Isolation, Characterization and IgE Binding of Two 2S Albumins of Pomegranate Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pomegranate Seed Extract Preparation
2.2.2. Purification of Two 2S Albumins (Pun g 2S-A1 and Pun g 2S-A2) from a Seed Extract
2.2.3. Analysis via SDS−PAGE
2.2.4. Amino Acid Sequencing
2.2.5. Mass Spectrometry Experiments
2.2.6. IgE Immunoblotting Analysis
2.2.7. Specific IgE Detection Using the FABER® Multiplex Testing System
2.2.8. Patients
2.2.9. Bioinformatic Investigations
3. Results
3.1. Analysis via Immunoblotting of Pomegranate Seed Extract
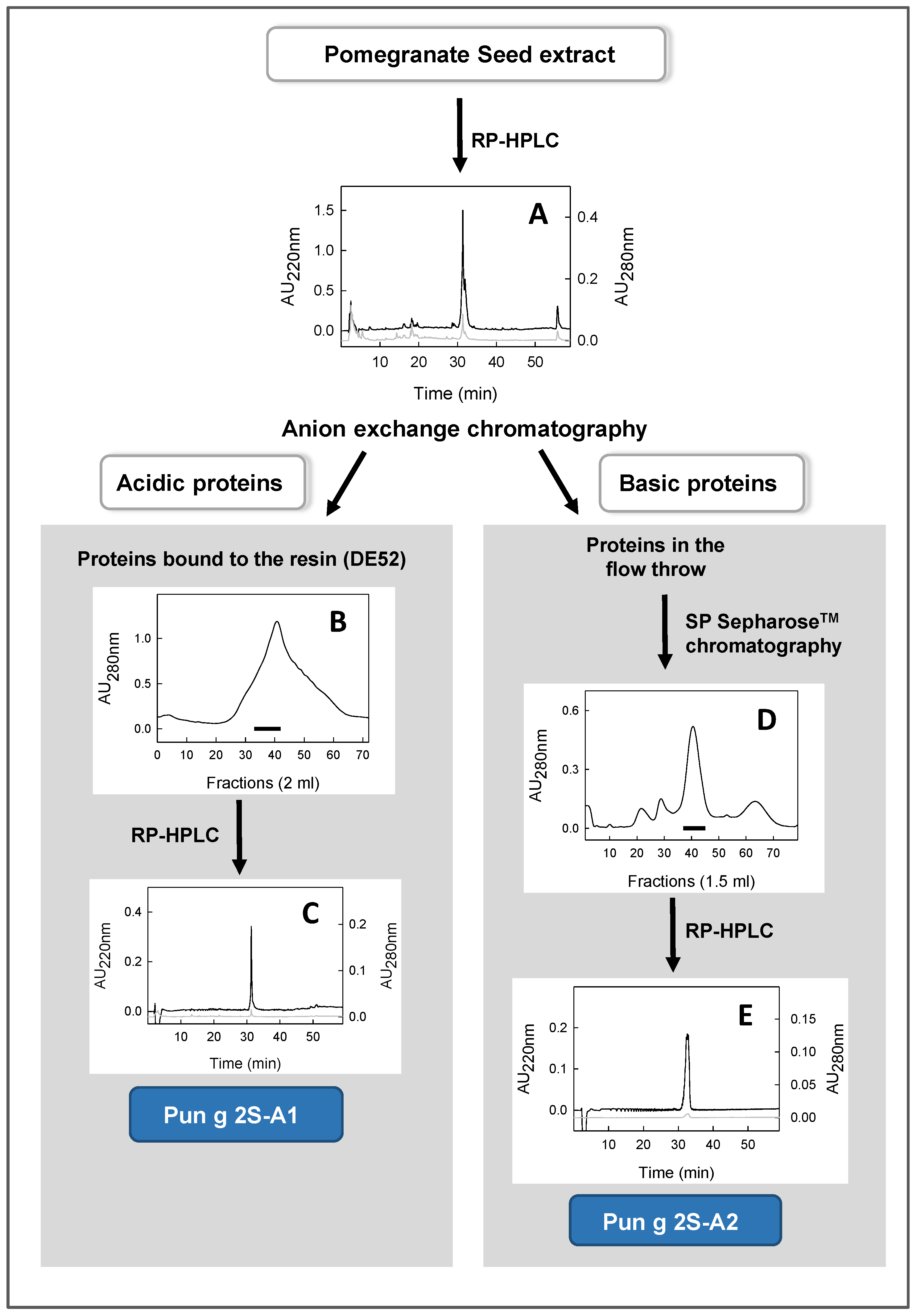
3.2. Isolation of Two Proteins Contained in The Seed Extract
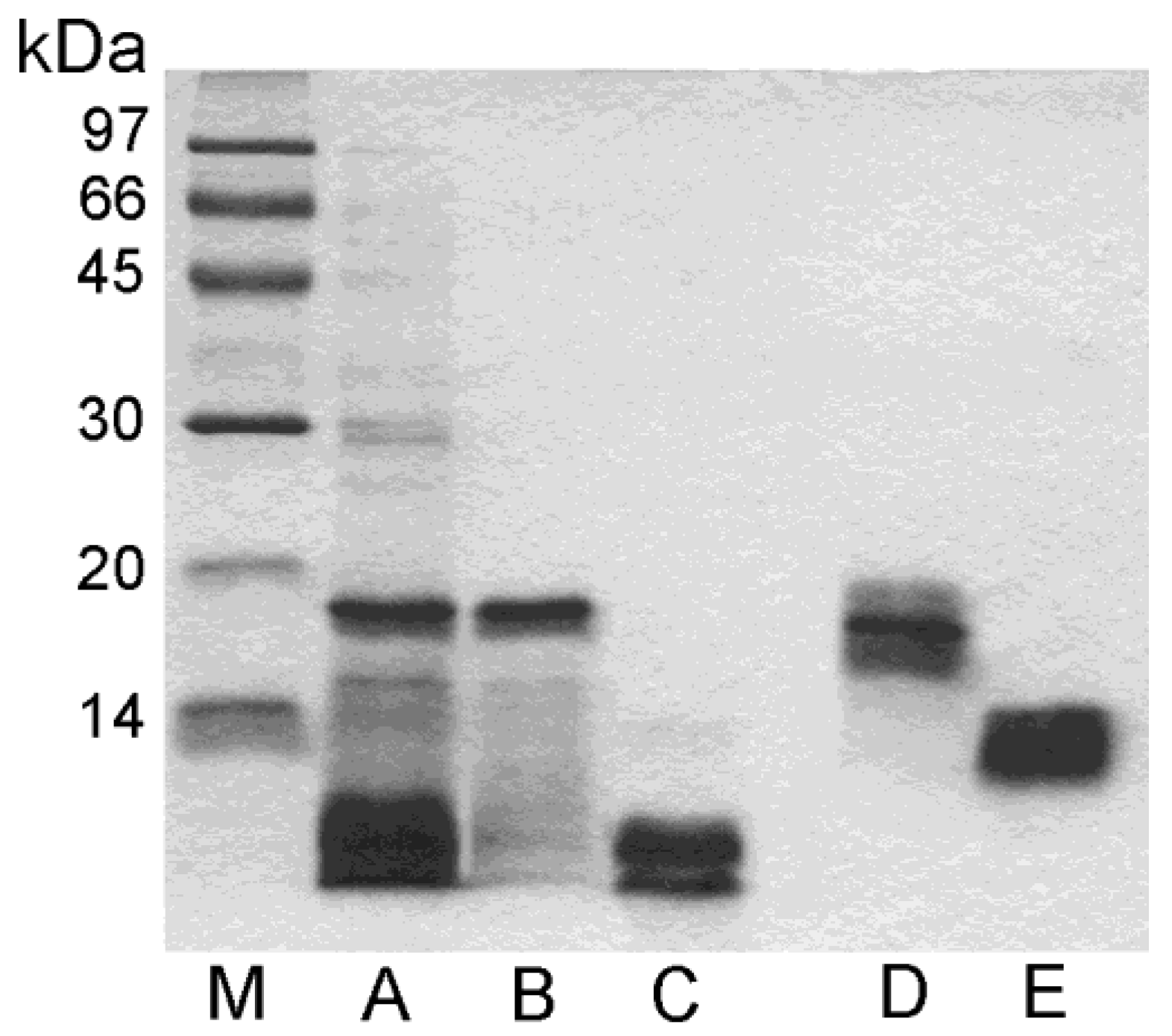
3.3. SDS-PAGE Analysis of Purified Proteins
3.4. Identification of the Isolated Proteins as 2S Albumins
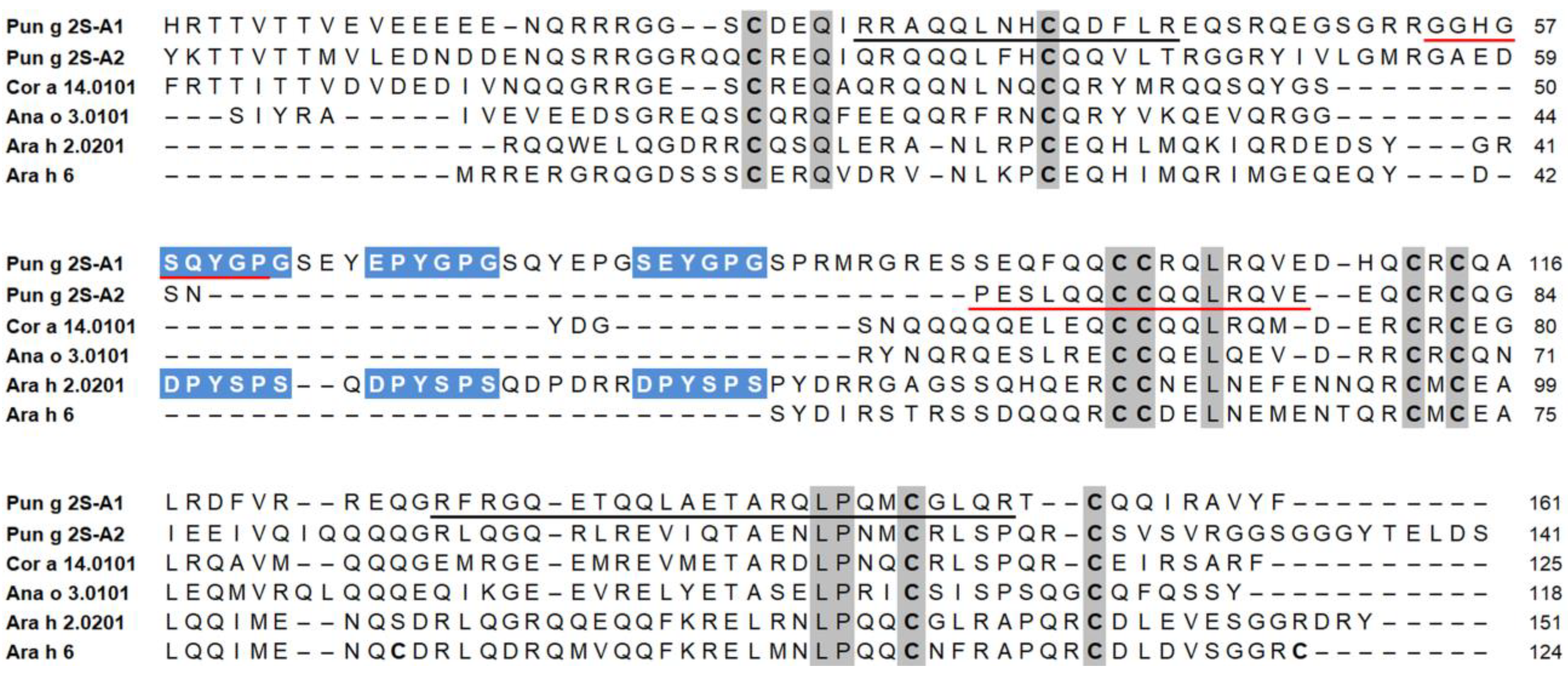
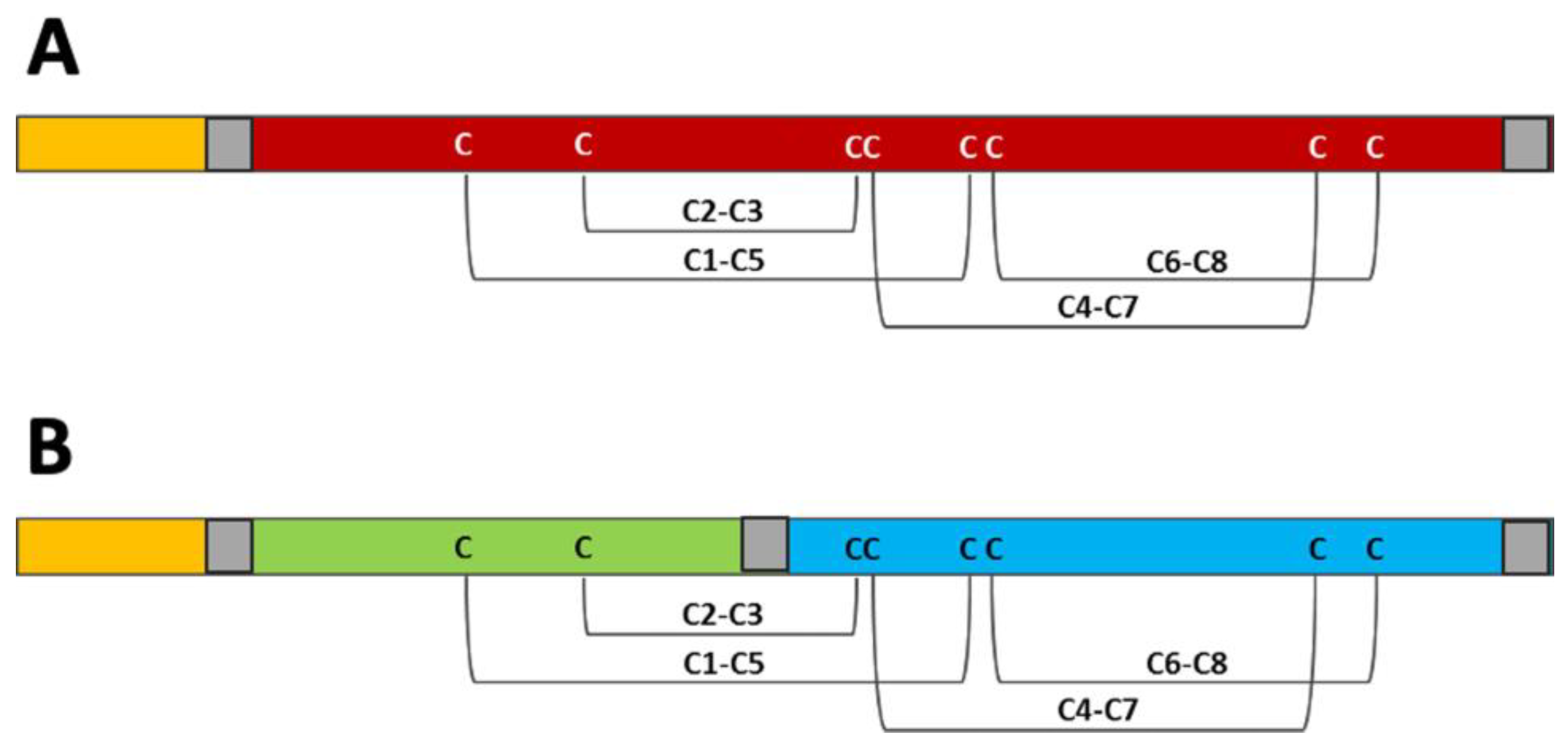
3.5. Structural Investigation of Isolated Pomegranate 2S Albumins in Comparison with Allergenic Homologs from Other Sources
3.6. Comparative Structural Investigations of Pomegranate 2S Albumins Found in UniProt Database
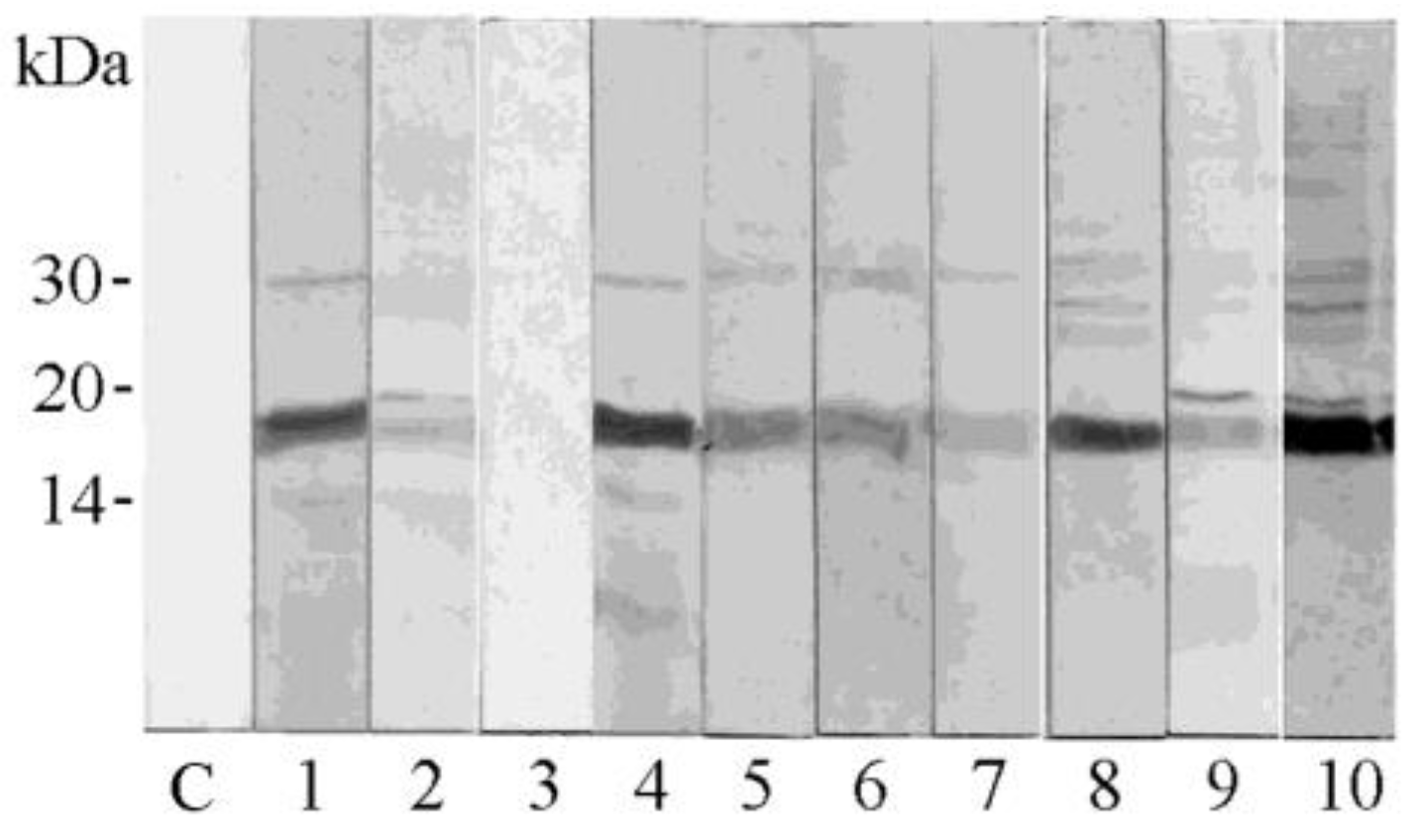
3.7. IgE Binding Detection and Sensitization Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stover, E.; Mercure, E.W. The Pomegranate: A New Look at the Fruit of Paradise. HortScience 2007, 42, 1088–1092. [Google Scholar] [CrossRef]
- Danesi, F.; Ferguson, L.R. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer Activity of Punica granatum (Pomegranate): A Review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Martinez-Perez, M.M.; Calderon-Ramos, Z.G.; Belefant-Miller, H.; Cancino-Diaz, J.C. Pomegranate juice increases levels of paraoxonasel (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res. Int. 2011, 44, 1381–1385. [Google Scholar] [CrossRef]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegrante (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Spilmont, M.; Léotoing, L.; Davicco, M.J.; Lebecque, P.; Mercier, S.; Miot-Noirault, E.; Pilet, P.; Rios, L.; Wittrant, Y.; Coxam, V. Pomegranate and its derivatives can improve bone health through decreased inflammation and oxidative stress in an animal model of postmenopausal osteoporosis. Eur. J. Nutr. 2014, 53, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; D’Souza, D.H. The pomegranate: Effects on bacteria and viruses that influence human health. Evid. Based Complement. Alternat. Med. 2013, 2013, 606212. [Google Scholar] [CrossRef] [PubMed]
