The Effect of Heat Stress on Sensory Properties of Fresh Oysters: A Comprehensive Study Using E-Nose, E-Tongue, Sensory Evaluation, HS–SPME–GC–MS, LC–MS, and Transcriptomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Heat Stress Treatment and Sample Collection
2.2. Analysis of Electronic Nose, Electronic Tongue, Sensory Evaluation and Proximate Composition
2.3. Metabolite Analysis by LC–MS
2.4. Volatile Metabolite Analysis by HS–SPME–GC–MS
2.5. Library Construction and RNA–Seq Analysis
2.6. DHE Staining and Analysis of Antioxidant Indexes
2.7. Statistical Analysis
3. Results and Discussion
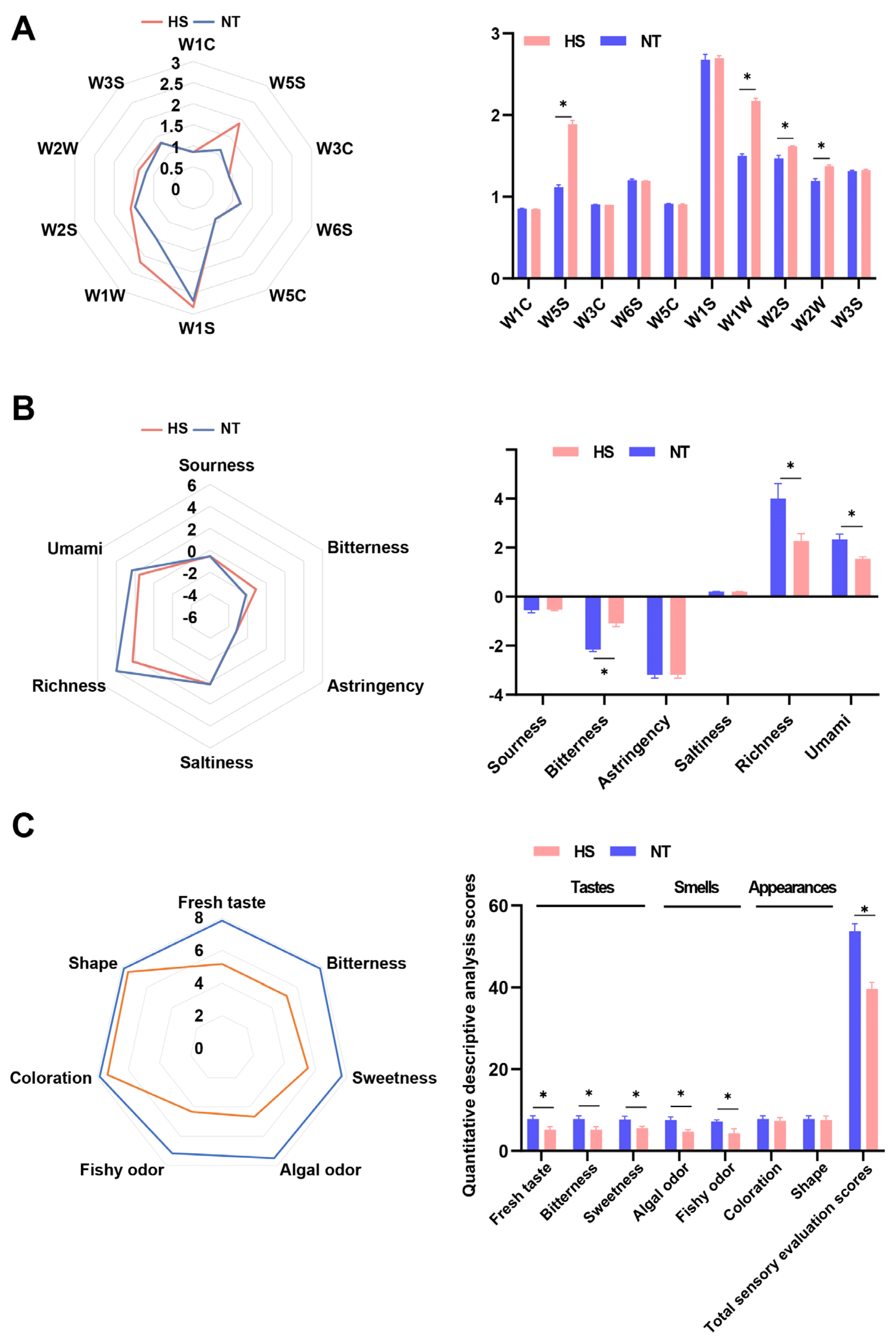
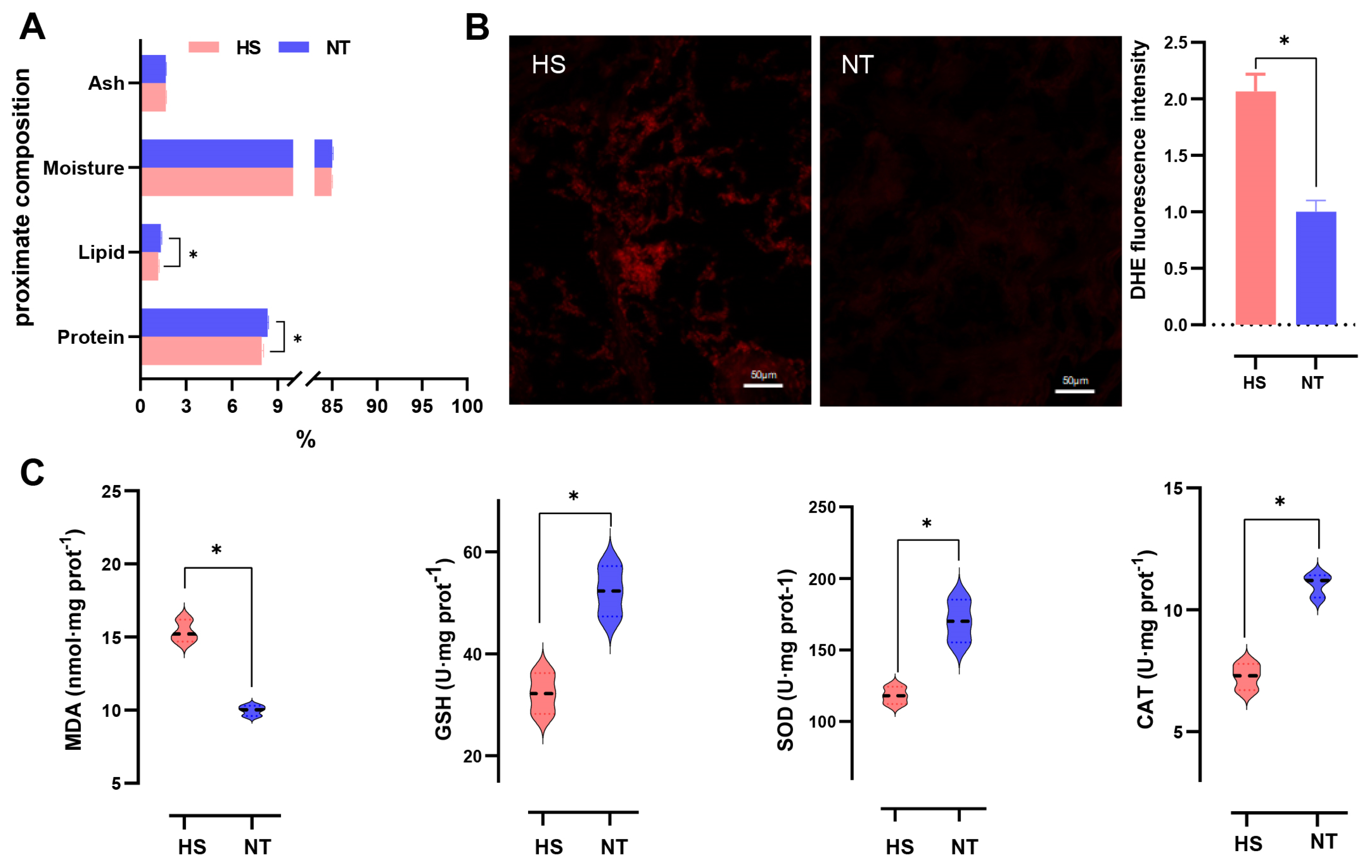
3.1. The Effect of Chronic Heat Stress on Sensory Properties and Proximate Composition of Oyster C. ariakensis
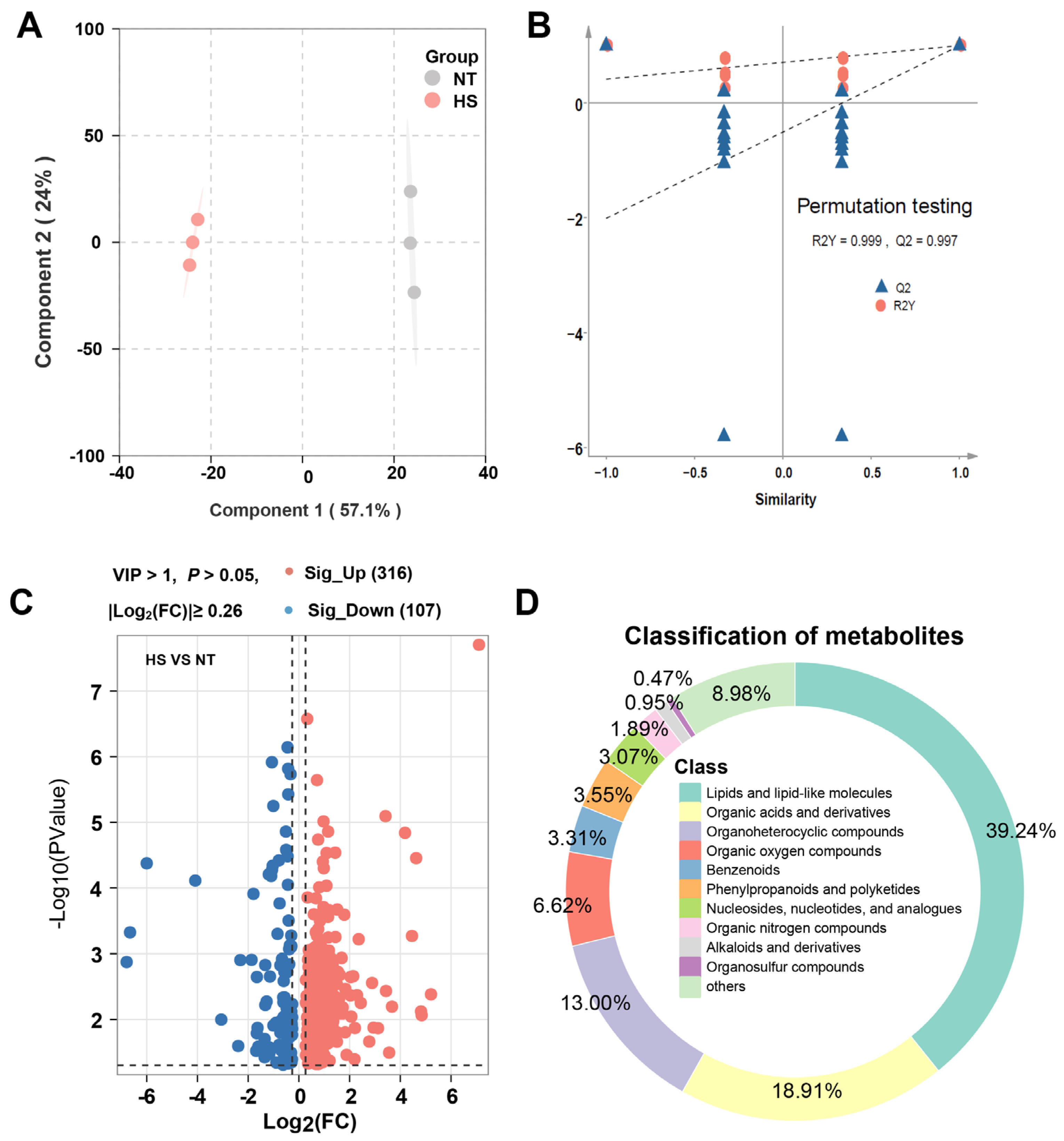
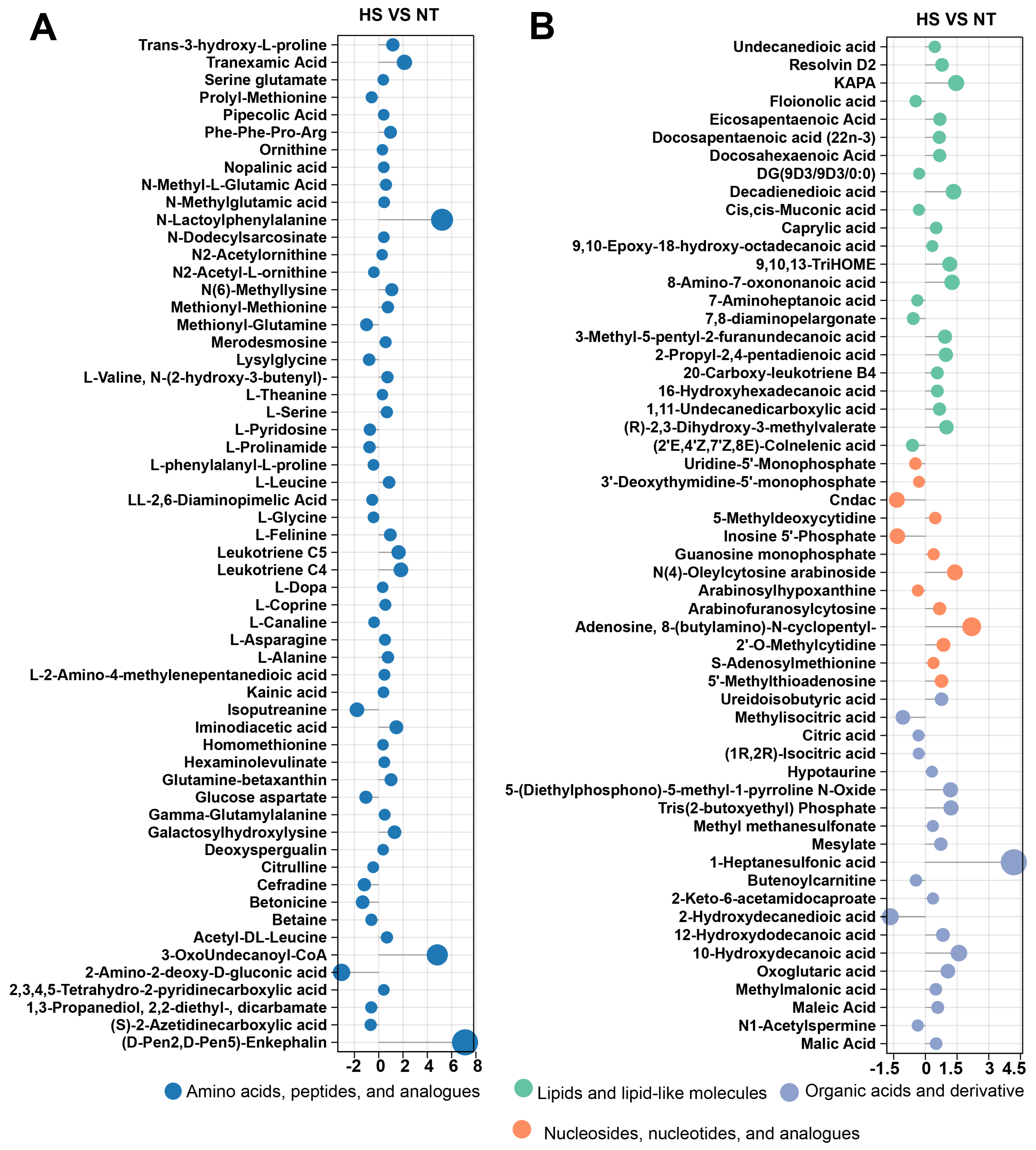
3.2. The Effect of Chronic Heat Stress on Non-Volatile Metabolites of Oyster C. ariakensis
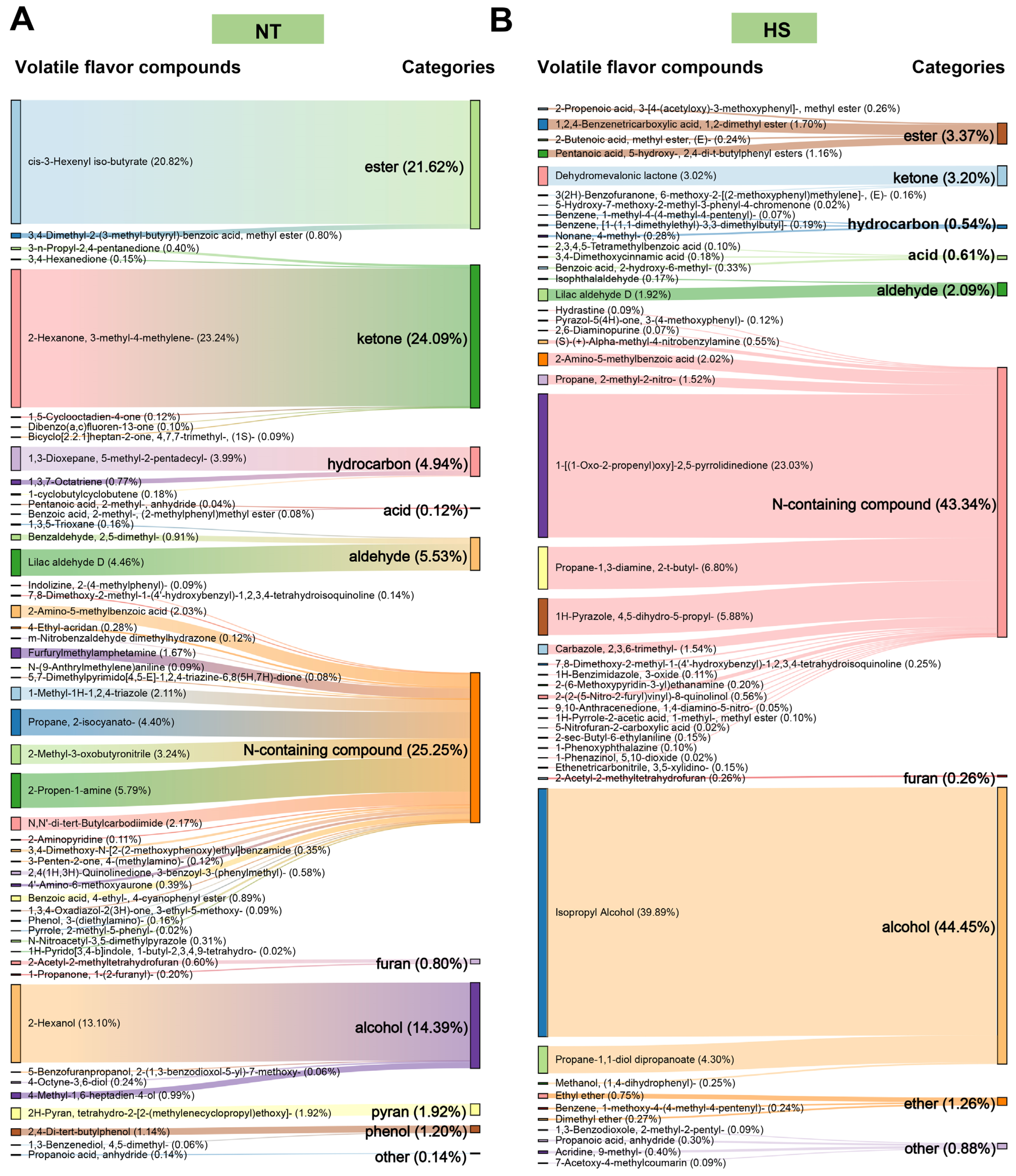
3.3. The Effect of Chronic Heat Stress on Volatile Compounds of Oyster C. ariakensis
3.4. Correlation Analysis between Differential Metabolites and Sensory Properties
3.5. The Effect of Heat Stress on Metabolic Pathways in Oyster C. ariakensis
