Research Progress and Production Status of Edible Insects as Food in China
Abstract
1. Introduction
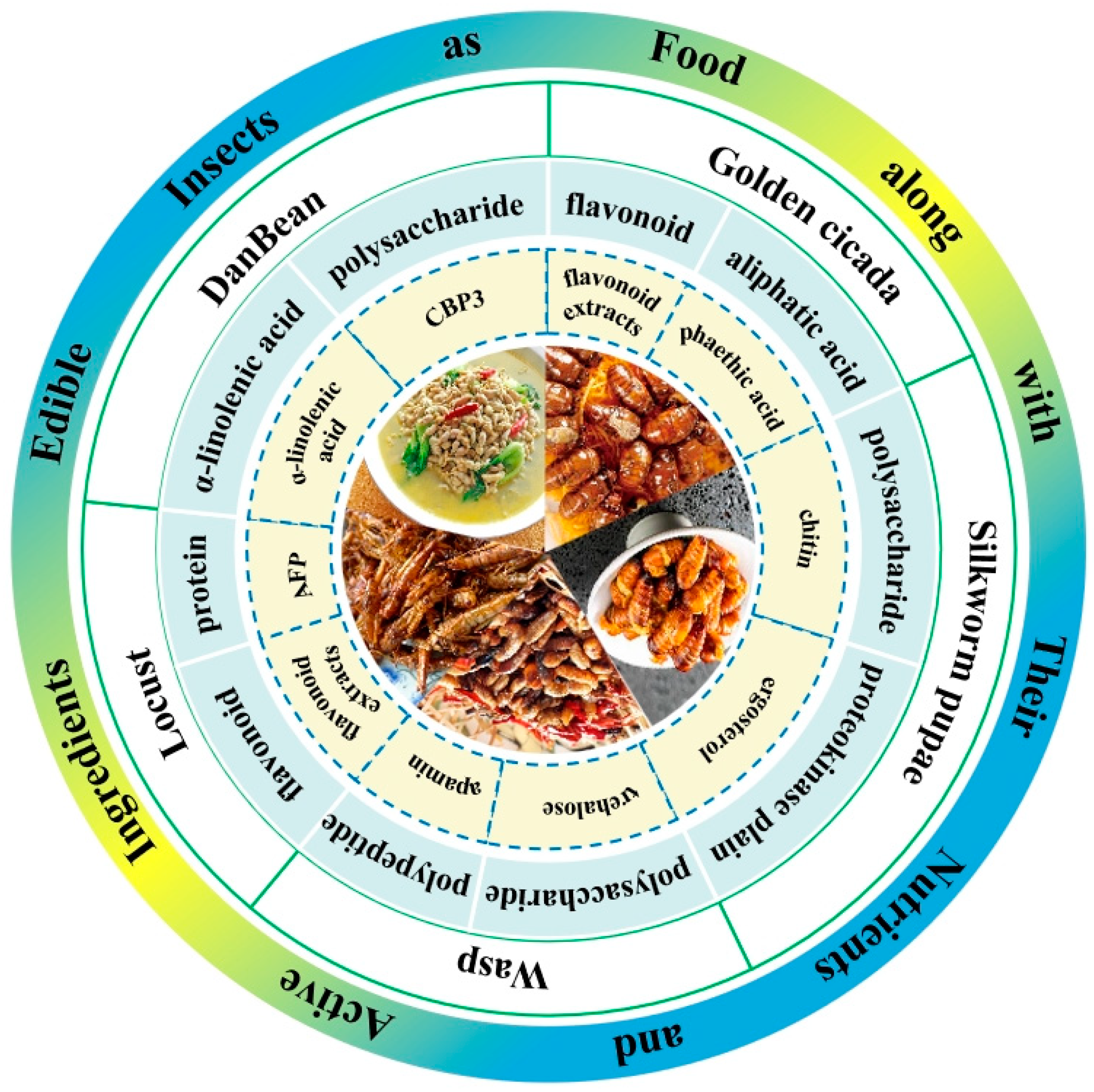
2. Major Edible Insect Species and Their Resource Utilization in China
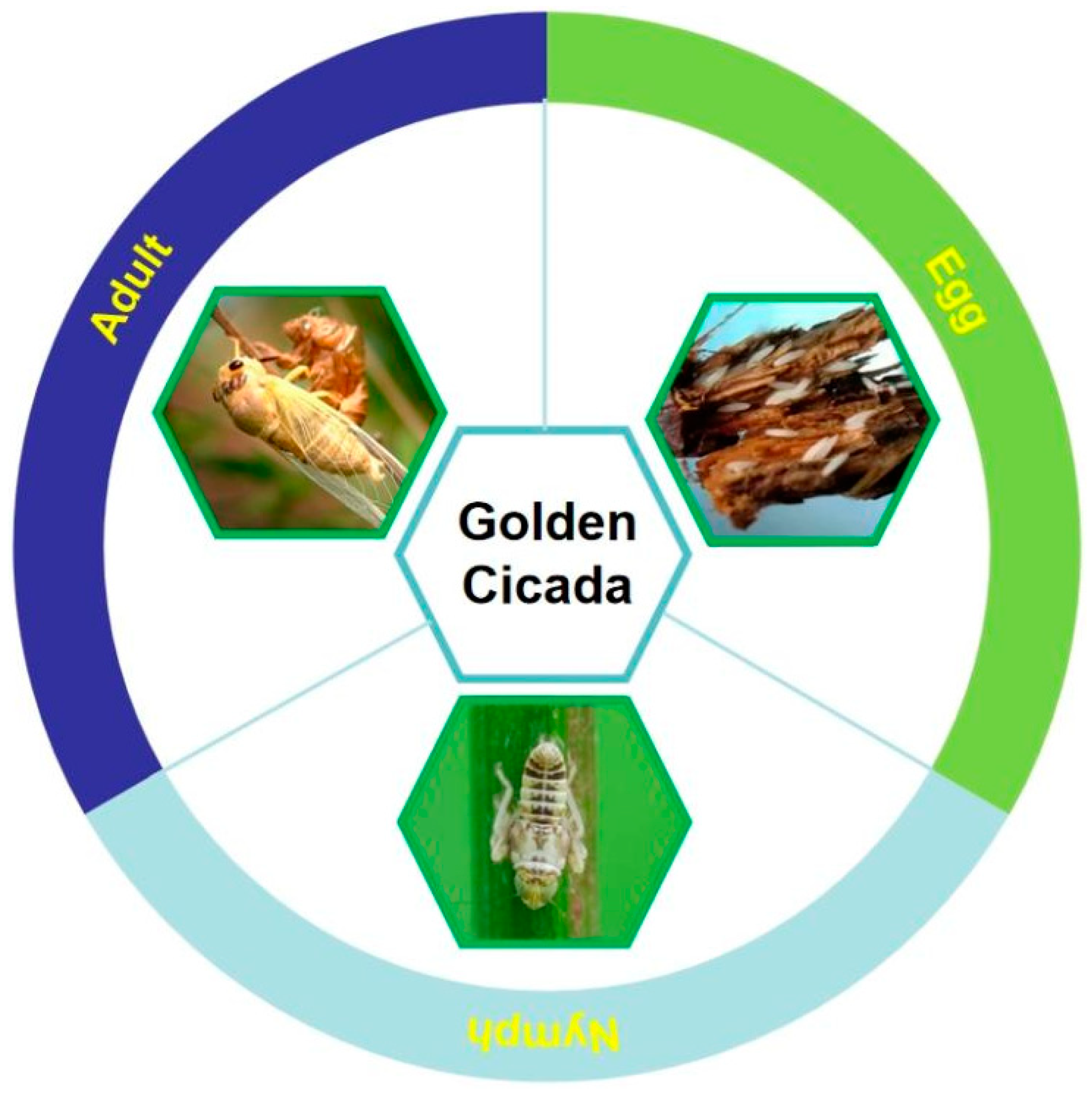
2.1. Golden Cicada
2.1.1. History and Status of Golden Cicada Consumption
2.1.2. Nutrient Composition and Medicinal Value of Golden Cicadas
- Nutrient Composition
- Medicinal Value
2.1.3. Artificial Rearing Technology of Golden Cicada
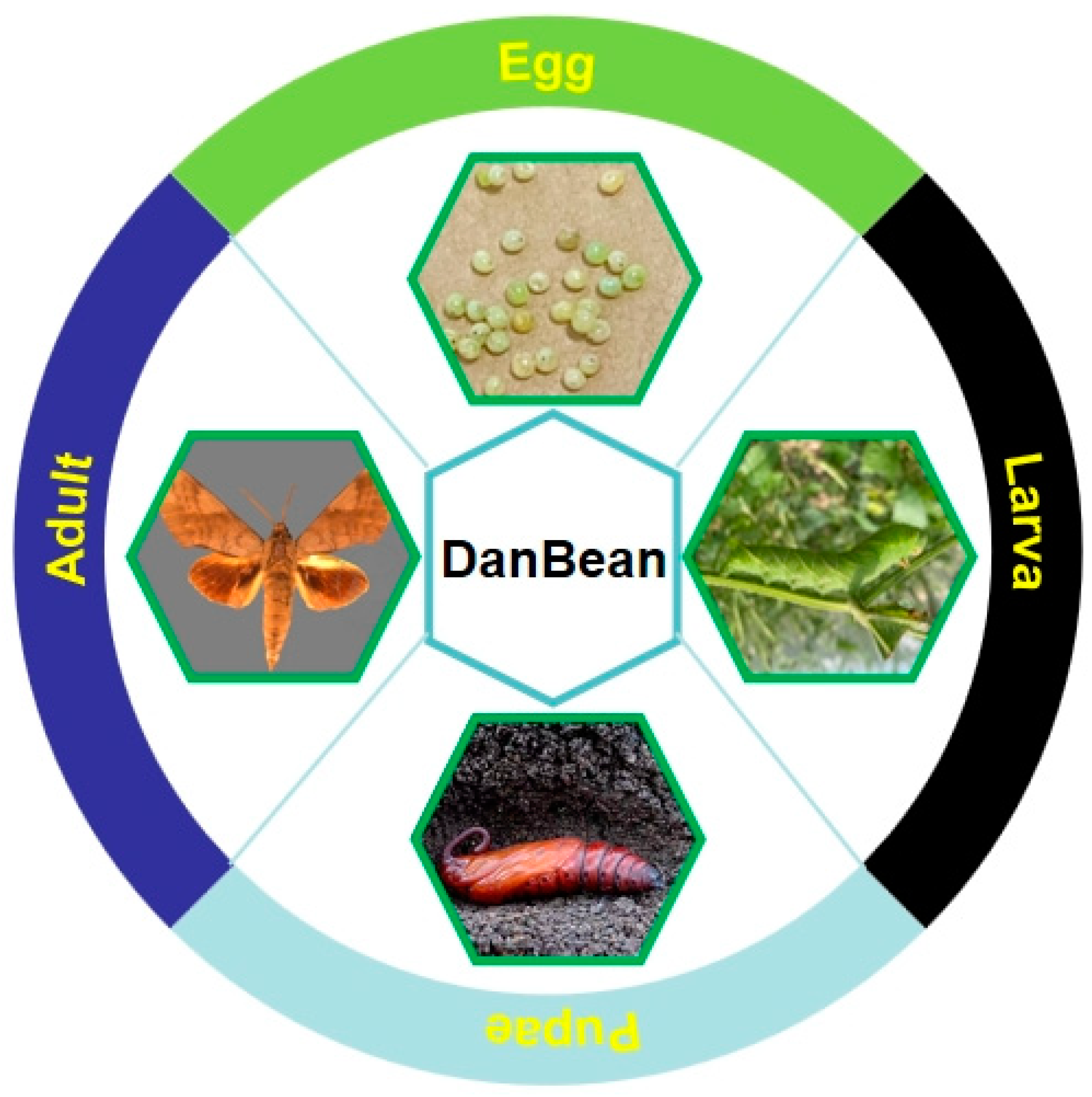
2.2. DanBean
2.2.1. History and Current Situation of DanBean Consumption
2.2.2. Nutrition and Medicinal Value of DanBean
- Nutritional Value
- Medicinal Value
2.2.3. Current Situation of DanBean Artificial Rearing
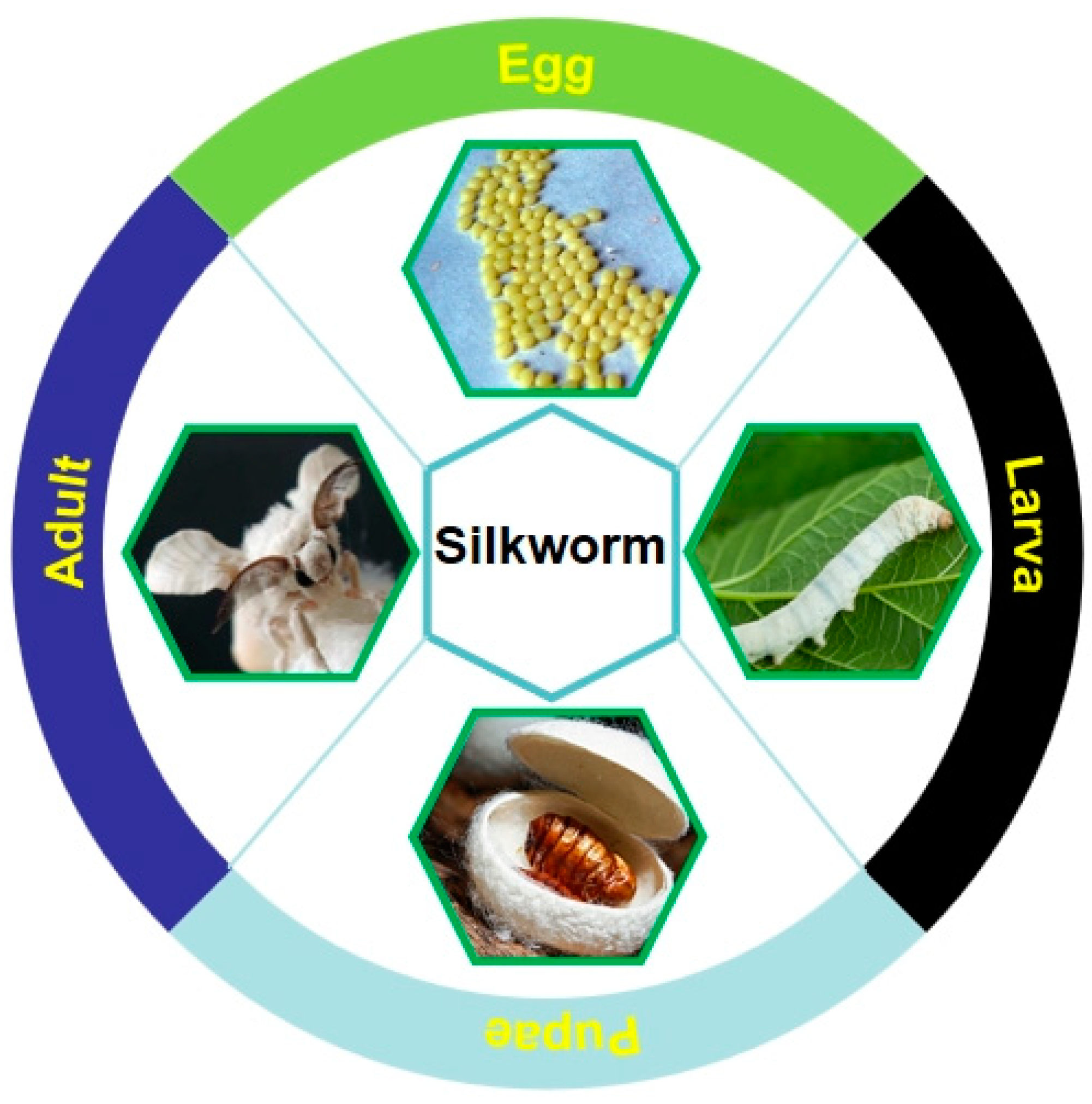
2.3. Silkworm Pupae
2.3.1. History and Status of Silkworm Pupae Consumption
2.3.2. Nutrition and Medicinal Value of Silkworm Pupae
2.3.3. Development Status of Silkworm-Rearing Technology and Industry
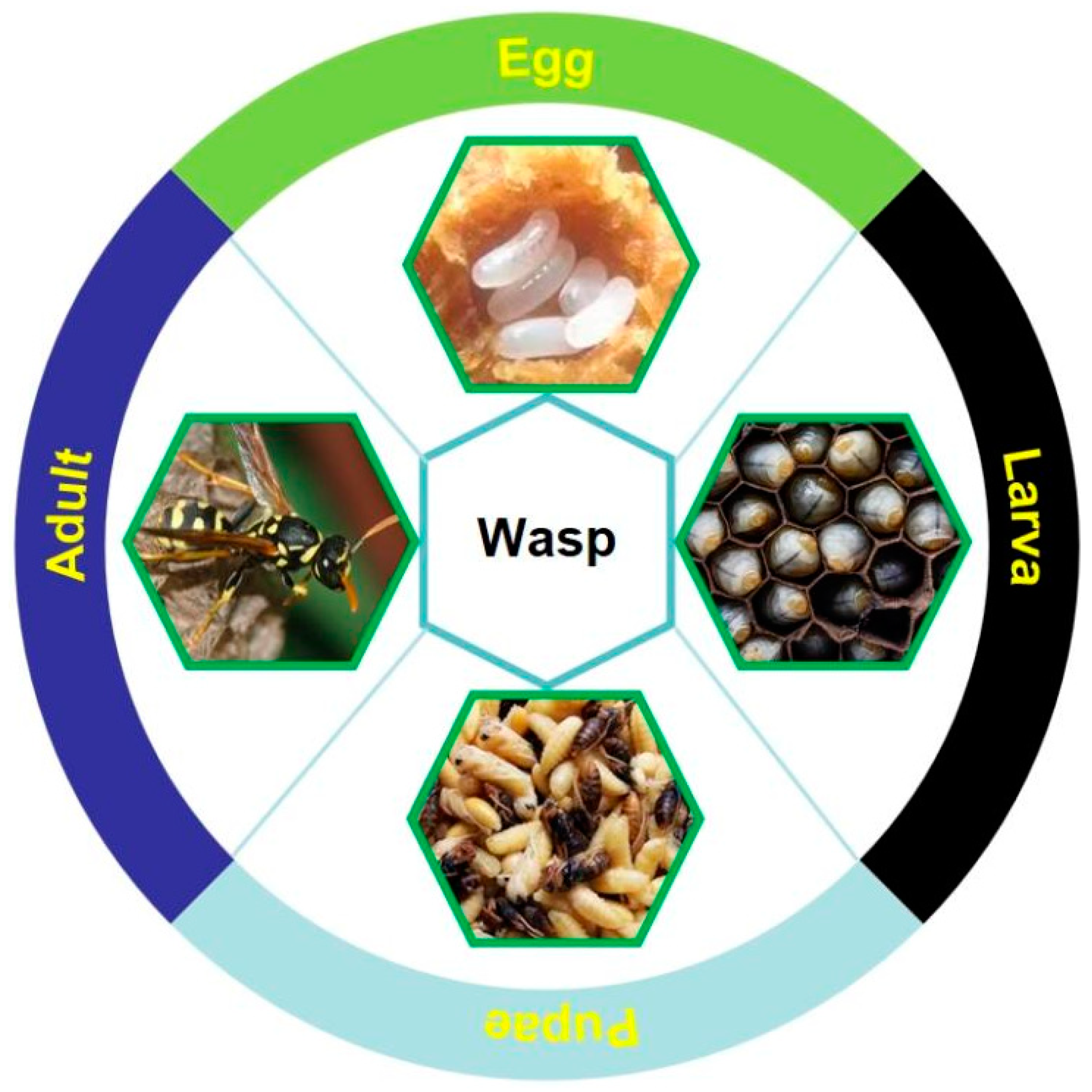
2.4. Wasps
2.4.1. History and Current Situation of Wasp Consumption
2.4.2. Nutritional and Medicinal Value of Wasps
2.4.3. Current Situation of Wasp Artificial Rearing