- Haghayeghi, K.; Shetty, K.; Labbé, R. Inhibition of foodborne pathogens by pomegranate juice. J. Med. Food 2013, 16, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Fraschetti, C.; Goci, E.; Nicolescu, A.; Cairone, F.; Carradori, S.; Filippi, A.; Palmieri, V.; Mocan, A.; Cesa, S. Pomegranate Fruit Cracking during Maturation: From Waste to Valuable Fruits. Foods 2023, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.; Zabini, F.; Tagliavento, L.; Meneguzzo, F.; Calderone, V.; Testai, L. An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate. Antioxidants 2023, 12, 1351. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Nikolaou, P.E.; Mitakou, S.; Skaltsounis, A.L. Exploitation of Vitis vinifera, Foeniculum vulgare, Cannabis sativa and Punica granatum By-Product Seeds as Dermo-Cosmetic Agents. Molecules 2021, 26, 731. [Google Scholar] [CrossRef] [PubMed]
- Iriti, G.; Bonacci, S.; Lopreiato, V.; Frisina, M.; Oliverio, M.; Procopio, A. Functional Compounds of Cold-Pressed Pomegranate Seed Oil: Fatty Acids and Phytosterols Profile as Quality Biomarkers for Origin Discrimination. Foods 2023, 12, 2599. [Google Scholar] [CrossRef] [PubMed]
- Cairone, F.; Salvitti, C.; Iazzetti, A.; Fabrizi, G.; Troiani, A.; Pepi, F.; Cesa, S. In-Depth Chemical Characterization of Punica granatum L. Seed Oil. Foods 2023, 12, 1592. [Google Scholar] [CrossRef] [PubMed]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Quality and Antioxidant Properties of Cold-Pressed Oil from Blanched and Microwave-Pretreated Pomegranate Seed. Foods 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Igea, J.M.; Cuesta, J.; Cuevas, M.; Elias, L.M.; Marcos, C.; Lazaro, M.; Compaired, J.A. Adverse reaction to pomegranate ingestion. Allergy 1991, 46, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Gaig, P.; Botey, J.; Gutiérrez, V.; Pena, M.; Eseverri, J.L.; Marín, A. Allergy to pomegranate (Punica granatum). J. Investig. Allergol. Clin. Immunol. 1992, 2, 216–218. Available online: https://pubmed.ncbi.nlm.nih.gov/1342903/ (accessed on 30 May 2024). [CrossRef] [PubMed]
- Gaig, P.; Bartolomé, B.; Lleonart, R.; García-Ortega, P.; Palacios, R.; Richart, C. Allergy to pomegranate (Punica granatum). Allergy 1999, 54, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Zoccatelli, G.; Dalla Pellegrina, C.; Consolini, M.; Fusi, M.; Sforza, S.; Aquino, G.; Dossena, A.; Chignola, R.; Peruffo, A.; Olivieri, M.; et al. Isolation and identification of two lipid transfer proteins in pomegranate (Punica granatum). J. Agric. Food Chem. 2007, 55, 11057–11062. [Google Scholar] [CrossRef]