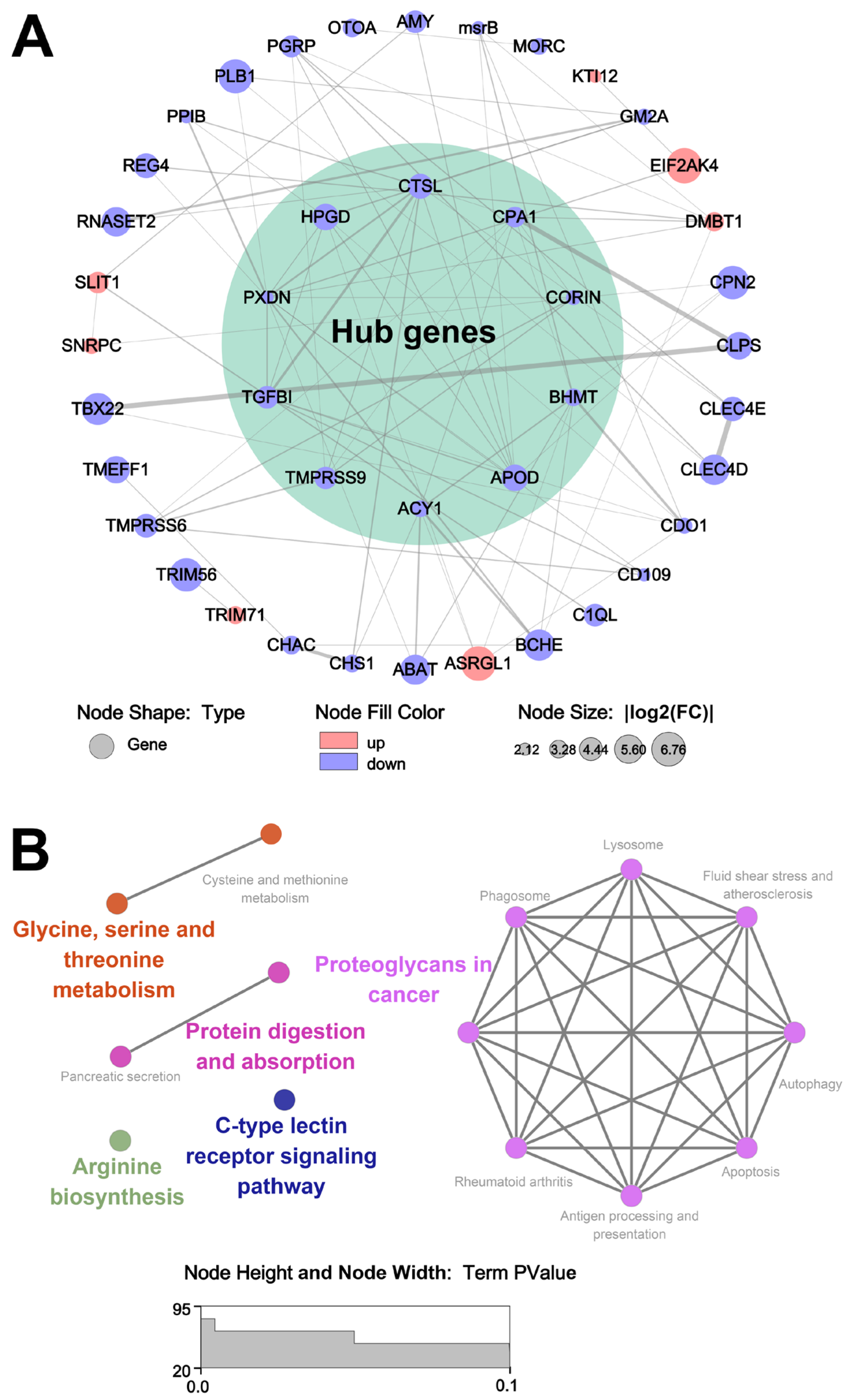
3.6. The Analysis of Key Genes Affecting Sensory Properties
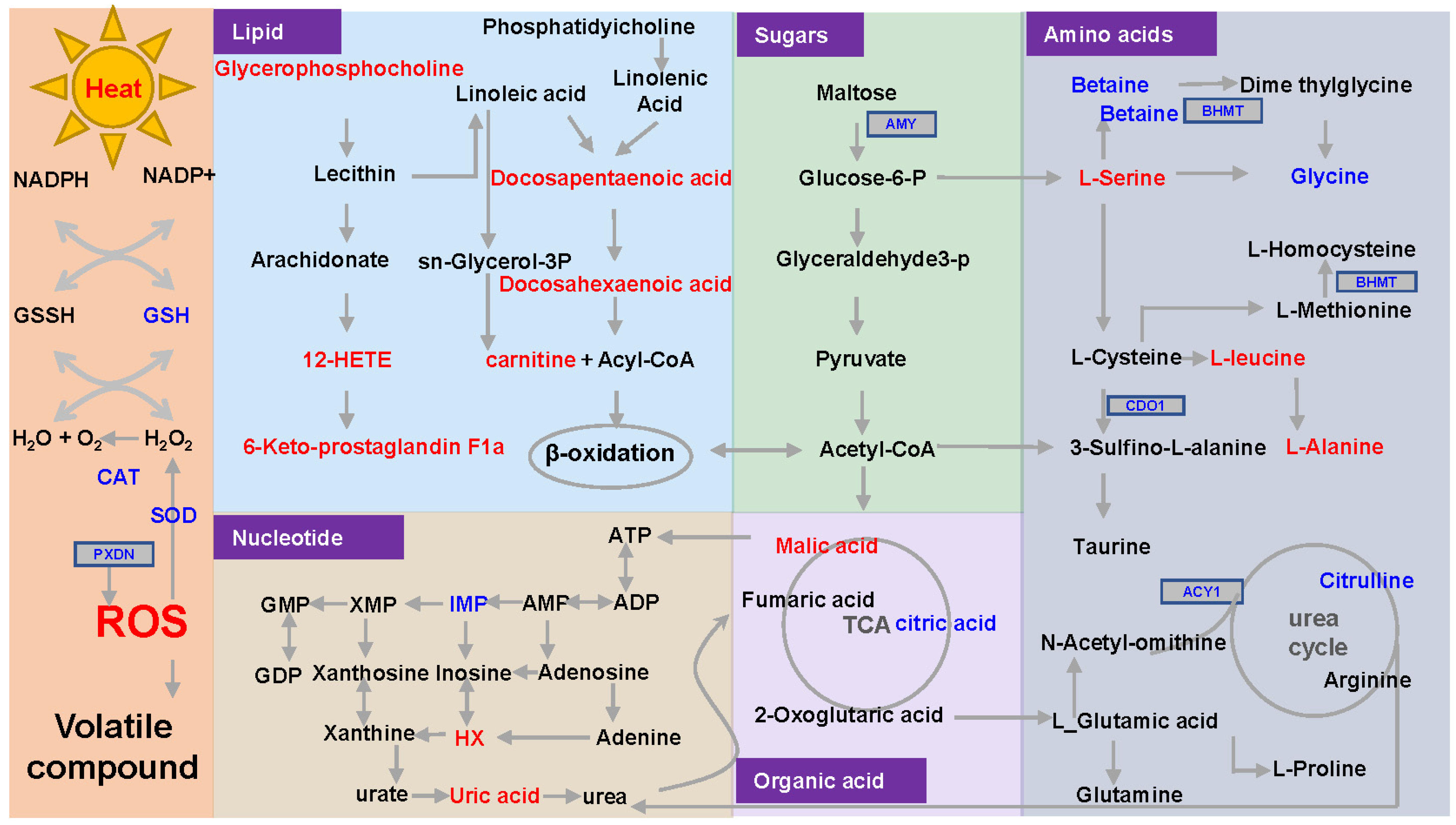
3.7. Proposed Formation Pathways for Sensory Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Retrieved from Rome. 2020. Available online: https://scholar.google.com/scholar_lookup?title=World%20fisheries%20and%20aquaculture&publication_year=2020&author=FAO (accessed on 20 February 2024).
- Shalders, T.C.; Champion, C.; Coleman, M.A.; Benkendorff, K. The nutritional and sensory quality of seafood in a changing climate. Mar. Environ. Res. 2022, 176, 105590. