Prunin Laurate Derived from Natural Substances Shows Antibacterial Activity against the Periodontal Pathogen Porphyromonas gingivalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Prunin Laurate and Its Analogues
2.3. Determination of Minimum Inhibitory Concentration (MIC)
2.4. Inhibition of Biofilm Formation
2.5. Cytotoxicity Test
2.6. Animal Experiments
2.7. Statistical Analysis
3. Results
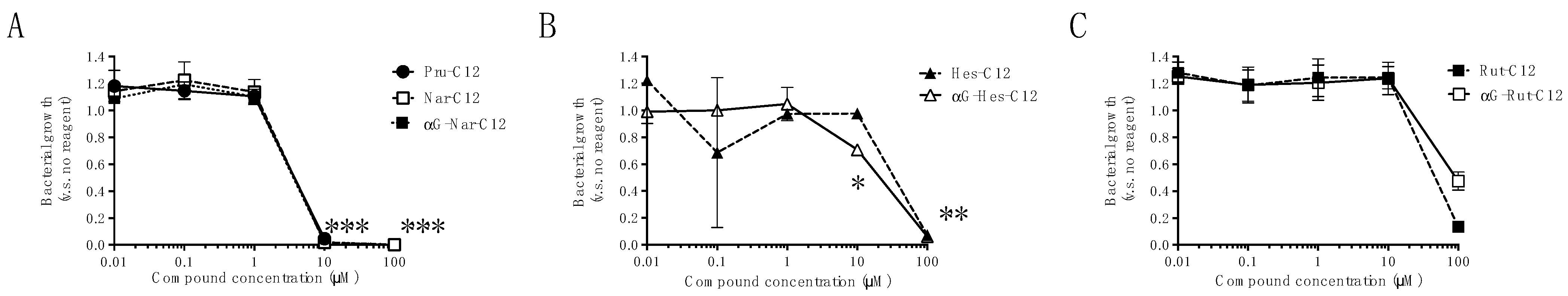
3.1. Growth Inhibitory Effects of Prunin Laurates and Their Analogues
3.2. Biofilm Formation Inhibitory Effect of Prunin Laurate and Its Analogs
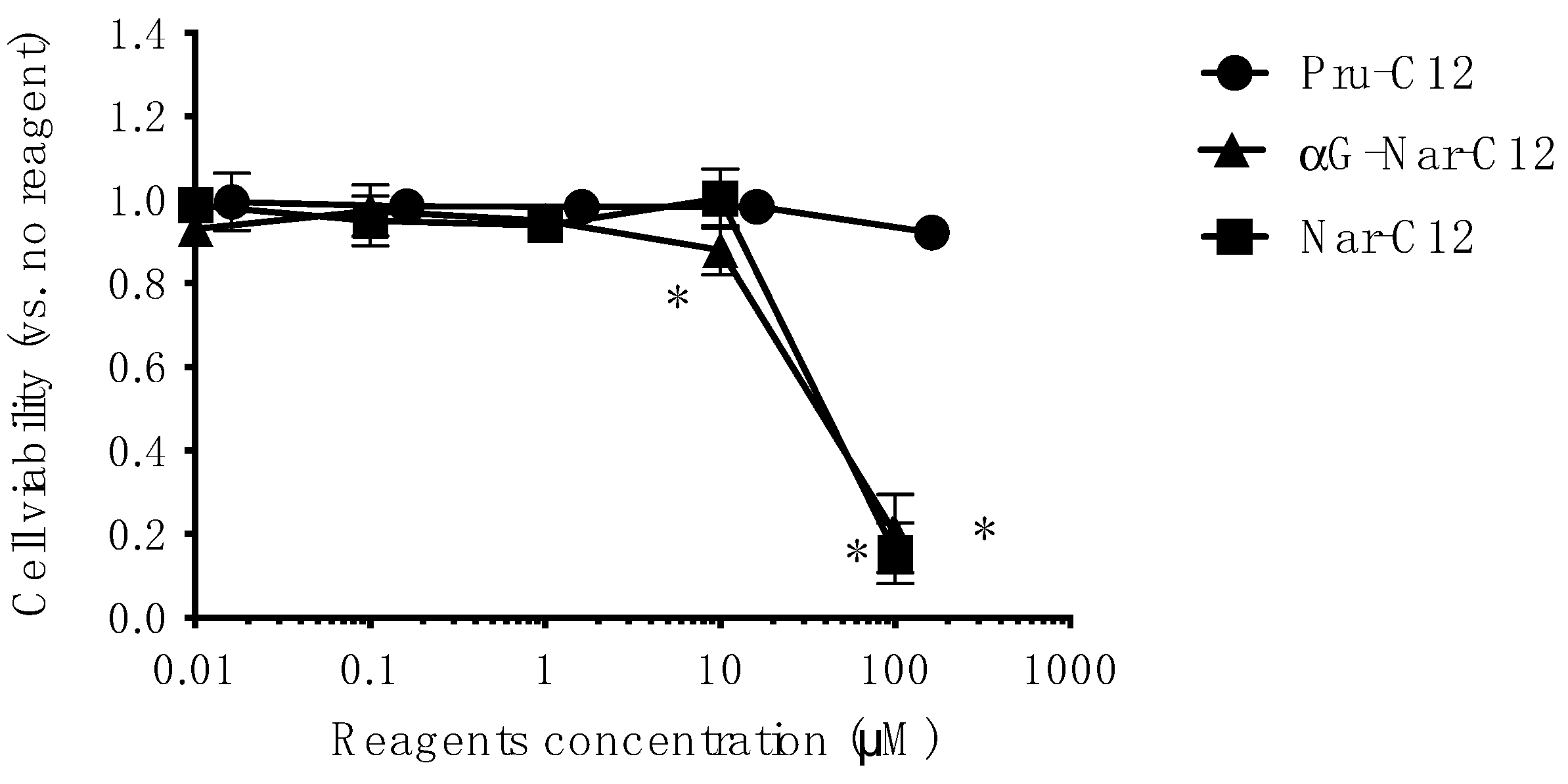
3.3. Cytotoxicity of Prunin Laurate and Its Analogs on Human-Derived Cells
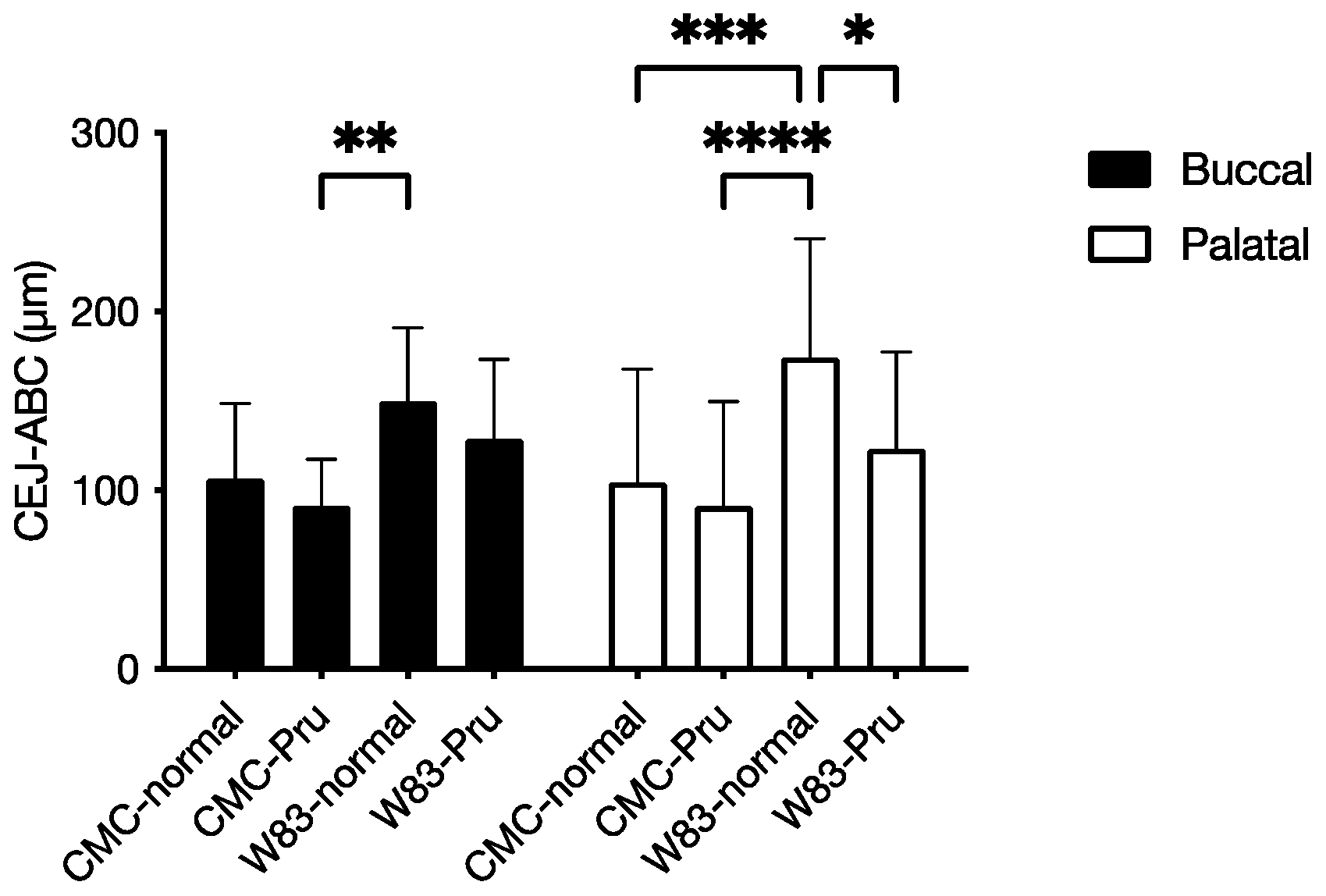
3.4. Investigation of Periodontal Disease Prevention Effect of Pru-C12 in a Mouse Animal Model of Periodontal Disease Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genco, R.J.; Sanz, M. Clinical and Public Health Implications of Periodontal and Systemic Diseases: An Overview. Periodontology 2000 2020, 83, 7–13. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2014, 15, 30–44. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators; Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A.B.; Crean, S.; Olsen, I.; Singhrao, S.K. Periodontitis, Microbiomes and Their Role in Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Elwishahy, A.; Antia, K.; Bhusari, S.; Ilechukwu, N.C.; Horstick, O.; Winkler, V. Porphyromonas gingivalis as a Risk Factor to Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. Rep. 2021, 5, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Leech, M.T.; Bartold, P.M. The Association between Rheumatoid Arthritis and Periodontitis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Geurs, N.C.; Lewis, C.E.; Jeffcoat, M.K. Osteoporosis and Periodontal Disease Progression. Periodontology 2000 2003, 32, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Terpenning, M.S.; Taylor, G.W.; Lopatin, D.E.; Kerr, C.K.; Dominguez, B.L.; Loesche, W.J. Aspiration Pneumonia: Dental and Oral Risk Factors in an Older Veteran Population. J. Am. Geriatr. Soc. 2001, 49, 557–563. [Google Scholar] [CrossRef]