- Tuppo, L.; Alessandri, C.; Pasquariello, M.S.; Petriccione, M.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Pomegranate Cultivars: Identification of the New IgE-Binding Protein Pommaclein and Analysis of Antioxidant Variability. J. Agric. Food Chem. 2017, 65, 2702–2710. [Google Scholar] [CrossRef]
- Tuppo, L.; Giangrieco, I.; Alessandri, C.; Ricciardi, T.; Rafaiani, C.; Ciancamerla, M.; Ferrara, R.; Zennaro, D.; Bernardi, M.L.; Tamburrini, M.; et al. Pomegranate chitinase III: Identification of a new allergen and analysis of sensitization patterns to chitinases. Mol. Immunol. 2018, 103, 89–95. [Google Scholar] [CrossRef]
- Bolla, M.; Zenoni, S.; Scheurer, S.; Vieths, S.; San Miguel Moncin, M.d.M.; Olivieri, M.; Antico, A.; Ferrer, M.; Berroa, F.; Enrique, E.; et al. Pomegranate (Punica granatum L.) expresses several nsLTP isoforms characterized by different immunoglobulin E-binding properties. Int. Arch. Allergy Immunol. 2014, 164, 112–121. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, A.; Pote, S.; Giangrieco, I.; Tuppo, L.; Gawlicka-Chruszcz, A.; Kowal, K.; Ciardiello, M.A.; Chruszcz, M. Structural Characterization of Act c 10.0101 and Pun g 1.0101—Allergens from the Non-Specific Lipid Transfer Protein Family. Molecules 2021, 26, 256. [Google Scholar] [CrossRef] [PubMed]
- Nahirñak, V.; Almasia, N.I.; Hopp, H.E.; Vazquez-Rovere, C. Snakin/GASA proteins: Involvement in hormone crosstalk and redox homeostasis. Plant Signal Behav. 2012, 7, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, T.; Masuda, T.; Lv, C.; Sun, L.; Qu, G.; Zhao, G. Chitinase III in pomegranate seeds (Punica granatum L.): A high-capacity calcium-binding protein in amyloplasts. Plant J. 2011, 68, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Baker, M.G. Pomegranate seed allergy in a child with multiple tree nut allergies. Ann. Allergy Asthma Immunol. 2023, 130, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.C.; Yates, J.R. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef]
- Bernardi, M.L.; Picone, D.; Tuppo, L.; Giangrieco, I.; Petrella, G.; Palazzo, P.; Ferrara, R.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Physico-chemical features of the environment affect the protein conformation and the immunoglobulin E reactivity of kiwellin (Act d 5). Clin. Exp. Allergy 2010, 40, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Ferrara, R.; Bernardi, M.L.; Zennaro, D.; Tuppo, L.; Giangrieco, I.; Ricciardi, T.; Tamburrini, M.; Ciardiello, M.A.; Mari, A. Molecular approach to a patient’s tailored diagnosis of the oral allergy syndrome. Clin. Transl. Allergy 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Giangrieco, I.; Ricciardi, T.; Alessandri, C.; Farina, L.; Crescenzo, R.; Tuppo, L.; Ciancamerla, M.; Rafaiani, C.; Bernardi, M.L.; Digilio, A.F.; et al. ENEA, a peach and apricot IgE-binding protein cross-reacting with the latex major allergen Hev b 5. Mol. Immunol. 2019, 112, 347–357. [Google Scholar] [CrossRef]
