Effects of Inoculating Autochthonous Starter Cultures on Changes of N-Nitrosamines and Their Precursors in Chinese Traditional Fermented Fish during In Vitro Human Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Starter Cultures
2.2. CTFPs Preparation
2.3. In Vitro Human Digestion System
2.4. Microbiological Analysis
2.5. BAs Determination
2.6. Nitrite Determination
2.7. NAs (N-Nitrosamines) Determination
2.8. Statistical Analysis
3. Results and Discussion
3.1. Changes in Microorganisms
3.2. Changes in BAs
3.3. Changes in Nitrite Contents
3.4. Changes in NAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hidajat, M.; Mcelvenny, D.; Ritchie, P.; Darnton, A.; Mueller, W.; Agius, R.M. Lifetime cumulative exposure to rubber dust, fumes and N-nitrosamines and non-cancer mortality: A 49-year follow-up of UK rubber factory workers. Occup. Environ. Med. 2020, 77, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, W. Biogenic amines and volatile N-nitrosamines in Chinese smoked-cured bacon (Larou) from industrial and artisanal origins. Food Addit. Contam. B 2023, 16, 143–160. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M. Risk assessment of N-nitrosamines in food. EFSA J. 2023, 21, e07884. [Google Scholar] [PubMed]
- Lu, S.; Jiang, C.; Xu, X.; Xu, C.J.; Li, K.; Shu, R.H. Improved screening procedure for biogenic amine production by lactic acid bacteria and Enterobacteria. Czech J. Food Sci. 2015, 33, 19–26. [Google Scholar]
- Lee, H. Literature compilation of volatile N-nitrosamines in processed meat and poultry products-an update. Food Addit. Contam. B 2019, 36, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Caplice, E.; Fitzgerald, G. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Essid, I.; Hassouna, M. Effect of inoculation of selected Staphylococcus xylosus and Lactobacillus plantarum strains on biochemical, microbiological and textural characteristics of a Tunisian dry fermented sausage. Food Control 2013, 32, 707–714. [Google Scholar] [CrossRef]
- Petrova, I.; Aasen, I.; Rustad, T.; Eikevik, T. Manufacture of dry-cured ham: A review. Part 1. Biochemical changes during the technological process. Eur. Food Res. Technol. 2015, 241, 587–599. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Yang, F.; Jiang, Q. Changes of biogenic amines in Chinese low-salt fermented fish pieces (Suan yu) inoculated with mixed starter cultures. Int. J. Food Sci. Technol. 2013, 48, 685–692. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J. Quality, functionality, and microbiology of fermented fish: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1228–1242. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, L.; Chen, J.; Wang, H.; Liao, E. Nitrite, biogenic amines and volatile N-nitrosamines in commercial Chinese traditional fermented fish products. Food Addit. Contam. B 2022, 15, 10–19. [Google Scholar] [CrossRef]