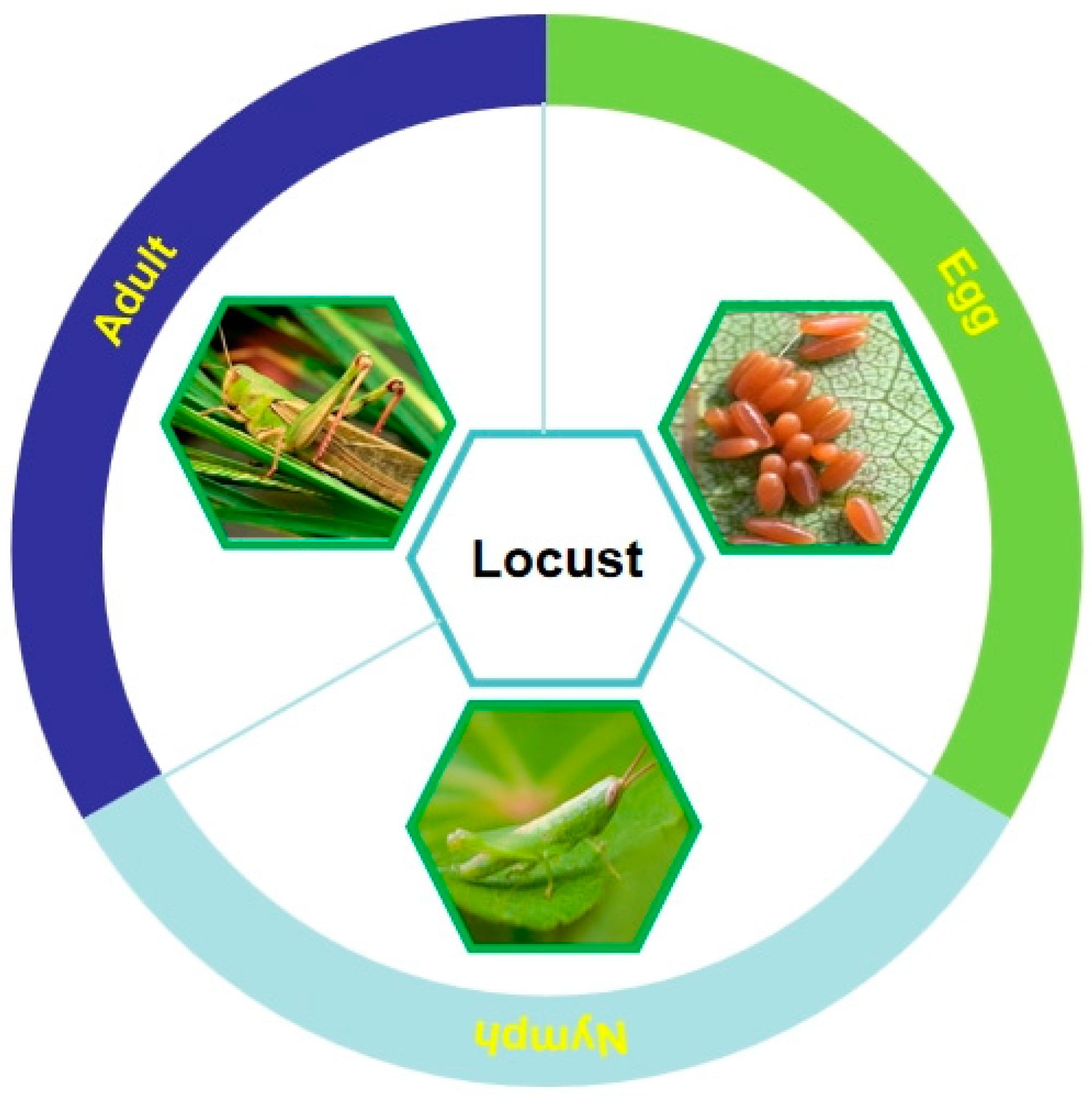
2.5. Locust
2.5.1. History and Current Situation of Locust Consumption
2.5.2. Nutritional and Medicinal Value of Locusts
2.5.3. Locust-Rearing Technique
2.5.4. Development Status of the Locust-Rearing Industry
2.6. Insect Products Used as Edible Insects
3. The Main Nutrients and Active Ingredients of Edible Insects
3.1. Proteins
3.1.1. Type and Content
3.1.2. Nutritional Value
3.1.3. Special Protein with Nutrition Function
3.2. Lipids
3.2.1. Type and Content
3.2.2. Nutritional Value
3.2.3. Nutritional Functions of Special Lipid
3.3. Amino Acids
3.3.1. Type and Content
3.3.2. Nutritional Value
3.4. Special Carbohydrates
3.4.1. Alginate
3.4.2. Chitin
3.4.3. Cordyceps Polysaccharide
3.5. Other Nutrients and Bio-Active Compounds
3.5.1. Minerals
3.5.2. Vitamin and Carotenoids
3.5.3. Specific Active Substances
4. Resource Utilization Advantage of Edible Insects
4.1. Characteristics of Edible Insect Production
4.2. Utilization Methods of Edible Insects
4.2.1. Direct Consumption
4.2.2. Consumption of Processed Edible Insects
4.2.3. Consumption of Nutritional and Health-Care Products Made from Edible Insects
4.2.4. Utilization of Edible Insects in the Yunnan Province of China
5. Production Advantages of Edible Insects over Traditional Livestock
5.1. Short Life Cycle
5.2. Low Production Costs
5.3. Improvement of the Ecological Environment
5.4. Water Saving
5.5. Reduced Land Use
6. Consumption of Edible Insects: Problems and Challenges
6.1. Limited-Scale Production of Edible Insects
6.2. Lack of Rearing Technology and Facilities
6.3. Immature Market and Lack of Industrial Alliance
6.4. Low Acceptability of Edible Insects and Their Products
6.5. Safety of Consumption
6.6. Legislation on Edible Insects in China
7. Developmental Prospect of Edible Insect Food
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- Posey, D.A. Kayapó Ethnoecology and Culture, 1st ed.; Plenderleith, K., Ed.; Routledge: London, UK, 2002. [Google Scholar] [CrossRef]
- Das, K. Entomophagy in Africa. In African Edible Insects As Alternative Source of Food, Oil, Protein and Bioactive Components; Adam Mariod, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 53–58. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO Forestry Paper No. 171; FAO: Rome, Italy, 2013; Available online: https://www.fao.org/3/i3253e/i3253e.pdf (accessed on 2 May 2024).