- Ricciardi, T.; Giangrieco, I.; Alessandri, C.; Rafaiani, C.; Tuppo, L.; Tamburrini, M.; Digilio, A.F.; Ciardiello, M.A.; Mari, A. Pattern of sensitization to Juniperus oxycedrus 4EF-hand polcalcin, Jun o 4, compared with the 2EF-hand grass homolog Phl p 7 in a general Italian population of subjects suffering from pollinosis. Clin. Immunol. 2022, 234, 108894. [Google Scholar] [CrossRef]
- Souza, P.F.N. The forgotten 2S albumin proteins: Importance, structure, and biotechnological application in agriculture and human health. Int. J. Biol. Macromol. 2020, 164, 4638–4649. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; de Jong, G.A.; Laaper-Ertmann, M.; Peeters, K.A.; Knulst, A.C.; Hefle, S.L.; Knol, E.F. Purification and immunoglobulin E-binding properties of peanut allergen Ara h 6: Evidence for cross-reactivity with Ara h 2. Clin. Exp. Allergy 2005, 35, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Spiric, J.; Koppelman, S.J.; Knulst, A.; Nordlee, J.A.; Taylor, S.L.; Joseph, L.; Baumert, J.L. In vitro digestion and characterisation of 2S albumin and digestion-resistant peptides in pecan. Int. J. Food Sci. Technol. 2018, 53, 1566–1578. [Google Scholar] [CrossRef]
- Nesbit, J.B.; Schein, C.H.; Braun, B.A.; Gipson, S.A.Y.; Cheng, H.; Hurlburt, B.K.; Maleki, S.J. Epitopes with similar physicochemical properties contribute to cross reactivity between peanut and tree nuts. Mol. Immunol. 2020, 122, 223–231. [Google Scholar] [CrossRef]
- Moreno, F.J.; Clemente, A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem. J. 2008, 2, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Nucera, E.; Rizzi, A.; Aruanno, A.; Uasuf, C.G.; Manzotti, G.; Villalta, D.; Conte, M.; Pastorello, E.A.; Losappio, L.; et al. Peanut allergy in Italy: A unique Italian perspective. J. Allergy Clin. Immunol. Glob. 2022, 1, 61–66. [Google Scholar] [CrossRef]
- Asero, R.; Piantanida, M.; Pinter, E.; Pravettoni, V. The clinical relevance of lipid transfer protein. Clin. Exp. Allergy 2018, 48, 6–12. [Google Scholar] [CrossRef]
- Romano, A.; Scala, E.; Rumi, G.; Gaeta, F.; Caruso, C.; Alonzi, C.; Maggioletti, M.; Ferrara, R.; Palazzo, P.; Palmieri, V.; et al. Lipid transfer proteins: The most frequent sensitizer in Italian subjects with food-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2012, 42, 1643–1653. [Google Scholar] [CrossRef]