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, A.J.; Hall-Spencer, J.M.; Kuri, V.; Knights, A.M. Changes in the biochemical and nutrient composition of seafood due to ocean acidification and warming. Mar. Environ. Res. 2019, 143, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, B.; Zhang, J.; Wang, G.; Gong, W.; Tian, J.; Li, H.; Zhang, K.; Xia, Y.; Li, Z.; et al. Effects of heat stress on the chemical composition, oxidative stability, muscle metabolism, and meat quality of Nile Tilapia (Oreochromis Niloticus). Food Chem. 2023, 426, 136590. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, X.; Tao, N.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese Mitten Crab (Eriocheir Sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, H.; Yan, X. Metabolomic analysis provides new insights into the heat-hardening response of Manila Clam (Ruditapes Philippinarum) to high temperature stress. Sci. Total Environ. 2023, 857, 159430. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gu, Z.; Lin, X.; Wang, Y.; Wang, A.; Sun, Y.; Shi, Y. Effects of high hydrostatic pressure (HHP) and storage temperature on bacterial counts, color change, fatty acids and non-volatile taste active compounds of oysters (Crassostrea Ariakensis). Food Chem. 2022, 372, 131247. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, X.; Liu, Y.; Shi, H.; Li, Z.; Xue, C. The dynamic change of flavor characteristics in Pacific Oyster (Crassostrea Gigas) during depuration uncovered by mass spectrometry-based metabolomics combined with gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2024, 434, 137277. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Guangxi Zhuang Autonomous Region Investment Promotion Bureau website Guangxi, China: Modern Agriculture. Available online: http://tzcjj.gxzf.gov.cn/tzzn/gxgkzrjjqwd/t16669936.shtml (accessed on 5 June 2024).