- Sun, F.; Kong, B.; Chen, Q.; Han, Q.; Diao, X. N-nitrosoamine inhibition and quality preservation of Harbin dry sausages by inoculated with Lactobacillus pentosus, Lactobacillus curvatus and Lactobacillus sake. Food Control 2017, 73, 1514–1521. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, M.; Zhang, Z.; Huang, P.; Xu, B.; Chen, C. N-nitrosodimethylamine reduction by Lactobacillus pentosus R3 in fermented cooked sausages. Food Control 2021, 124, 107869. [Google Scholar] [CrossRef]
- Fan, T.; Sun, G.; Zhao, L.; Cui, X.; Zhong, R. QSAR and classification study on prediction of acute oral toxicity of N-nitroso compounds. Int. J. Mol. Sci. 2018, 19, 3015. [Google Scholar] [CrossRef]
- Hecht, S. N-nitroso compounds and man: Sources of exposure, endogenous formation and occurrence in body fluids. Eur. J. Cancer Prev. Eur. J. Cancer Prev. 1997, 6, 226–268. [Google Scholar]
- Mirvish, S. The etiology of gastric cancer: Intragastric N-nitrosamide formation and other theories. J. Natl. Cancer Inst. 1983, 71, 629–647. [Google Scholar] [PubMed]
- Kim, S.; Kim, S.; Kang, K.; Lee, S.; Kim, S.; Kim, J. Kimchi probiotic bacteria contribute to reduced amounts of N-nitrosodimethylamine in lactic acid bacteria-fortified kimchi. LWT-Food Sci. Technol. 2017, 84, 196–203. [Google Scholar] [CrossRef]
- Kim, H.; Hur, S. Changes in the mutagenicity of heterocyclic amines, nitrite, and N-nitroso compound in pork patties during in vitro human digestion. LWT-Food Sci. Technol. 2018, 92, 47–53. [Google Scholar] [CrossRef]
- Niklas, A.; Borge, G.; Rodbotten, R.; Berget, I.; Muller, M.; Herrmann, S. Levels of nitrate, nitrite and nitrosamines in model sausages during heat treatment and in vitro digestion-The impact of adding nitrite and spinach (Spinacia oleracea L.). Food Res. Int. 2023, 166, 112595. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Jiang, Q.; Yang, F. Effect of autochthonous starter cultures on microbiological and physico-chemical characteristics of Suan yu, a traditional Chinese low salt fermented fish. Food Control 2013, 33, 344–351. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.; Yu, D.; Yang, F. Correlations between microbiota succession and flavor formation during fermentation of Chinese low-salt fermented common carp (Cyprinus carpio L.) inoculated with mixed starter cultures. Food Microbiol. 2020, 90, 103487. [Google Scholar] [CrossRef] [PubMed]