- Vereda, A.; van Hage, M.; Ahlstedt, S.; Ibañez, M.D.; Cuesta-Herranz, J.; van Odijk, J.; Wickman, M.; Sampson, H.A. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J. Allergy Clin. Immunol. 2011, 127, 603–607. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S. The prolamin storage proteins of cereal seeds: Structure and evolution. Biochem. J. 1990, 267, 1–12. [Google Scholar] [CrossRef]
- Maciel, F.M.; Laberty, M.A.; Oliveira, N.D.; Felix, S.P.; Soares, A.M.; Verícimo, M.A.; Machado, O.L. A new 2S albumin from Jatropha curcas L. seeds and assessment of its allergenic properties. Peptides 2009, 30, 2103–2107. [Google Scholar] [CrossRef]
- Youle, R.J.; Huang, A.H. Albumin storage proteins in the protein bodies of castor bean. Plant Physiol. 1978, 61, 13–16. [Google Scholar] [CrossRef]
- Freire, J.E.C.; Moreno, F.B.M.B.; Monteiro-Júnior, J.E.; Sousa, A.J.S.; Vasconcelos, I.M.; Oliveira, J.T.A.; Monteiro-Moreira, A.C.O.; Rocha, B.A.M.; Grangeiro, T.B. Mo-CBP, a 2S albumin from Moringa oleifera, is a complex mixture of isoforms that arise from different post-translational modifications. Plant Physiol. Biochem. 2019, 140, 68–77. [Google Scholar] [CrossRef]
- Li, X.; Franceschi, V.R.; Okita, T.W. Segregation of storage protein mRNAs on the rough endoplasmic reticulum membranes of rice endosperm cells. Cell 1993, 72, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Sharief, F.S.; Li, S.S. Amino acid sequence of small and large subunits of seed storage protein from Ricinus communis. J. Biol. Chem. 1982, 257, 24. Available online: https://pubmed.ncbi.nlm.nih.gov/7174664/ (accessed on 28 September 2023). [CrossRef]
- Kortt, A.A.; Caldwell, B.C.; Lilley, G.G.; Higgins, T.J.V. Amino acid and cDNA sequences of a methionine-rich 2s protein from sunflower seed (Helianthus annuus L.). Eur. J. Biochem. 1991, 195, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Mylne, J.S.; Hara-Nishimura, I.; Rosengren, K.J. Seed storage albumins: Biosynthesis, trafficking and structures. Funct. Plant Biol. 2014, 41, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Otegui, M.S.; Herder, R.; Schulze, J.; Jung, R.; Staehelin, L.A. The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 2006, 18, 2567–2581. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.T.; Leu, W.M.; Yang, H.H.; Chen, B.C.M.; Tzen, J.T.C. Sesame oleosin and prepro-2S albumin expressed as a fusion polypeptide in transgenic rice were split, processed and separately assembled into oil bodies and protein bodies. J. Cereal Sci. 2006, 44, 333–341. [Google Scholar] [CrossRef]