- Lv, Y.L.; Hu, J.; Wang, L. Market potential analysis of edible insect products. China Mark. 2013, 20, 87–89. [Google Scholar]
- Zhang, J.C.; Zhou, X.Y.; Cai, M.M.; Zheng, L.; Yu, Z.; Zhang, J. Research and application of edible insects. Biot. Resour. 2018, 40, 232–239. [Google Scholar] [CrossRef]
- Liu, M.; Zhai, R.H. The history and industrial development of Chinese edible insect culture. Agric. Archaeol. 2017, 37, 223–232. [Google Scholar]
- Kelemu, S.; Niassy, S.; Torto, B.; Fiaboe, K.; Affognon, H.; Tonnang, H.; Maniania, N.K.; Ekesi, S. African edible insects for food and feed: Inventory, diversity, commonalities and contribution to food security. J. Insects Food Feed 2015, 1, 103–119. [Google Scholar] [CrossRef]
- Magara, H.J.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible crickets (Orthoptera) around the world: Distribution, nutritional value, and other benefits—A review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef] [PubMed]
- Svanberg, I.; Berggren, Å. Ant schnapps for health and pleasure: The use of Formica rufa L. (Hymenoptera: Formicidae) to flavour aquavit. J. Ethnobiol. Ethnomed. 2019, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Svanberg, I.; Berggren, Å. Insects as past and future food in entomophobic Europe. Food Cult. Soc. 2021, 24, 624–638. [Google Scholar] [CrossRef]
- Xing, X.C. Development and utilization of insect protein food. Territ. Nat. Resour. Study 1994, 16, 56–59+67. [Google Scholar]
- Virginia, M.R.; Tomás, Q.B.; Rafael, D.G.; César, G.R. Consumption of escamoles (Liometopum apiculatum M.): A source of vitamins A and E. J. Appl. Life Sci. Int. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.J.; Jiang, S.T. Progress of the exploitation and utilization of silkworm in food field. Food Sci. 2005, 26, 574–578. [Google Scholar]
- Zhang, F.; Zhang, Z.N. Study on edible insect resources and their exploitation and utilization. Resour. Sci. 2001, 25, 58–61+97. [Google Scholar]
- Qian, L.; Deng, P.; Chen, F.; Cao, Y.; Sun, H.; Liao, H. The exploration and utilization of functional substances in edible insects: A review. Food Prod. Process. Nutr. 2022, 4, 11. [Google Scholar] [CrossRef]
- Xu, H.Q.; Xia, Q.Q. Nutritional value of edible insects and their development and utilization. J. Res. Diet. Sci. Cult. 2004, 21, 20–23. [Google Scholar]
- Omuse, E.R.; Tonnang, H.E.Z.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Mohamed, S.F.; Kassie, M.; Subramanian, S.; Onditi, J.; et al. The global atlas of edible insects: Analysis of diversity and commonality contributing to food systems and sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Hou, M.T.; Hu, J.X.; Liu, A.J. Research on the development status and problems of the world insect food industry. World Agric. 2019, 41, 13–19. [Google Scholar] [CrossRef]
- Yao, R.H.; Zhou, Q.F. The meaning, classification, and evaluation of functional foods. Food Sci. Technol. 1996, 5, 2–3. [Google Scholar]
- Feng, Y.; Chen, X.M. Resource value of edible insects and utilizable ways. For. Res. 2002, 15, 105–110. [Google Scholar]
- Wang, T.Y. Primary study on culture technology of golden cicada under forest. Spec. Econ. Anim. Plants 2020, 23, 13+18. [Google Scholar]
- Wang, C.N.; Cong, N. Study on nutritional composition of larva of Cryptotympana pustulata. Acta Nutr. Sin. 2002, 47, 447–448. [Google Scholar]
- Wang, J.W.; Lu, J.Z.; Jing, Y.L.; Liu, Y.Z. The key technology of cicada culture. Spec. Econ. Anim. Plants 2018, 21, 5–6. [Google Scholar]
- Chen, X.M.; Chen, H.; Zhao, M.; Yang, Z.X.; Feng, Y. Insect industrialization and prospect in commerce: A case of China. Entomol. Res. 2022, 52, 178–194. [Google Scholar] [CrossRef]
- Pu, Z.H. Talking about cicadas from ancient and modern times. Health Preserv. 2005, 26, 73–76. [Google Scholar]
- Harpaz, I. Frederick Simon Bodenheimer (1897–1959): Idealist, scholar, scientist. Annu. Rev. Entomol. 1984, 29, 1–24. [Google Scholar] [CrossRef]
- Wang, F.Y.; Tang, Y.Z.; Jin, H.X. Application of protein of nymph of Cryptotympana atrata Fabricius in the food and medicine. Shaanxi For. Sci. Technol. 2012, 40, 99–101. [Google Scholar]
- Feng, Y.; Chen, X.M. Review on nutritive value of edible insects. For. Res. 1999, 12, 662–668. [Google Scholar]
- Wu, X.; Zhong, L.; Dong, B. The utilization value and market analysis of golden cicada. New Rural Area 2011, 3, 60–61. [Google Scholar]
- Liu, M.; Peng, G.L.; Li, P.C.; Liu, C.; Yi, X. Determination and analysis of cicada’s nutritional content from different developmental phases. J. Jilin Med. Univ. 2017, 38, 167–169. [Google Scholar]
- Zhu, B.H. Food and medicine: Golden cicada. In Proceedings of the Collection of Articles from the 2011 National Forum on Traditional Chinese Medicine Science Popularization; China Association of Chinese Medicine: Beijing, China, 2011; pp. 203–204. [Google Scholar]
- Lu, J.Z.; Wang, J.W.; Liu, Y.Z.; Jing, Y.L. Preliminary study on the technology of cultivating wild cicadas in forest land. Spec. Econ. Anim. Plants 2019, 22, 4–6. [Google Scholar]
- Yan, M.H. Clanis bilineata tsingtauica-Feed. J. Biol. 2001, 19, 25–33. [Google Scholar]
- Lv, F.; Liu, Y.S.; Wang, Z.P.; Zhang, X.B. Advances in production and comprehensive utilization of Clanis bilineata tsingtauica. J. Biosaf. 2006, 15, 192–195. [Google Scholar]
- Xu, Y.H.; Shen, J.; Li, T.Y. The current situation and prospects of the comprehensive utilization of soybean armyworm in Lianyungang. Sci. Technol. Inf. 2008, 25, 30. [Google Scholar]
- Liu, L.C. Insect food anecdotes. Chin. J. Appl. Entomol. 1995, 32, 126–127. [Google Scholar]
- Xu, G.W.; Li, D.W.; Li, J.L. Research on the dilemma and countermeasures of Clanis bilineata industrialization development. Food Sci. Technol. 2022, 28, 96–99. [Google Scholar]
- Song, K.X. Edible insect—Bean moth. Middle Sch. Biol. 2006, 22, 11–12. [Google Scholar]
- Wang, T.T. Study on the Vacuum Freeze-Drying and Quality of the Bean Worm. Master’s Dissertation, Nanjing Agricultural University, Nanjing, China, 2018. [Google Scholar]
- Wu, S.J.; Meng, X.; Chen, S.J. The analysis and evaluation of main nutrient components for Herse bilineata tsingtauica. J. Jiangsu Ocean Univ. Sci. Ed. 2000, 9, 60–63. [Google Scholar]
- Tian, H.; Zhang, Y.M. The anti-oxidant activity of polysaccharide CBP3 in Clanis bilineata tsingtauica Mell. Food Sci. Technol. 2012, 37, 228–231. [Google Scholar]
- Wang, H.J. The occurrence of the bean moth is severe in Hebei Province. Plant Prot. 1988, 1, 55. [Google Scholar]
- Jiang, J. Promoting advantageous technologies to promote the upgrading of sericulture industry. Friends Farmers Becom. Rich. 2013, 57, 74. [Google Scholar]
- Jiang, X.R.; Huo, X.Y.; Huang, J.H.; Sun, Z.T. Technology of Biotech Fermentation Industry; China Light Industry Press: Beijing, China, 2016. [Google Scholar]
- Li, M.L. Resource Entomology; China Forestry Publishing House: Beijing, China, 2004. [Google Scholar]
- Liu, X.W.; Chen, X.L.; Zhang, Y.; Li, Y.Q.; Chen, F.L. Studies on anti-fatigue effect of alcohol extract of Clanis bilineata Walker. Sci. Technol. Food Ind. 2014, 35, 362–363+368. [Google Scholar]
- Sun, Z.W.; Fan, J.W.; Wang, K.J.; Li, Q.; Guo, M.M. The current situation and prospects of breeding bean moth larvae. Agric. Dev. Equip. 2019, 25, 64+67. [Google Scholar]
- Liu, Y.S.; Ye, B.H. Fine Breeding of Economic Insects; Shandong Science Press: Jinan, China, 2001. [Google Scholar]
- Wang, Y.N. Manual of Artificial Feed for Insects; Shanghai Scientific & Technical Publishers: Shanghai, China, 1984. [Google Scholar]