- Liao, E.; Xu, Y.; Jiang, Q.; Xia, W. Effects of inoculating autochthonous starter cultures on biogenic amines accumulation of Chinese traditional fermented fish. J. Food Process Preserv. 2018, 42, e13694. [Google Scholar] [CrossRef]
- Kim, H.; Hur, S. Effect of in vitro human digestion on biogenic amine (tyramine) formation in various fermented sausages. J. Food Prot. 2018, 81, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Capasso, G.; Rando, A.; Perna, A. Antioxidant activity of beef, pork and chicken burgers before and after cooking and after in vitro intestinal digestion. Foods 2023, 12, 4100. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, Y.; Chen, J.; Xia, W.; Liao, E.; Wang, H. Effects of superchilling on quality of crayfish (Procambarus clarkii): Water migration, biogenic amines accumulation, and nucleotides catabolism. Int. J. Food Sci. Technol. 2022, 57, 506–515. [Google Scholar] [CrossRef]
- Ito, Y.; Yodoshi, M.; Tanaka, J.; Iwaida, M. Comparison of two methods and improvements for colorimetric determination of nitrite in cod roe. J. Food Prot. 1979, 42, 715–718. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Cui, Y.; Wang, L.; Duan, J.; Yang, X.; Gao, X. Characteristics of lactic acid bacteria as potential probiotic starters and their effects on the quality of fermented sausages. Foods 2024, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Adamberg, K.; Kask, S.; Laht, T.; Paalme, T. The effect of temperature and pH on the growth of lactic acid bacteria: A pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Aristoy, M.; Cavella, S.; Di Monaco, R.; Ercolini, D.; Toldrá, F.; Villani, F. Biochemical and sensory characteristics of traditional fermented sausages of Vallo di Diano (Southern Italy) as affected by the use of starter cultures. Meat Sci. 2007, 76, 295–307. [Google Scholar] [CrossRef]
- Chrun, R.; Born, P.; Huon, T.; Buntong, B.; Chay, C.; Inatsu, Y. Application of lactic acid bacteria for enhanced food safety of Cambodian fermented small fish (Pha-ork kontrey). Food Sci. Technol. Res. 2020, 26, 687–694. [Google Scholar] [CrossRef]
- Turna, N.; Chung, R.; McIntyre, L. A review of biogenic amines in fermented foods: Occurrence and health effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Sivamaruthi, B.; Kesika, P.; Chaiyasut, C. A narrative review on biogenic amines in fermented fish and meat products. J. Food Sci. Technol.-Mysore 2021, 58, 1623–1639. [Google Scholar] [CrossRef]