- D’Hondt, K.; Bosch, D.; Van Damme, J.; Goethals, M.; Vandekerckhove, J.; Krebbers, E. An aspartic proteinase present in seeds cleaves Arabidopsis 2S albumin precursors in vitro. J. Biol. Chem. 1993, 268, 20884–20891. Available online: https://pubmed.ncbi.nlm.nih.gov/8407921/ (accessed on 28 September 2023). [CrossRef]
- Pandya, M.J.; Sessions, R.B.; Williams, P.B.; Dempsey, C.E.; Tatham, A.S.; Shewry, P.R.; Clarke, A.R. Structural characterization of a methionine-rich, emulsifying protein from sunflower seed. Proteins 2000, 38, 341–349. Available online: https://pubmed.ncbi.nlm.nih.gov/10713993/ (accessed on 15 January 2018). [CrossRef]
- Moreno, F.J.; Jenkins, J.A.; Mellon, F.A.; Rigby, N.M.; Robertson, J.A.; Wellner, N.; Clare Mills, E.N. Mass spectrometry and structural characterization of 2S albumin isoforms from Brazil nuts (Bertholletia excelsa). Biochim. Biophys. Acta 2004, 1698, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, P.M.; Krzyzaniak, A.; Barciszewski, J.; Biemann, K. Mass spectrometric amino acid sequencing of a mixture of seed storage proteins (napin) from Brassica napus, products of a multigene family. Proc. Natl. Acad. Sci. USA 1996, 93, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Huang, A.H. Evidence that the castor bean allergens are the albumin storage proteins in the protein bodies of castor bean. Plant Physiol. 1978, 61, 1040–1042. [Google Scholar] [CrossRef] [PubMed]
- Giangrieco, I.; Ciardiello, M.A.; Tamburrini, M.; Tuppo, L.; Rafaiani, C.; Mari, A.; Alessandri, C. Comparative Analysis of the Immune Response and the Clinical Allergic Reaction to Papain-like Cysteine Proteases from Fig, Kiwifruit, Papaya, Pineapple and Mites in an Italian Population. Foods 2023, 12, 2852. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Uceda, D.; Bruix, M.; Santoro, J.; Rico, M.; Monsalve, R.; Villalba, M. Solution structure of allergenic 2 S albumins. Biochem. Soc. Trans. 2002, 30, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Uceda, D.; Shewry, P.R.; Bruix, M.; Tatham, A.S.; Santoro, J.; Rico, M. Solution structure of a methionine-rich 2S albumin from sunflower seeds: Relationship to its allergenic and emulsifying properties. Biochemistry 2004, 43, 6976–6986. [Google Scholar] [CrossRef] [PubMed]
- Üzülmez, Ö.; Kalic, T.; Mayr, V.; Lengger, N.; Tscheppe, A.; Radauer, C.; Hafner, C.; Hemmer, W.; Breiteneder, H. The Major Peanut Allergen Ara h 2 Produced in Nicotiana benthamiana Contains Hydroxyprolines and Is a Viable Alternative to the E. Coli Product in Allergy Diagnosis. Front. Plant Sci. 2021, 12, 723363. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.S.; King, N.; Burks, A.W.; Huang, S.K.; Sampson, H.; Cockrell, G.; Helm, R.M.; West, C.M.; Bannon, G.A. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch. Biochem. Biophys. 1997, 342, 244–253. [Google Scholar] [CrossRef]