- Pace, C.C.; McCullough, G.H. The Association between Oral Microorgansims and Aspiration Pneumonia in the Institutionalized Elderly: Review and Recommendations. Dysphagia 2010, 25, 307–322. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Liu, X.; Zhang, W.; Li, Y. Interrelationship between Diabetes and Periodontitis: Role of Hyperlipidemia. Arch. Oral Biol. 2015, 60, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association Between Periodontal Disease and Atherosclerotic Cardiovascular Diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef] [PubMed]
- Ong, G. Periodontal Disease and Tooth Loss. Int. Dent. J. 1998, 48, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.E.; Rahim-Wöstefeld, S.; Stocker, F.; Behnisch, R.; Eickholz, P.; Pretzl, B. The 2018 Classification of Periodontal Diseases: Its Predictive Value for Tooth Loss. J. Periodontol. 2022, 93, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental Biofilms: Difficult Therapeutic Targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis Disturbs Host–Commensal Homeostasis by Changing Complement Function. J. Oral Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Hajishengallis, G. Porphyromonas gingivalis Virulence Factors Involved in Subversion of Leukocytes and Microbial Dysbiosis. Virulence 2015, 6, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Tsutsuki, H.; Nakazawa, M.; Ueda, M.; Ihara, H.; Sakamoto, T. Naringin Lauroyl Ester Inhibits Lipopolysaccharide-Induced Activation of Nuclear Factor ΚB Signaling in Macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Céliz, G.; Audisio, M.C.; Daz, M. Antimicrobial Properties of Prunin, a Citric Flavanone Glucoside, and Its Prunin 6″-O-lauroyl Ester. J. Appl. Microbiol. 2010, 109, 1450–1457. [Google Scholar] [CrossRef]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial Activity of Naringin Derivatives against Pathogenic Strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M.H.H.B. Citrus Flavonoids as Potential Therapeutic Agents: A Review. Phytotherapy Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Krzeminski, R.; Gralak, M.; Delgado-Licon, E.; Ayala, A.L.M.; Katrich, E.; Trakhtenberg, S. Changes in Plasma Lipid and Antioxidant Activity in Rats as a Result of Naringin and Red Grapefruit Supplementation. J. Agric. Food Chem. 2005, 53, 3223–3228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, W.