- Gui, Z.Z.; Zhuang, D.H.; Dai, J.Y.; Hu, J.; Wuang, G.Y. Research progress on the edible and medicinal value of resource insects (Silkworms). China Seric. 2002, 23, 63–65. [Google Scholar]
- Chai, J.P.; Chen, S.L.; Bai, X.R.; Xie, D.Y. Exploitation and utilization of silkworm pupa as a resource insect. Yunnan Agric. Sci. Technol. 2002, 31, 18–20. [Google Scholar]
- Fan, L.S.; Li, D.S.; Ma, L. New processing technology for convenient silkworm pupae food. Jiangsu Food Ferment. 1998, 26, 34–36. [Google Scholar]
- Song, X.Y.; Dong, T.X. Research on the processing technology of canned silkworm pupae. China Seric. 2004, 25, 87–88. [Google Scholar]
- Xu, J.L. The efficacy and consumption of silkworm pupae. Newsl. Seric. Tea 2021, 2, 15+17. [Google Scholar]
- Zhu, K.K.; Chen, W.P.; He, Z.L. The effects of silkworm pupa extract on the blood glucose and lipids levels in diabetic rats. China Prev. Med. J. 2008, 20, 9–11+13. [Google Scholar] [CrossRef]
- Wang, S.M.; Chen, Y.Q.; Li, Q.; Lu, Z.X.; Guo, Z.X. The dynamics and spatial distribution of mixed-population of ants on two host plant species of Kerria. yunnanensis (Hemiptera: Kerridae). In Proceedings of the 2009 Annual Meeting of the Yunnan Entomological Society, Kunming, China, 1 October 2009; pp. 12–18. [Google Scholar]
- Zhou, G.; Xie, F.; Chen, Z.H.; Zhang, R.R. Research progress on Ophiocordyceps sinensis compounds. Guangzhou Chem. Ind. 2023, 51, 5–8. [Google Scholar]
- Wei, X.F. A brief talk about the key technical measures of silkworm breeding. Guangdong Seric. 2021, 55, 7–8. [Google Scholar]
- Zou, S.W. The History of Chinese Entomology; Science Press: Beijing, China, 1982; 247p. [Google Scholar]
- Wen, L.Z. Nutrient composition of edible insects in Mexico. Chin. Bull. Entomol. 1998, 35, 58–61. [Google Scholar]
- Yang, D.R. Resources of edible insects and insect knowledge of national insectivorous culture in Yunnan. Entomol. Knowl. 1999, 36, 122–125. [Google Scholar]
- Feng, Y.; Chen, X.M.; Ye, S.D.; Wang, S.Y.; Chen, Y.; Wang, Z.L. The common edible species of wasps in Yunnan and their value as food. For. Res. 2001, 14, 578–581. [Google Scholar]
- Feng, Y.; Chen, X.M.; Sun, L.; Chen, Z.Y. Common edible wasps in Yunnan Province of China and their nutritional value. In Forest Insects as Food: Human Bite Back, Proceedings of the Workshop on Asia-Pacific Resources and Their Potential for Develop, Chiang Mai, Thailand, 19–21 February 2008; Durst, P., Johnson, D., Leslie, R., Shono, K., Eds.; FAO: Rome, Italy, 2010; pp. 92–98. ISBN 978-92-5-106488-7. [Google Scholar]
- Zhang, Y.J. Resource advantage of the development and utilization of edible insects. Technol. Inf. 2012, 29, 228–229. [Google Scholar]
- Wang, X.W.; Li, T.; Zhuo, Z.H.; Deng, Z.B.; Yang, W.; Yang, C.P. Analysis of resource value and harm of wasps. J. Sichuan For. Sci. Technol. 2015, 36, 42–47. [Google Scholar]
- Yang, H.; Chen, D.; Xiao, P.Y.; Yang, Y.S.; Hu, K.; He, M.; Li, C.G.; Zhang, C.G. Heavy metals, constant and trace elements analysis of Vespa velutina auraria Smith adults. Guangzhou Chem. Ind. 2016, 44, 141–143+168. [Google Scholar]
- Ghosh, S.; Mohamadzade Namin, S.; Meyer-Rochow, V.; Jung, C. Chemical composition and nutritional value of different species of Vespa hornets. Foods 2021, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.B.; Yang, W.; Yang, C.P.; Yang, H.; Yang, X.Z.; Huang, Q. Nutritional analysis and evaluation of Vespa basalis Smith. Acta Nutr. Sin. 2013, 35, 514–515+518. [Google Scholar]
- Huang, F.K. The current situation and prospects of insect food development. Light Ind. Sci. Technol. 2011, 27, 11–12. [Google Scholar]
- Wu, R.; Li, D.; Tang, Q.; Wang, W.; Xie, G.; Dou, P. A novel peptide from Vespa ducalis induces apoptosis in osteosarcoma cells by activating the p38 MAPK and JNK signaling pathways. Biol. Pharm. Bull. 2018, 41, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.X. Interpretation of “Shennong Book”. Read. Benef.-Seek. Med. Treat. 2019, 39, 53. [Google Scholar]
- Zhang, Y.Z.; Wu, J.H.; Miu, J.L.; Chen, X.B. Analysis of the liposoluble constituents from the Vespa velutina by GC-MS. Chin. J. Ethnomed. Ethnopharm. 2020, 29, 19–21. [Google Scholar]
- Wang, Q.; Zhou, G.L.; Zhou, W.L.; Huang, P.; Guan, S.Q. Research Progress on nidus vespae and predictive analysis on its quality marker. Chin. J. Ethnomed. Ethnopharm. 2021, 39, 254–258. [Google Scholar]
- Jin, F.M.; Zhang, Z.X.; Wang, Y.; Zhao, H.R.; Yang, Y.Y.; Huang, X.; Zhang, C.G. Protective effect of Ento-i plastic against cerebral ischemia-reperfusion injury in rats. J. Int. Pharm. Res. 2016, 43, 504–508+528. [Google Scholar]
- Feng, R.; Wang, Y.; Zhu, F.; Zhao, H.R.; Li, H.M.; Zhang, Z.X.; Wu, X.M. Effects of Ento-I plastic on analgesia, anticoagulation and anti-thrombosis. J. Int. Pharm. Res. 2016, 43, 509–513. [Google Scholar]
- Wang, Y.; Zhang, Y.; Li, C.G.; Gao, P.F.; Zhao, H.R.; Zhao, Y.; Zhang, C.G. Effects of Ento-i plastic on anti-anoxia and myocardial ischemia in mice: A preliminary study. J. Int. Pharm. Res. 2017, 44, 251–256. [Google Scholar]
- He, J.G. Wasp assisted migration method. Plant Prot. 1981, 7, 11. [Google Scholar]
- Li, T.S.; He, J.G. Artificial early nest construction by wasps can improve the effectiveness of pest control in cotton fields by increasing generations. Chin. J. Appl. Entomol. 1988, 25, 108–109. [Google Scholar]
- Liu, N. The medicinal and artificial breeding of wasps. Farmers Consult 1997, 15, 24. [Google Scholar]
- Wang, Y.X. A leader in artificial wasp breeding—The first “Poisonous Wasp Breeding Research Association” in Shuangbai county, China. Priv. Technol. 2001, 7, 42–44. [Google Scholar]
- Zheng, G.Q.; Tan, K. The biological characteristics and feeding management of the Concave wasp. Spec. Econ. Anim. Plants 2008, 11, 10–12. [Google Scholar]
- Guo, Y.J. Experimental study on artificial assisted migration and breeding of Vespa mandarinia Smith. Mod. Agric. Sci. Technol. 2012, 41, 328–329+332. [Google Scholar]
- Guo, Y.J.; Gao, S.; Luo, Z.W. Experimental study on artificial breeding of Vespa velutina auraria Smith. Silicon Val. 2013, 6, 150–152. [Google Scholar]
- Guo, Y.J.; Huang, G.Z. Preliminary study on artificial science breeding of Vespa mandarinia Smith. Agric. Technol. 2013, 33, 138–139. [Google Scholar]
- He, Q.J.; Yi, C.H.; Zhang, Z.W.; Ren, X.P.; Lin, Z.Y. The status and problems of wasps artificial breeding. Hubei Agric. Sci. 2018, 57, 10–13. [Google Scholar]
- Cui, X.Y. Locust species and breeding techniques. Mod. Agric. Sci. Technol. 2010, 5, 305+307. [Google Scholar]
- Egonyu, J.P.; Subramanian, S.; Tanga, C.M.; Dubois, T.; Ekesi, S.; Kelemu, S. Global overview of locusts as food, feed and other uses. Glob. Food Secur. 2021, 31, 100574. [Google Scholar] [CrossRef]
- Meng, T.; Ren, B.Z. The research advance of developing and utilization of the grasshopper resource. J. Beihua Univ. Sci. 2002, 3, 485–490. [Google Scholar]
- Kuper, R. After 5000 BC: The Libyan desert in transition. C. R. Palevol 2006, 5, 409–419. [Google Scholar] [CrossRef]
- Mitsuhashi, J. Edible Insects of the World, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Yhoung-Aree, J. Edible insects in Thailand: Nutritional values and health concerns. In Forest Insects as Food: Human Bites Back; Durst, P.B., Johnson, D.V., Leslie, R.N., Shono, K., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy; Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; pp. 93–98. [Google Scholar]