- Li, J.; Shefcheck, K.; Callahan, J.; Fenselau, C. Primary sequence and site-selective hydroxylation of prolines in isoforms of a major peanut allergen protein Ara h 2. Protein Sci. 2010, 19, 174–182. [Google Scholar] [CrossRef]
- Blazowski, L.; Majak, P.; Kurzawa, R.; Kuna, P.; Jerzynska, J. Food allergy endotype with high risk of severe anaphylaxis in children-Monosensitization to cashew 2S albumin Ana o 3. Allergy 2019, 74, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.; Chen, X.; Lew, J.; Wang, Q.; Patel, O.P.; Zhuang, Y.; Murray, K.S.; Duncan, M.W.; Porterfield, H.S.; Burks, A.W.; et al. The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin. Exp. Allergy 2012, 42, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, S.; Bublin, M.; Dubiela, P.; Hummel, K.; Wortmann, J.; Hofer, G.; Keller, W.; Radauer, C.; Hoffmann-Sommergruber, K. Cor a 14, the allergenic 2S albumin from hazelnut, is highly thermostable and resistant to gastrointestinal digestion. Mol. Nutr. Food Res. 2015, 59, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Robotham, J.M.; Wang, F.; Seamon, V.; Teuber, S.S.; Sathe, S.K.; Sampson, H.A.; Beyer, K.; Seavy, M.; Roux, K.H. Ana o 3, an important cashew nut (Anacardium occidentale L.) allergen of the 2S albumin family. J. Allergy Clin. Immunol. 2005, 115, 1284–1290. [Google Scholar] [CrossRef]
- Hazebrouck, S.; Patil, S.U.; Guillon, B.; Lahood, N.; Dreskin, S.C.; Adel-Patient, K.; Bernard, H. Immunodominant conformational and linear IgE epitopes lie in a single segment of Ara h 2. J. Allergy Clin. Immunol. 2022, 150, 131–139. [Google Scholar] [CrossRef]
| Date | Mr (kDa) | Tissue | Allergen | Bibliography |
|---|---|---|---|---|
| 1999 | 29 | pulp | Not identified | [17] |
| 2007 | 9 | pulp | Pun g 1 | [18] |
| 2017 | 7 | pulp | Pun g 7 | [19] |
| 2018 | 29 | Pulp and seeds | Pun g 14 | [20] |
| Protein/Allergen Name | Protein Accession Number (in UniProt) | Theoretical a Mr (Da) | Experimental Mr (Da) b | Theoretical pI a |
|---|---|---|---|---|
| Pun g 2S-A1 | A0A218XU94 | 18,716.43 | 16,398.67 | 7.74 |
| Pun g 2S-A2 | A0A2I0JHZ1 | 16,116.97 | 12,350.50 | 6.77 |
| Cor a 14.0101 | D0PWG2 | 14,916.49 | N/A | 5.61 |
| Ana o 3.0101 | Q8H2B8 | 14,234.74 | N/A | 5.37 |
| Ara h 2.0201 | Q6PSU2 | 17,993.59 | N/A | 5.51 |
| Ara h 6 | A5Z1R0 | 14,845.61 | N/A | 5.49 |
| Pun g 2S-A1 | Pun g 2S-A2 | Cor a 14.0101 | Ana o 3.0101 | Ara h 2.0201 | Ara h 6 | |
