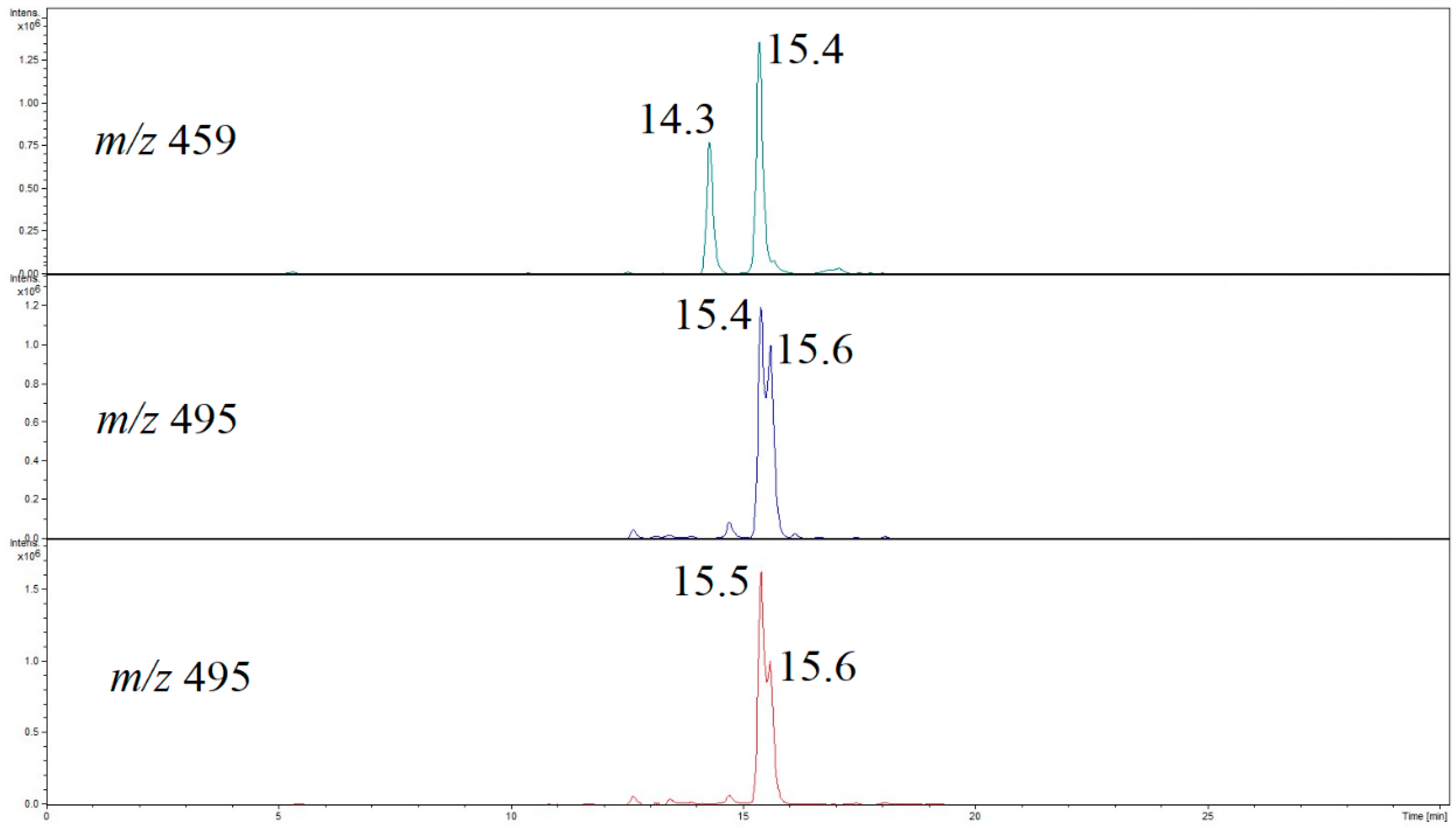
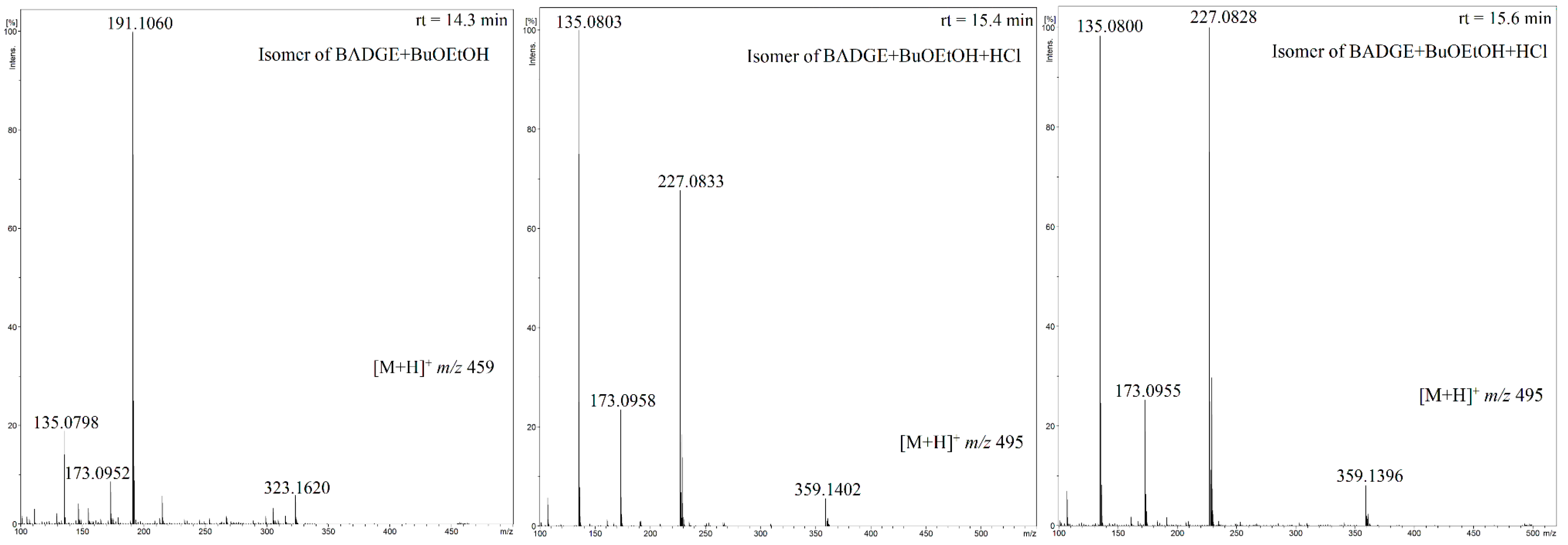
| Compound | Composition of [M + H]+ | Exact Mass | Measured Mass | Error (ppm) |
|---|---|---|---|---|
| BAMGE + BuOEtOH | C24H35O5 | 403.2479 | 403.2470 | −2.2 |
| BADGE + BuOEtOH | C27H39O6 | 459.2742 | 459.2726 | −3.5 |
| BADGE + BuOEtOH + H2O | C27H41O7 | 477.2847 | 477.2840 | −1.6 |
| BADGExBuOEtOH + HCl | C27H40O6Cl | 495.2508 | 495.2499 | −1.8 |
| BADGE + BuOEtOH + BuOH | C31H49O7 | 533.3473 | 533.3464 | −1.7 |
| BADGE + 2BuOEtOH | C33H53O8 | 577.3735 | 577.3725 | −1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beszterda-Buszczak, M.; Kasperkowiak, M.; Teżyk, A.; Augustynowicz, N.; Frański, R. Mass Spectrometric Study of the Most Common Potential Migrants Extractible from the Inner Coatings of Metallic Beverage Cans. Foods 2024, 13, 2025. https://doi.org/10.3390/foods13132025
Beszterda-Buszczak M, Kasperkowiak M, Teżyk A, Augustynowicz N, Frański R. Mass Spectrometric Study of the Most Common Potential Migrants Extractible from the Inner Coatings of Metallic Beverage Cans. Foods. 2024; 13(13):2025. https://doi.org/10.3390/foods13132025
Chicago/Turabian StyleBeszterda-Buszczak, Monika, Małgorzata Kasperkowiak, Artur Teżyk, Natalia Augustynowicz, and Rafał Frański. 2024. "Mass Spectrometric Study of the Most Common Potential Migrants Extractible from the Inner Coatings of Metallic Beverage Cans" Foods 13, no. 13: 2025. https://doi.org/10.3390/foods13132025
APA StyleBeszterda-Buszczak, M., Kasperkowiak, M., Teżyk, A., Augustynowicz, N., & Frański, R. (2024). Mass Spectrometric Study of the Most Common Potential Migrants Extractible from the Inner Coatings of Metallic Beverage Cans. Foods, 13(13), 2025. https://doi.org/10.3390/foods13132025