- Costa-Neto, E.M.; Dunkel, F.V. Insects as food: History, culture, and modern use around the world. In Insects as Sustainable Food Ingredients: Production, Processing and Food Applications; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 29–60. [Google Scholar]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Han, J.M.; Song, H.-Y.; Byun, E.-H.; Seo, H.S.; Byun, E.-B. Edible Oxya chinensis sinuosa—Derived protein as a potential nutraceutical for anticancer immunity improvement. Nutrients 2020, 12, 3236. [Google Scholar] [CrossRef]
- Pang, L.Y.; Duan, Y.F.; Chen, J.P.; Yao, L. Functional ingredients of grasshopper and their developing prospect. J. Econ. Anim. 2004, 8, 54–56+59. [Google Scholar]
- Duan, Y.F. The Study on Nutritional Value and Biological Function of Locust. Ph.D. Thesis, Shaanxi Normal University, Xi’an, China, 2005. [Google Scholar]
- Liu, S.P.; Li, Q.X.; He, F.; Feng, W.J. Advances in research on edible insects of Orthoptera. Light Ind. Sci. Technol. 2016, 32, 9–11. [Google Scholar]
- Chen, Y.L. Artificial breeding technology of grasshoppers and their market prospect analysis. Guizhou For. Sci. Technol. 2012, 40, 52–55. [Google Scholar]
- Peng, W.X.; Ma, N.L.; Zhang, D.Q.; Zhou, Q.; Yue, X.C.; Khoo, S.C.; Yang, H.; Guan, R.R.; Chen, H.L.; Zhang, X.F.; et al. A review of historical and recent locust outbreaks: Links to global warming, food security and mitigation strategies. Environ. Res. 2020, 191, 110046. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Cheseto, X.; Kuate, S.P.; Tchouassi, D.P.; Ndung’u, M.; Teal, P.E.A.; Torto, B. Potential of the desert locust Schistocerca gregaria (Orthoptera: Acrididae) as an unconventional source of dietary and therapeutic sterols. PLoS ONE 2015, 10, e0127171. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.J.; Zhang, J.N.; Liu, Y.; Ma, L.K.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A Review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, L.; Smagghe, G.; Herregods, G.; Van Camp, J. ACE inhibitory activity in enzymatic hydrolysates of insect protein. J. Agric. Food Chem. 2005, 53, 5207–5211. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Liu, Y.S. Lecture on new technologies for economic insect development and utilization (Seventeen), Artificial breeding and utilization technology of locust (Part 2). Agric. Knowl. 2005, 27, 23–25. [Google Scholar]
- Zhao, J.Y. Locust breeding technology. Nongcun Xinjishu 2012, 3, 17–18. [Google Scholar]
- Zhang, Y. Locust breeding management four attention. Nongmin Keji Peixun 2010, 8, 36. [Google Scholar]
- Cao, C.Q. Several key links in locust farming (Part 1). Agric. Knowl. 2006, 57, 40–41. [Google Scholar]
- Cao, C.Q. Current situation and development counter measures of locust culture in China. Agric. Knowl. 2008, 9, 32–33. [Google Scholar]
- Cao, C.Q.; Nie, S.X.; Chen, S.Z.; Ye, B.H.; Liu, Y.S. The recyclical exploitation and use of grasshopper. J. Econ. Anim. 2007, 11, 116–120. [Google Scholar]
- Li, L.T.; Wang, H.; Bai, W.D.; Dong, H.; Li, N.W.; Xiao, G.S.; Liu, G.L.; Lai, Q.P. Research progress on bioactive ingredients, biological activity and wall-breaking technology of bee pollen. Sci. Technol. Food Ind. 2022, 43, 408–417. [Google Scholar]
- Liu, Y.B.; Wu, D.Q.; Lin, Z.G.; Ji, T. Review on biological function of royal jelly. Acta Vet. Zootech. Sin. 2021, 52, 1498–1510. [Google Scholar]
- Wang, K.L.; LI, S.S.; Hu, F.L. Research status of bee venom in 2021. J. Bee 2022, 42, 1–8. [Google Scholar]
- Wang, Q.Q.; Du, X.Y.; Gao, X.B.; Lin, X.; Zhang, G.S. Research progress on functional activities and medicinal value of honey. J. Food Saf. Food Qual. 2022, 13, 5849–5854. [Google Scholar]
- Tian, Q. Silk food. Food Health 2002, 12, 19. [Google Scholar]
- Wang, H.; Yang, W.; Yang, C.P.; Yang, H.; Yang, X.Z.; Zhang, H. Analysis and evaluation of nutritional components of four species of Conocephalidae. Acta Nutr. Sin. 2011, 33, 627–629. [Google Scholar]
- Li, Y.C.; Li, K.; Dai, R.H. Analysis and evaluation of nutrition of Opisthoplata orientalis Buron. J. Environ. Entomol. 2013, 35, 466–472. [Google Scholar]
- Raheem, D.; Raposo, A.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Carrascosa, C. Entomophagy: Nutritional, ecological, safety and legislation aspects. Food Res. Int. 2019, 126, 108672. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Mu, L.X.; Liao, S.T.; Zou, Y.X.; Liu, F.; Shen, W.Z.; Liu, J.P. Research progress on main nutritional and active substances of some edible insects at different developmental stages. Acta Sericologica Sin. 2014, 40, 737–742. [Google Scholar]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-protein conversion factors for edible insects on the Swiss market: T. Molitor, A. Domesticus, and L. Migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.Z.; Luo, H.R.; Wang, K.Q. Present status and development counter measures on insect food industry. Hunan Agric. Sci. 2003, 33, 69–71. [Google Scholar]
- Gou, M.X.; Li, J.J.; Zhao, Y.X.; Nie, Q.Y. Application and research progress of insect protein in food. Meat Ind. 2020, 41, 50–54. [Google Scholar]
- Ai, H.; Wang, F.R.; Zhang, N.; Zhang, L.Y.; Lei, C.L. Antiviral, Immunomodulatory, and free radical scavenging activities of a protein-enriched fraction from the larvae of the housefly, Musca domestica. J. Insect Sci. 2013, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.Y.; Xu, H.N.; Yu, C.; Mintah, B.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.L.; Dai, C.H.; He, R.H.; et al. Recent insight on edible insect protein: Extraction, functional properties, allergenicity, bioactivity, and applications. Foods 2022, 11, 2931. [Google Scholar] [CrossRef]
- Zhuo, Z.H.; Yang, W.; Xu, D.P.; Yang, C.P.; Yang, H.; Chen, C.Y. Analysis of resource value of edible insect. Sci. Technol. Food Ind. 2014, 35, 390–394. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.Q.; Liu, W.X. Advances in the research and the exploitation of the edible insects. Food Res. Dev. 2006, 27, 89–91+97. [Google Scholar]
- Xie, X.Y.; Luo, S.Y.; Liu, S.Q.; Yuan, Z.H.; An, J.H.; Shang, L.C.; Deng, L.L. Application and research progress of yellow mealworms in the food industry. Food Ferment. Ind. 2022, 48, 300–305. [Google Scholar]
- Siddiqui, S.A.; Zhao, T.; Fitriani, A.; Rahmadhia, S.N.; Alirezalu, K.; Fernando, I. Acheta domesticus (house cricket) as human foods—An approval of the European Commission—A systematic review. Food Front. 2024, 5, 435–473. [Google Scholar] [CrossRef]
- Yongsawas, R.; Chaimanee, V.; Pettis, J.S.; Boncristiani, H.F.; Lopez, D.; In-on, A.; Chantawannakul, P.; Disayathanoowat, T. Impact of sacbrood virus on larval microbiome of Apis mellifera and Apis cerana. Insects 2020, 11, 439. [Google Scholar] [CrossRef]
- Tian, H. Research overview of amino acids and fatty acids of edible insects. Mod. Agric. Sci. Technol. 2015, 44, 292–293. [Google Scholar]
- Wang, D.; Bai, Y.Y.; Zhang, C.X. A review on nutrition value of oak silkworm pupae and its exploitation. Chin. J. Appl. Entomol. 2004, 41, 418–421. [Google Scholar]
- Longvah, T.; Mangthya, K.; Ramulu, P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; Li, H.J.; Yang, J.; Tong, H.R. Utilization and research progress of edible resource insects. Food Sci. Technol. 2002, 28, 29–31. [Google Scholar]
- Ren, H.B.; Zhao, G.Y.; Li, Y.X.; Meng, Q.X. Influence of dietary lysine level on whole-body protein turnover, plasma IGF-I, GH and insulin concentration in growing pigs. Livest. Sci. 2007, 110, 126–132. [Google Scholar] [CrossRef]