-W.; Wang, S.; Li, P.-B. Naringin Inhibits Chemokine Production in an LPS-Induced RAW 264.7 Macrophage Cell Line. Mol. Med. Rep. 2012, 6, 1343–1350. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Bérard, A.M. Naringin, the Major Grapefruit Flavonoid, Specifically Affects Atherosclerosis Development in Diet-Induced Hypercholesterolemia in Mice. J. Nutr. Biochem. 2012, 23, 469–477. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Chen, M.; Chen, T.; Chen, Z.; He, B. Biological Activities, Molecular Mechanisms, and Clinical Application of Naringin in Metabolic Syndrome. Pharmacol. Res. 2024, 202, 107124. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.M.; Gentili, B. Flavonoids of Citrus. IV. Isolation of Some Aglycones from the Lemon (Citrus Limon). J. Org. Chem. 1960, 25, 2183–2187. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective Effects of Flavonoids against Microbes and Toxins: The Cases of Hesperidin and Hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Bernard, F.X.; Sablé, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J.; Blanche, F. Glycosylated Flavones as Selective Inhibitors of Topoisomerase IV. Antimicrob. agents Chemother. 1997, 41, 992–998. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suzuki, K. Enzymatic Formation of 4G-α-d-Glucopyranosyl-Rutin. Agric. Biol. Chem. 1991, 55, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tanabe, F.; Arai, N.; Mitsuzumi, H.; Miwa, Y.; Kubota, M.; Chaen, H.; Kibata, M. Bioavailability of Glucosyl Hesperidin in Rats. Biosci. Biotechnol. Biochem. 2006, 70, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Darise, M.; Mizutani, K.; Kasai, R.; Tanaka, O.; Kitahata, S.; Okada, S.; Ogawa, S.; Murakami, F.; Chen, F.-H. Enzymic Transglucosylation of Rubusoside and the Structure-Sweetness Relationship of Steviol-Bisglycosides. Agric. Biol. Chem. 1984, 48, 2483–2488. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Miyata, T.; Nakayasu, N.; Mizutani, K.; Kasai, R.; Tanaka, O. Enzymic Transglucosylation Products of Stevioside: Separation and Sweetness-Evaluation. Agric. Biol. Chem. 1989, 53, 1603–1607. [Google Scholar] [CrossRef]