|---|---|---|---|---|---|---|
| Pun g 2S-A1 | 100 | |||||
| Pun g 2S-A2 | 46.03 | 100 | ||||
| Cor a 14.0101 | 45.16 | 44.80 | 100 | |||
| Ana o 3.0101 | 31.30 | 36.84 | 41.23 | 100 | ||
| Ara h 2.0201 | 28.17 | 28.81 | 28.83 | 21.93 | 100 | |
| Ara h 6 | 26.05 | 28.70 | 33.33 | 22.52 | 60.66 | 100 |
| Patients | FABER® 244 (FIU) a | ||||||
|---|---|---|---|---|---|---|---|
| N° | Gender | Pun g 2S-A1 | Pun g 2S-A2 | Cor a 14 | Ana o 3 | Ara h 2 | Ara h 6 |
| 1 | F | 0.5 | 0 | 0 | 0 | 0 | 0 |
| 2 | F | 1.0 | 0 | 0 | 0 | 0 | 0 |
| 3 | F | 0.9 | 0 | 0 | 0 | 0 | 0 |
| 4 | F | 0.7 | 0 | 0 | 0 | 0 | 0 |
| 5 | F | 0.5 | 0 | 0 | 0 | 0 | 0 |
| 6 | F | 4.3 | 0 | 0 | 0 | 0 | 0 |
| 7 | M | 0.2 | 0 | 0 | 0 | 0 | 0 |
| 8 | M | 0.5 | 0 | 0 | 0 | 0 | 0 |
| 9 | M | 1.3 | 0 | 0 | 0 | 0 | 0 |
| 10 | M | 0.5 | 0 | 0 | 0 | 0 | 0 |
| 11 | M | 14.3 | 0 | 0 | 0 | 0 | 14.3 |
| 12 | F | 2.6 | 2.4 | 3.8 | 1.8 | 2.0 | 1.3 |
| 13 | F | 0 | 0.7 | 0 | 0 | 0 | 0 |
| 14 | F | 0 | 0 | 1.2 | 0 | 0 | 0 |
| 15 | M | 0 | 0 | 0 | 1.4 | 0 | 0 |
| 16 | F | 0 | 0 | 0 | 3.8 | 0 | 0 |
| 17 | M | 0 | 0 | 0 | 2.6 | 0 | 0 |
| 18 | M | 0 | 0 | 67.0 | 27.8 | 91.0 | 22.9 |
| 19 | M | 0 | 0 | 0 | 0 | 4.3 | 0 |
| 20 | F | 0 | 0 | 0 | 0 | 1.6 | 0 |
| 21 | F | 0 | 0 | 0 | 0 | 0.4 | 0 |
| 22 | F | 0 | 0 | 0 | 0 | 0 | 1.0 |
| 23 | F | 0 | 0 | 0 | 0 | 0 | 0.4 |
| 24 | F | 0 | 0 | 1.2 | 0 | 0 | 0 |
| 25 | F | 0 | 0 | 8.9 | 0 | 0 | 0 |
| 26 | F | 0 | 0 | 0.2 | 0 | 0 | 0 |
| 27 | M | 0 | 0 | 5.5 | 0 | 0 | 0 |
| 28 | M | 0 | 0 | 3.9 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuppo, L.; Alessandri, C.; Zaccaro, L.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Isolation, Characterization and IgE Binding of Two 2S Albumins of Pomegranate Seeds. Foods 2024, 13, 1965. https://doi.org/10.3390/foods13131965
Tuppo L, Alessandri C, Zaccaro L, Giangrieco I, Tamburrini M, Mari A, Ciardiello MA. Isolation, Characterization and IgE Binding of Two 2S Albumins of Pomegranate Seeds. Foods. 2024; 13(13):1965. https://doi.org/10.3390/foods13131965
Chicago/Turabian StyleTuppo, Lisa, Claudia Alessandri, Laura Zaccaro, Ivana Giangrieco, Maurizio Tamburrini, Adriano Mari, and Maria Antonietta Ciardiello. 2024. "Isolation, Characterization and IgE Binding of Two 2S Albumins of Pomegranate Seeds" Foods 13, no. 13: 1965. https://doi.org/10.3390/foods13131965
APA StyleTuppo, L., Alessandri, C., Zaccaro, L., Giangrieco, I., Tamburrini, M., Mari, A., & Ciardiello, M. A. (2024). Isolation, Characterization and IgE Binding of Two 2S Albumins of Pomegranate Seeds. Foods, 13(13), 1965. https://doi.org/10.3390/foods13131965