- Chen, D.H. Research progress on pharmacological functions of Cordyceps sinensis. Chin. J. Urban Rural Ind. Hyg. 2008, 23, 92–94. [Google Scholar]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Food. 2014, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhao, J.; Feng, K.; Hu, D.J.; Li, S.P. Comparison and characterization of polysaccharides from natural and cultured Cordyceps using saccharide mapping. Anal. Bioanal. Chem. 2011, 399, 3465–3474. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, W.Q.; Li, L.; Wu, J.Y. Physiochemical properties and antitumor activities of two α-glucans isolated from hot water and alkaline extracts of Cordyceps (Cs-HK1) fungal mycelia. Carbohydr. Polym. 2011, 85, 753–758. [Google Scholar] [CrossRef]
- Gao, S.S.; Chen, Y.Z. A study on the liver and kidney protection and immune function regulation of Cordyceps sinensis. Tradit. Chin. Med. Res. 2015, 28, 77–80. [Google Scholar]
- Peng, W.B.; Wang, J.C.; Chu, Z.Y. Research progress on the anti-osteoporosis effect of Cordyceps sinensis. J. Navy Med. 2022, 43, 449–452. [Google Scholar]
- Chen, X.N. Analysis of Composition, Immune and Antioxidant Activities of Polysaccharide and Preliminary Study on PGM Gene of Cordyceps guangdongensis. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2020. [Google Scholar]
- Zhong, W.; Guo, L.X.; Qi, Y. Research progress on extraction and biological activity of Cordyceps militaris polysaccharides. J. Liaoning Univ. Tradit. Chin. Med. 2017, 19, 219–221. [Google Scholar]
- Qin, L.X.; Ding, Y.C.; Zhao, J.F.; Gao, Y.J. Optimization of extraction process of Cordyceps militaris polysaccharides and its antibacterial and antioxidant activities. Food Res. Dev. 2023, 44, 159–165. [Google Scholar]
- Qiao, T.S.; Tang, H.C.; Liu, J.X.; Li, L. Nutrient analysis and protein evaluation of Oxya chinensis. Chin. J. Appl. Entomol. 1992, 29, 113–117. [Google Scholar]
- Rong, B.X.; Gan, S.Y.; Chen, J.H.; Liang, W.F. Analysis of trace elements in ants and their preparations. Chin. Tradit. Herb. Drugs 1987, 18, 47. [Google Scholar]
- Mo, Y.H. Experimental Study on the Food Safety of Male Rhinoceros Beetle and the Analgesic Effect of Aqueous Extract. Master’s Dissertation, Zhejiang A&F University, Hangzhou, China, 2022. [Google Scholar]
- Liu, G.Q.; Wei, M.C.; Wang, X.L. Exploitation trend and strategy on edible insects in China. J. Northwest For. Univ. 2004, 19, 105–107. [Google Scholar]
- Wang, X.L.; Liu, G.Q.; Zhou, G.Y.; Wei, M.C. Antitumor functional factors in insect bodies. Food Res. Dev. 2004, 25, 124–125. [Google Scholar]
- Feng, Y. Studies on the Nutritive and Health Care Value of White Wax Scale (Ericerus pela Chavannes) and Evaluation of Its Functional Factors. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2005. [Google Scholar]
- Liu, A.Q. The Study on the Extraction and Seperation of Flavonoids from Locust and Its Bioactivity. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2008. [Google Scholar]
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and characterization of edible cricket peptides on hypertensive and glycemic in vitro inhibition and their anti-inflammatory activity on RAW 264.7 macrophage cells. Nutrients 2020, 12, 3588. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Wang, X.L. On the development trends of insect food in China. Food Res. Dev. 2003, 24, 88–90. [Google Scholar]
- Zhu, Z.; Bao, Y.M. Using fresh tussah pupae to produce high protein nutrient solution. Food Sci. 1995, 16, 45–47. [Google Scholar]
- Wei, M.C.; Liu, G.Q. The research and exploitation of insect protein. J. Cent. South Univ. For. Technol. 2001, 21, 86–90. [Google Scholar]
- Long, Z.Q.; Yang, Z.S. The nutritional characteristics of edible insects and their development and utilization. Sci. Technol. Innov. Her. 2011, 8, 230. [Google Scholar]
- Yang, Y.; Zhou, C.Z. Comprehensive utilization of silkworm pupae. Food Sci. 1993, 14, 31–33. [Google Scholar]
- Xiong, R.C.; Li, X.L.; Cheng, W.N. Edible insects in China. J. Northwest A & F Univ. Sci. Ed. 2003, 31, 179–182. [Google Scholar] [CrossRef]
- Han, D. The potential and challenges of insects as food and feed proteins. China Feed. 2020, 31, 125–128. [Google Scholar]
- Li, S.Y.; Bi, T.J. Preliminary exploration on ethnic insectivorous culture in Puer Yunan. J. Anhui Agric. Sci. 2012, 40, 1028–1030. [Google Scholar]
- Wang, R.; Shao, Y.X. Development, application and industrial development of edible insects in Yunnan Province. China Mark. 2019, 26, 27–28. [Google Scholar]
- Liu, Y.Q.; Zhang, X.; Du, L.L.; Wang, Y.R.; Liu, Y.B.; Li, Y. Resources and development prospect of edible insects in China. Food Res. Dev. 2023, 44, 206–211. [Google Scholar]
- Wang, Y.H. Study on the Insects of Hemiptera, Orthoptera and Odonata from Zushan Nature Reserve, Qinhuangdao. Master’s Thesis, Northwest A&F University, Xianyang, China, 2009. [Google Scholar]
- Wang, Q.; Wang, X.Y.; Wang, Z.; Li, Y.Q. Composition analysis and effect on lowering high blood fat of Tussah pupa oil and polyunsaturated fatty acid products. Acta Sericologica Sin. 2009, 35, 436–440. [Google Scholar]
- Wang, S.M.; Chen, Y.Q. Interactions and ecological consequences of interactions between ants and honeydew-producing homopteran. Chin. J. Appl. Entomol. 2011, 48, 183–190. [Google Scholar]
- Lan, Y.G. Eucalyptus seedling hazard: Large crickets and their control. Sci. Technol. Inf. 2008, 26, 133. [Google Scholar]
- Zhang, R. Effects of Host Plants on Enzymes of Oxya chinensis (Thunberg) and Its Susceptibility to Malathion. Master’s Thesis, Shanxi Agricultural University, Taiyuan, China, 2009. [Google Scholar]
- Fu, X.F.; Li, G.P.; Huang, J.X.; Cheng, J.H.; Lv, Y.L.; Li, Y.N.; Hu, F.G. Research advance in occurrence of major coffee insect pest, Xylotrechus quadripes. Acta Agric. Jiangxi 2020, 32, 50–56. [Google Scholar] [CrossRef]
- Chen, Z.M.; Guo, F.W.; Deng, Z.; Zhang, E.N.; Li, M. Host selection for feeding of Brontispa longissima (Gestro). Anhui Agric. Sci. 2018, 46, 141–142. [Google Scholar]
- Ji, W.X.; Chen, Z.; Li, H.M.; Lou, D.J.; Liu, X.B.; Dai, X.H.; Shi, H. Morphological and biological characteristics of Coridius chinensis (Hemiptera:Pentatomidae). J. Environ. Entomol. 2022, 44, 194–203. [Google Scholar]
- Gao, Y.; Han, Q.; Xu, B. Catalogue of host plants of Cicadella viridi L. (Hemiptera:Cicadellidae). Hubei Agric. Sci. 2015, 54, 3454–3459. [Google Scholar]
- Guo, T.D.; Qin, Y.J.; Li, Z.H. Advances in host selection and response of Tephritidae insects. Plant Prot. 2023, 49, 196–206. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.M. The nutritional elements analysis of bamboo insect and review on its development and utilization value. For. Res. 2000, 2, 188–191. [Google Scholar]
- Li, X.S.; Wu, Y.; Guo, T.K.; Zhao, S.W.; Mu, X.Q.; Chen, Z.L.; Zhao, N.; Teng, X.Y.; Dong, X.G.; Si, S.P. Check list of major oak pests in China (I). Sci. Seric. 2010, 36, 330–336. [Google Scholar]
- Lv, F. Preliminary Study of Intestinal Bacteriae of Clanis bilineata tsingtauica Mell. Master’s Thesis, Shandong Agricultural University, Taian, China, 2009. [Google Scholar]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental impact of the production of mealworms as a protein source for humans: A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed]
- Tasneem Abbasi, T.A.; Abbasi, S.A. Reducing the global environmental impact of livestock production: The minilivestock option. J. Clean. Prod. 2016, 112, 1754–1766. [Google Scholar] [CrossRef]
- Li, L.; He, J. Potential analysis of insect food industry. J. Anhui Agric. Sci. 2015, 43, 326–327+329. [Google Scholar]