- Liao, E.; Xu, Y.; Jiang, Q.; Xia, W. Characterisation of dominant autochthonous strains for nitrite degradation of Chinese traditional fermented fish. Int. J. Food Sci. Technol. 2018, 53, 2633–2641. [Google Scholar] [CrossRef]
- Williams, D. Nitrosation Mechanisms. Adv. Phys. Org. Chem. 1983, 19, 381–428. [Google Scholar]
- Nakamura, M.; Katoh, K.; Kawabata, T. Precursors to nitrosopyrrolidine and nitrosopiperidine in black pepper treated with nitrous acid. Agric. Biol. Chem. 1981, 45, 1257–1259. [Google Scholar]
- Warthesen, J.; Scanlan, R.; Bills, D.; Libbey, L. Formation of heterocyclic N-nitrosamines from the reaction of nitrite and selected primary diamines and amino acids. Commun. Nonlinear Sci. 1975, 20, 516–535. [Google Scholar] [CrossRef]
- Bills, D.; Hildrum, K.; Scanlan, R.; Libbey, L. Potential precursors of N-nitrosopyrrolidine in bacon and other fried foods. J. Agric. Food Chem. 1973, 21, 876–877. [Google Scholar] [CrossRef]
- Domanska, K.; Kowalski, B. Occurrence of volatile N-nitrosamines in Polish processed meat products. Bull.-Vet. Inst. Pulawy 2003, 47, 507–514. [Google Scholar]
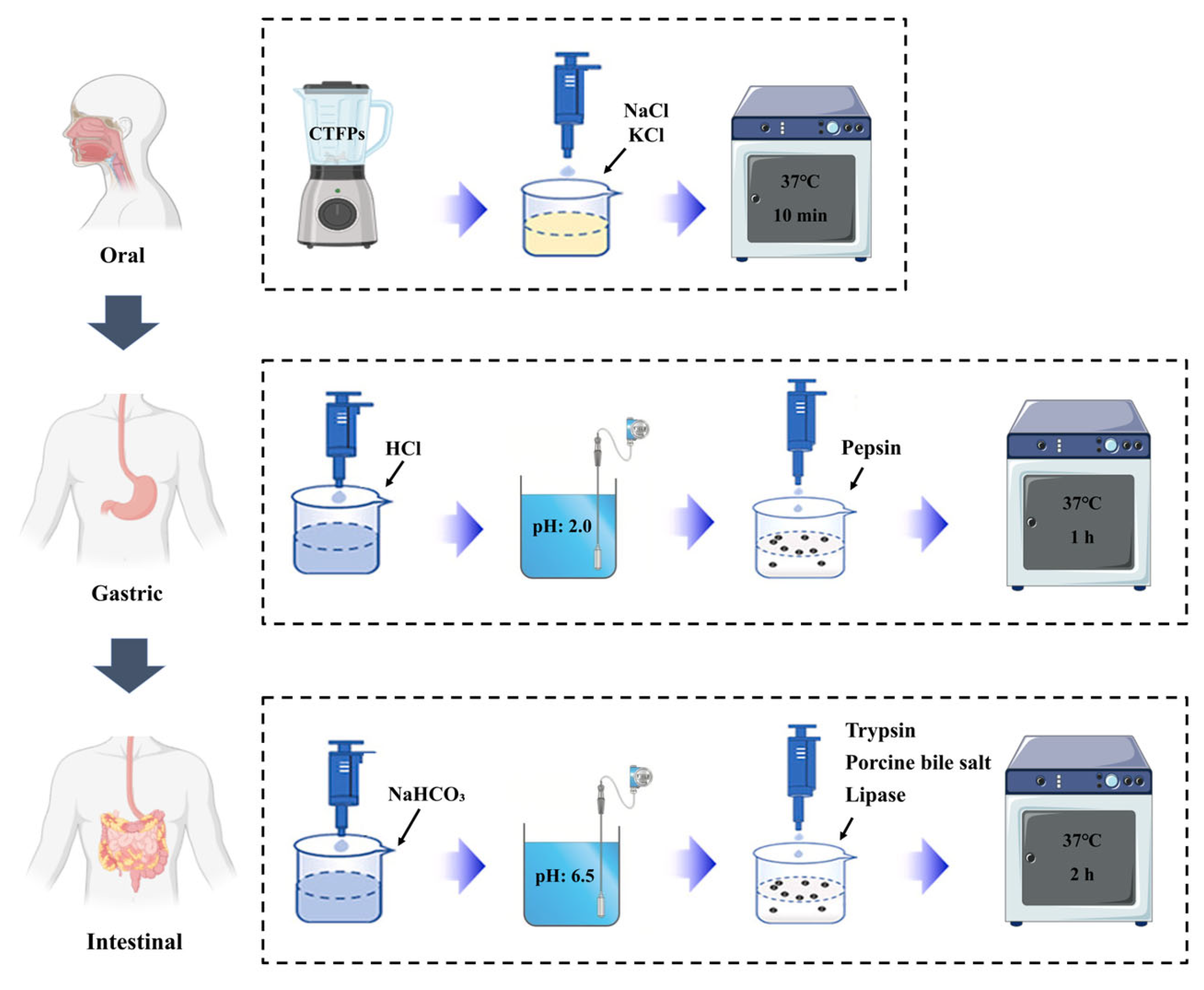



| Saliva (Mouth Step) | Gastric Juice (Stomach Step) | Duodenal Juice (Small Intestine Step) | |
|---|---|---|---|
| Organic and inorganic components | 10 mL salt solution (140 mmol/L NaCl and 5 mol/L KCl) | 5 mL 0.1 mol/L HCl | 37.5 mL 0.1 mol/L NaHCO3 |
| Enzymes | — | 200 mg pepsin | 75 mg pancreatin 450 mg porcine bile salt 25 mg lipase |
| pH | 6.8 ± 0.2 | 2 ± 0.02 | 6.5 ± 0.2 |
| Conditions | 37 °C/200 rmp/10 min | 37 °C/200 rmp/1 h | 37 °C/200 rmp/2 h |
| Digestion Steps | Groups | NDMA | NMEA | NPIP | NPYR | NDPheA | NMOR |
|---|---|---|---|---|---|---|---|
| Before digestion | NS | 3.15 ± 0.16 Da | ND | 0.49 ± 0.03 Aa | 1.15 ± 0.06 Da | 0.06 ± 0.01 Ba | 0.32 ± 0.02 Aa |
| Lp-120 | 1.54 ± 0.08 Ba | ND | 0.59 ± 0.05 Ca | 0.74 ± 0.08 Ca | 0.05 ± 0.01 Ba | 0.34 ± 0.03 Aa | |
| Sc-2018 | 2.32 ± 0.07 Ca | ND | 0.45 ± 0.02 Aa | 1.21 ± 0.05 Da | ND | 0.32 ± 0.04 Aab | |
| Sx-135 | 3.07 ± 0.03 Da | ND | 0.56 ± 0.03 BCa | 0.57 ± 0.03 Ba | 0.02 ± 0.01 Aa | 0.37 ± 0.03 ABa | |
| MS | 1.21 ± 0.02 Ab | ND | 0.51 ± 0.02 ABa | 0.29 ± 0.04 Aa | ND | 0.41 ± 0.02 Ba | |
| Mouth | NS | 3.21 ± 0.05 Ea | ND | 0.51 ± 0.02 Aa | 1.21 ± 0.07 Da | 0.06 ± 0.01 Ba | 0.31 ± 0.03 Aa |
| Lp-120 | 1.45 ± 0.06 Ba | ND | 0.61 ± 0.03 Ba | 0.81 ± 0.02 Cab | 0.05 ± 0.02 Ba | 0.40 ± 0.06 Bab | |