- Kelly, C.J.; Laramore, S.E.; Scarpa, J.; Newell, R.I.E. Seasonal comparison of physiological adaptation and growth of Suminoe (Crassostrea Ariakensis) and Eastern (Crassostrea Ariakensis) oysters. J. Shellfish Res. 2011, 30, 737–749. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rahman, M.S. Elevated seasonal temperature disrupts prooxidant-antioxidant homeostasis and promotes cellular apoptosis in the American Oyster, Crassostrea Virginica, in the Gulf of Mexico: A Field Study. Cell Stress Chaperon 2021, 26, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Gao, L.; Liu, R.; Yang, Q.; Li, Q.; Wang, L.; Song, L. The oxidative stress of the Pacific Oyster Crassostrea Gigas under high-temperature stress. Aquaculture 2023, 577, 739998. [Google Scholar] [CrossRef]
- IPCC. The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Fu, B.; Zheng, M.; Yang, H.; Zhang, J.; Li, Y.; Wang, G.; Tian, J.; Zhang, K.; Xia, Y.; Li, Z.; et al. The effect of broad bean diet on structure, flavor and taste of fresh grass Carp: A comprehensive study using E-Nose, E-Tongue, TPA, HS-SPME-GC-MS and LC-MS. Food Chem. 2024, 436, 137690. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Liu, Y.; Chen, L.; Xue, C.; Li, Z. Effects of liquid nitrogen freezing at different temperatures on the quality and flavor of Pacific Oyster (Crassostrea Gigas). Food Chem. 2023, 422, 136162. [Google Scholar] [CrossRef] [PubMed]
- Podduturi, R.; da Silva David, G.; da Silva Reinaldo, J.; Hyldig, G.; Jørgensen, N.O.G.; Agerlin Petersen, M. Characterization and finding the origin of off-flavor compounds in Nile Tilapia cultured in net cages in hydroelectric reservoirs, São Paulo State, Brazil. Food Res Int 2023, 173, 113375. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Wang, Q.; Sun, C.; Yu, F.; Chen, L.; Sun, Z.; Shi, H.; Xue, C.; Li, Z. Temperature effects on the nutritional quality in Pacific Oysters (Crassostrea Gigas) during ultraviolet depuration. J. Sci. Food Agr. 2022, 102, 1651–1659. [Google Scholar] [CrossRef]
- Subasinghe, M.M.; Jinadasa, B.K.K.K.; Navarathne, A.N.; Jayakody, S. Variations in proximate composition and percentage edibility of wild and cultured Indian backwater oyster (Crassostrea Madrasensis) in Sri Lanka. Aquac. Res. 2021, 52, 3949–3957. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one fastq preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrus Medica L. Var. Sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Tate, R.D.; Benkendorff, K.; Ab Lah, R.; Kelaher, B.P. Ocean acidification and warming impacts the nutritional properties of the Predatory Whelk, Dicathais Orbita. J. Exp. Mar. Biol. Ecol. 2017, 493, 7–13. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Flavor compounds of traditional fermented bean condiments: Classes, synthesis, and factors involved in flavor formation. Trends Food Sci. Tech. 2023, 133, 160–175. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, X.; Wei, S.; Li, S. Multi-omics revealed the formation mechanism of flavor in salted egg yolk induced by the stages of lipid oxidation during salting. Food Chem. 2023, 398, 133794. [Google Scholar] [CrossRef]
- Cheng, S.; He, Y.; Zeng, T.; Wang, D.; He, J.; Xia, Q.; Zhou, C.; Pan, D.; Cao, J. Heat stress induces various oxidative damages to myofibrillar proteins in ducks. Food Chem. 2022, 390, 133209. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xia, C.; He, Y.; Pan, D.; Cao, J.; Sun, Y.; Zeng, X. Proteomic responses to oxidative damage in meat from ducks exposed to heat stress. Food Chem. 2019, 295, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.C.; Bremner, H.A.; Olley, J.; Statham, J.A. Umami revisited: The relationship between inosine monophosphate, hypoxanthine and smiley scales for fish flavor. Food Rev. Int. 1990, 6, 489–503. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Sakaguchi, M.; Kawai, F.; Kanamori, M. Changes in concentration of ATP-related compounds in various tissues of oyster during ice storage. Nippon Suisan Gakk 1992, 58, 2125–2136. [Google Scholar] [CrossRef]
- Liu, C.; Meng, F.; Tang, X.; Shi, Y.; Wang, A.; Gu, Z.; Pan, Z. Comparison of nonvolatile taste active compounds of wild and cultured Mud Crab Scylla Paramamosain. Fish. Sci. 2018, 84, 897–907. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Cao, W.; Zhou, L.; Zhang, C.; Qin, X.; Zheng, H.; Lin, H.; Gao, J. Novel insight into the role of processing stages in nutritional components changes and characteristic flavors formation of Noble Scallop Chlamys Nobilis Adductors. Food Chem. 2022, 378, 132049. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J.; Franco, D. Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry-cured ham from Celta pig breed. J. Integr. Agr. 2013, 12, 2002–2012. [Google Scholar] [CrossRef]