- Tauseef, S.M.; Premalatha, M.; Abbasi, T.; Abbasi, S.A. Methane capture from livestock manure. J Environ. Manag. 2013, 117, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Araque, R.; Egas-Montenegro, E. Edible insects: A food alternative for the sustainable development of the planet. Int. J. Gastron. Food Sci. 2021, 23, 100304. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- Miiller, A.; Evans, J.; Payne, C.L.R.; Roberts, R. Entomophagy and power. J. Insects Food Feed. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Food, Energy and Society; Colorado University Press: Niwot, CO, USA, 1996. [Google Scholar]
- Pimentel, D. Canadian society of animal science. In Proceedings of the Canadian Society of Animal Science (Montreal), Montréal, QC, Canada, 24–26 July 1997. [Google Scholar]
- Pimentel, D.; Pimentel, M.H. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; Lin, J.L. Analysis of the edible value and future prospects of insect proteins. China Food Saf. Mag. 2020, 14, 93–94. [Google Scholar]
- Wang, L.; Hu, J.; Lv, Y.L. Research on market development strategies for edible insect products in China. Mark. Res. 2013, 61, 15–17. [Google Scholar]
- Zhu, F.; Shi, Z.H. Resource characteristics and development prospects of the proteins from edible insects. J. Chin. Inst. Food Sci. Technol. 2022, 22, 44–52. [Google Scholar]
- Hu, J.; Wang, L.; Lv, Y.L. Consumer cognition and attitudes towards edible insects products: Analysis based on 230 consumer surveys. Mark. Forum 2013, 35, 73–75. [Google Scholar]
- Zhou, H.Y.; Zhu, J.F.; Qi, J.J.; Li, C.J.; Chen, T.; Hu, B.; Hu, W.F. Safety analysis of edible and feeding insects. Guangdong Feed. 2021, 30, 27–31. [Google Scholar]
- Lähteenmäki-Uutela, A.; Repka, S.; Haukioja, T.; Pohjola, T. How to recognize and measure the economic impacts of environmental regulation: The sulphur emission control area case. J. Clean Prod. 2017, 154, 553–565. [Google Scholar] [CrossRef]
- Belluco, S.; Halloran, A.; Ricci, A. New protein sources and food legislation: The case of edible insects and EU law. Food Secur. 2017, 9, 803–814. [Google Scholar] [CrossRef]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Wang, J.; Tao, J.; Chu, M. Behind the label: Chinese consumers’ trust in food certification and the effect of perceived quality on purchase intention. Food Control 2020, 108, 106825. [Google Scholar] [CrossRef]
- Tanga, C.M.; Egonyu, J.P.; Beesigamukama, D.; Niassy, S.; Emily, K.; Magara, H.J.O.; Omuse, E.R.; Subramanian, S.; Ekesi, S. Edible insect farming as an emerging and profitable enterprise in east Africa. Curr. Opin. Insect Sci. 2021, 48, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Song, P.J.; Zhao, S.; He, Q.; Tang, S.Y.; Lv, X.Y.; Gou, M.X. Nutritional functional characteristics of insects and its application in food field. Meat Ind. 2022, 43, 48–52. [Google Scholar]
- Ran, W.B.; Zhao, C.X. Research progress on the development and utilization of proteins in edible insects. Jiangsu Agric. Sci. 2013, 41, 13–15. [Google Scholar]
| Scientific Name | Compounds | Type | Function Values | References |
|---|---|---|---|---|
| Isopterans | Interferon | Protein | Inhibit tumor formation | [149] |
| Bombyx mori | Pupal protein | Protein | Reduce the risk of cardiovascular diseases and cancer | [126] |
| Ericerus pela | Phosphatide | Lipid | Invigorate brain functions, lower blood lipids, cholesterol removal, treatment of fatty liver, cirrhosis and anti-aging. | [132,151] |
| Apis mellifera carnica | Trehalose | Polysaccharide | Enhance body immunity and anti-radiation | [46] |
| Dendrolimus houi | Chitin | Polysaccharide | Health care functions due to dietary fiber and anti-thrombotic | [17,151] |
| Hepiaua larva and Bombyx mori | Cordyceps polysaccharide | Polysaccharide | Antioxidant and immune regulation, anti-tumor activities, protect kidney and liver, reduce blood glucose and lipids, anti-radiation, anti-aging. | [143,144] |
| Hyalophora cecropia and Sarcophaga percgrina | Antibacterial peptide | Peptide | Inhibit tumor formation | [150] |
| Gryllodes sigillatus | Anti-inflammatory peptide | Peptide | Angiotensin-converting enzyme inhibitor | [153] |
| Oxya chinensis | Flavonoid extracts | Flavonoid | Hypolipidemic, anti-fatigue and antioxidation functions | [152] |
| Insect Order | Scientific Name | Special Cuisine | Edible Stage | Ethnic Minority | Host Plants | Distribution Regions | References |
|---|---|---|---|---|---|---|---|
| Megaloptera | Corydalus cornutus | Soft-fried hellgrammites | Adult, larva | Bouyei | Rice (Oryza sativa) plants (Megalopterans larvae feed on Nilaparvata lugens, Aphidoidea, Odontota scapularis) | All over Yunnan | [164] |
| Hymenoptera | Apis cerana, Vespa sp., Polistes sp., Provespa sp. | Deep-fried pupae | Larva, pupa | Basically all | Cotton (Gossypium hirsutum) plants (Apis sp. are generalist herbivores while Vespa sp., Polistes sp. and Provespa sp. are predatory wasps) | Xishuangbanna, Dehong | [161] |
| Pupa paste | Dai | ||||||
| Dai-taste pupae | |||||||
| Crunchy wasps | Zhuang | ||||||
| Orthoptera | Oecophylla sp. | Ant eggs salad | Spawn | Dai | Dalbergia hupeana, Ficus carica, Vachellia farnesiana | Lincang, Pu ‘er, Xishuangbanna | [165,166] |
| Ant egg pastry | Va | ||||||
| Fried flying ants | Miao | ||||||
| Acid ant vinegar | Larva | Dai, Jingpo, Miao, Jinuo | |||||
| Gryllus bimaculatus | Cricket jam | Adult | Dai | Glycine max, Oryza sativaZea mays, Setaria italica | All over Yunnan | [167] | |
| Fried cricket | |||||||
| Locusta migratoria, Oxya chinensis | Fried locust | Adult, larva | Yi | Triticum aestivum, Oryza sativa, Zea mays, Sorghum bicolor | All over Yunnan | [168] | |
| Grasshopper jam | Hani | ||||||
| Coleoptera | Xylotrechus quadripes | Fried bamboo worm | Larva | Va | Coffea arabica, Lannea A., Tectona grandis | Xishuangbanna, Pu’er, Lincang, Dehong, Baoshan, Wenshan | [169] |
| Brontispa longissima | Fried coconut worm | Adult | Dai | Cocos nucifera, Phoenix canariensis, Washingtonia filifera, Phoenix roebelenii | East-south regions | [170] | |
| Hemiptera | Aspongopus sp. | Deep-fried aspongopus | Adult | Dai | Cucurbits | Honghe, Baoshan, Wenshan | [171] |
| Cryptotympana atrata | Fermented cicada | Nymph | Dai | Sophora japonica, Salix babylonica | Xishuangbanna, Dehong | [172] | |
| Cicada pupa cake | |||||||
| Cicadas chop raw | |||||||
| Cicada meat balls | |||||||
| Diptera | Musca domestica | Fried granulation | Larva | Hani | Doesn’t have a specific host plants as it is a scavenger | Dehong, Xishuangbanna basin valley | [173] |
| Lepidoptera | Omphisa fuscidentalis | Fried bamboo worm | Larva | Dai, Hani, Jino, Zhuang, Buyi | Bambusoideae | Xishuangbanna, Dehong, Pu ‘er | [174] |
| Antherea pernyi | Crispy silkworm pupa | Pupa | Yi | Quercus sp. | Qujing, Dehong, Zhaotong, Pu ‘er | [175] | |
| Clanis bilineata | Spiced bean worm | Larva | Zhuang | Oryza sativa, Vigna radiata, Vigna unguiculata, Robinia pseudoacacia | All over Yunnan | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, B.; Zhu, Y.; Chu, X.; Pokharel, S.S.; Qian, L.; Chen, F. Research Progress and Production Status of Edible Insects as Food in China. Foods 2024, 13, 1986. https://doi.org/10.3390/foods13131986
Xie B, Zhu Y, Chu X, Pokharel SS, Qian L, Chen F. Research Progress and Production Status of Edible Insects as Food in China. Foods. 2024; 13(13):1986. https://doi.org/10.3390/foods13131986
Chicago/Turabian StyleXie, Boxuan, Yuxuan Zhu, Xiaoyi Chu, Sabin Saurav Pokharel, Lei Qian, and Fajun Chen. 2024. "Research Progress and Production Status of Edible Insects as Food in China" Foods 13, no. 13: 1986. https://doi.org/10.3390/foods13131986
APA StyleXie, B., Zhu, Y., Chu, X., Pokharel, S. S., Qian, L., & Chen, F. (2024). Research Progress and Production Status of Edible Insects as Food in China. Foods, 13(13), 1986. https://doi.org/10.3390/foods13131986