| Sc-2018 | 2.21 ± 0.06 Ca | ND | 0.49 ± 0.03 Aa | 1.26 ± 0.06 Da | ND | 0.30 ± 0.02 Aa | |
| Sx-135 | 2.98 ± 0.07 Da | ND | 0.59 ± 0.04 Ba | 0.58 ± 0.02 Ba | 0.02 ± 0.01 Aa | 0.39 ± 0.02 Bab | |
| MS | 1.05 ± 0.03 Aa | ND | 0.52 ± 0.03 Aa | 0.35 ± 0.03 Aa | ND | 0.35 ± 0.03 ABa | |
| Stomach | NS | 5.46 ± 0.21 Eb | ND | 0.85 ± 0.02 Ab | 1.29 ± 0.11 Da | 0.07 ± 0.02 Ba | 0.36 ± 0.02 Aab |
| Lp-120 | 2.24 ± 0.11 Bb | ND | 1.02 ± 0.06 Bb | 0.90 ± 0.11 Cb | 0.06 ± 0.01 Ba | 0.41 ± 0.03 ABab | |
| Sc-2018 | 3.08 ± 0.03 Cc | ND | 0.82 ± 0.04 Ab | 1.18 ± 0.06 Da | ND | 0.38 ± 0.03 ABb | |
| Sx-135 | 5.14 ± 0.17 Db | ND | 1.14 ± 0.10 Cb | 0.54 ± 0.07 Ba | 0.03 ± 0.02 Aa | 0.44 ± 0.05 Bb | |
| MS | 1.40 ± 0.03 Ac | ND | 0.9 ± 0.03 Ab | 0.34 ± 0.03 Aa | ND | 0.39 ± 0.03 ABa | |
| Small intestine | NS | 5.39 ± 0.03 Eb | ND | 0.87 ± 0.03 Bb | 1.19 ± 0.03 Da | 0.06 ± 0.01 Ba | 0.39 ± 0.03 ABb |
| Lp-120 | 2.12 ± 0.02 Bb | ND | 1.05 ± 0.02 Db | 0.85 ± 0.02 Cab | 0.05 ± 0.02 Ba | 0.42 ± 0.02 Bb | |
| Sc-2018 | 2.93 ± 0.05 Cb | ND | 0.80 ± 0.05 Ab | 1.23 ± 0.05 Da | ND | 0.39 ± 0.05 ABb | |
| Sx-135 | 5.09 ± 0.02 Db | ND | 1.12 ± 0.02 Eb | 0.59 ± 0.02 Ba | 0.02 ± 0.02 Aa | 0.41 ± 0.02 ABab | |
| MS | 1.35 ± 0.04 Ac | ND | 0.94 ± 0.04 Cb | 0.35 ± 0.04 Aa | ND | 0.35 ± 0.04 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, Q.; Wang, Q.; Chen, J.; Xia, W.; Liao, E. Effects of Inoculating Autochthonous Starter Cultures on Changes of N-Nitrosamines and Their Precursors in Chinese Traditional Fermented Fish during In Vitro Human Digestion. Foods 2024, 13, 2021. https://doi.org/10.3390/foods13132021
Li H, Li Q, Wang Q, Chen J, Xia W, Liao E. Effects of Inoculating Autochthonous Starter Cultures on Changes of N-Nitrosamines and Their Precursors in Chinese Traditional Fermented Fish during In Vitro Human Digestion. Foods. 2024; 13(13):2021. https://doi.org/10.3390/foods13132021
Chicago/Turabian StyleLi, Han, Qian Li, Qi Wang, Jiwang Chen, Wenshui Xia, and E Liao. 2024. "Effects of Inoculating Autochthonous Starter Cultures on Changes of N-Nitrosamines and Their Precursors in Chinese Traditional Fermented Fish during In Vitro Human Digestion" Foods 13, no. 13: 2021. https://doi.org/10.3390/foods13132021
APA StyleLi, H., Li, Q., Wang, Q., Chen, J., Xia, W., & Liao, E. (2024). Effects of Inoculating Autochthonous Starter Cultures on Changes of N-Nitrosamines and Their Precursors in Chinese Traditional Fermented Fish during In Vitro Human Digestion. Foods, 13(13), 2021. https://doi.org/10.3390/foods13132021