- Ning, M.; Rui, G.; Rentang, Z.; Yue, G. GC-IMS-based preliminary analysis of volatile flavor compounds in Ejiao at different processing stag. J. Food Qual. 2022, 2022, 3961593. [Google Scholar] [CrossRef]
- Cengiz, N.; Guclu, G.; Kelebek, H.; Selli, S. GC–MS-olfactometric characterization of key odorants in rainbow trout by the application of aroma extract dilution analysis: Understanding locational and seasonal effects. Food Chem. 2023, 407, 135137. [Google Scholar] [CrossRef] [PubMed]
- Sérot, T.; Regost, C.; Prost, C.; Robin, J.; Arzel, J. Effect of dietary lipid sources on odour-active compounds in muscle of Turbot (Psetta Maxima). J. Sci. Food Agr. 2001, 81, 1339–1346. [Google Scholar] [CrossRef]
- Mirata, M.-A.; Wüst, M.; Mosandl, A.; Sell, D.; Schrader, J. Lilac aldehydes and lilac alcohols as metabolic by-products of fungal linalool biotransformation. In Developments in Food Science; Bredie, W.L.P., Petersen, M.A., Eds.; Flavour Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 43, pp. 121–124. [Google Scholar]
- Sarnoski, P.J.; O’Keefe, S.F.; Jahncke, M.L.; Mallikarjunan, P.; Flick, G.J. Analysis of crab meat volatiles as possible spoilage indicators for Blue Crab (Callinectes Sapidus) meat by gas chromatography–mass spectrometry. Food Chem. 2010, 122, 930–935. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; Jurado, A.; Timón, M.L.; García, C. Odor-active compounds of Iberian hams with different aroma characteristics. J. Agric. Food Chem. 2002, 50, 6453–6458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile flavour components and the mechanisms underlying their production in Golden Pompano (Trachinotus Blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Liu, L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE–GC–MS, E-nose and sensory evaluation. Food Chem. 2016, 207, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhang, J.Y.; Liu, S.L.; Wang, Y.; Ding, Y.T. Chemical, Microbiological and sensory changes of dried Acetes Chinensis during accelerated storage. Food Chem. 2011, 127, 159–168. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC × GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Li, S.; Deng, B.; Tian, S.; Guo, M.; Liu, H.; Zhao, X. Metabolic and transcriptomic analyses reveal different metabolite biosynthesis profiles between leaf buds and mature leaves in Ziziphus Jujuba Mill. Food Chem. 2021, 347, 129005. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Shi, R. Mammalian peroxidasin (PXDN): From physiology to pathology. Free. Radical. Bio. Med. 2022, 182, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lourenço dos Santos, S.; Petropoulos, I.; Friguet, B. The oxidized protein repair enzymes methionine sulfoxide reductases and their roles in protecting against oxidative stress, in ageing and in regulating protein function. Antioxidants 2018, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Nava, V.E.; Milstien, S.; Spiegel, S. Aminoacylase 1 is a sphingosine kinase 1-interacting protein. Febs. Lett. 2004, 568, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.W.; Jun, D.S.; Na, J.D.; Choi, Y.J.; Kim, Y.C. Alleviation of hepatic fat accumulation by betaine involves reduction of homocysteine via up-regulation of betaine-homocysteine methyltransferase (BHMT). Biochem. Bioph. Res. Co. 2016, 477, 440–447. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, J.-Y.; Mu, W.-J.; Guo, L. Cysteine dioxygenase type 1 (CDO1): Its functional role in physiological and pathophysiological processes. Genes Dis. 2023, 10, 877–890. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, B.; Fang, C.; Li, Z.; Zeng, Z.; He, Y.; Chen, S.; Yang, H. The Effect of Heat Stress on Sensory Properties of Fresh Oysters: A Comprehensive Study Using E-Nose, E-Tongue, Sensory Evaluation, HS–SPME–GC–MS, LC–MS, and Transcriptomics. Foods 2024, 13, 2004. https://doi.org/10.3390/foods13132004
Fu B, Fang C, Li Z, Zeng Z, He Y, Chen S, Yang H. The Effect of Heat Stress on Sensory Properties of Fresh Oysters: A Comprehensive Study Using E-Nose, E-Tongue, Sensory Evaluation, HS–SPME–GC–MS, LC–MS, and Transcriptomics. Foods. 2024; 13(13):2004. https://doi.org/10.3390/foods13132004
Chicago/Turabian StyleFu, Bing, Chang Fang, Zhongzhi Li, Zeqian Zeng, Yinglin He, Shijun Chen, and Huirong Yang. 2024. "The Effect of Heat Stress on Sensory Properties of Fresh Oysters: A Comprehensive Study Using E-Nose, E-Tongue, Sensory Evaluation, HS–SPME–GC–MS, LC–MS, and Transcriptomics" Foods 13, no. 13: 2004. https://doi.org/10.3390/foods13132004
APA StyleFu, B., Fang, C., Li, Z., Zeng, Z., He, Y., Chen, S., & Yang, H. (2024). The Effect of Heat Stress on Sensory Properties of Fresh Oysters: A Comprehensive Study Using E-Nose, E-Tongue, Sensory Evaluation, HS–SPME–GC–MS, LC–MS, and Transcriptomics. Foods, 13(13), 2004. https://doi.org/10.3390/foods13132004