- Ohtani, K.; Aikawa, Y.; Ishikawa, H.; Kasai, R.; Kitahata, S.; Mizutani, K.; Doi, S.; Nakaura, M.; Tanaka, O. Further Study on the 1,4-Alpha-Transglucosylation of Rubusoside, a Sweet Steviol-Bisglucoside from Rubus suavissimus. Agric. Biol. Chem. 1991, 55, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Schifferle, R.E.; Shostad, S.A.; Bayers-Thering, M.T.; Dyer, D.W.; Neiders, M.E. Effect of Protoporphyrin IX Limitation on Porphyromonas gingivalis. J. Endod. 1996, 22, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T.; Simpson, W.; Liu, X.; Genco, C.A. Iron and Heme Utilization in Porphyromonas gingivalis. FEMS Microbiol. Rev. 2005, 29, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Macdonald, J.B. Hemin and Vitamin K Compounds as Required Factors for the Cultivation of Certain Strains of Bacteroides melaninogenicus. J. Bacteriol. 1960, 80, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Tsai, T.-H.; Chuang, L.-T.; Li, Y.-Y.; Zouboulis, C.C.; Tsai, P.-J. Anti-Bacterial and Anti-Inflammatory Properties of Capric Acid against Propionibacterium Acnes: A Comparative Study with Lauric Acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the Tea Catechin Epigallocatechin Gallate on Porphyromonas gingivalis Biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef]
- Karched, M.; Bhardwaj, R.G.; Inbamani, A.; Asikainen, S. Quantitation of Biofilm and Planktonic Life Forms of Coexisting Periodontal Species. Anaerobe 2015, 35, 13–20. [Google Scholar] [CrossRef]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A Water-Soluble Tetrazolium Salt Useful for Colorimetric Cell Viability Assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Suomi, J.D. Prevention and Control of Periodontal Disease. J. Am. Dent. Assoc. 1971, 83, 1271–1287. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Gershovich, E. The Prevention of Periodontal Disease—An Overview. Periodontology 2000 2020, 84, 9–13. [Google Scholar] [CrossRef]
- Janakiram, C.; Dye, B.A. A Public Health Approach for Prevention of Periodontal Disease. Periodontology 2000 2020, 84, 202–214. [Google Scholar] [CrossRef]
- de Sousa, J.M.; Balbontín, R.; Durão, P.; Gordo, I. Multidrug-Resistant Bacteria Compensate for the Epistasis between Resistances. PLoS Biol. 2017, 15, e2001741. [Google Scholar] [CrossRef]
- Eick, S.; Pfister, W. Efficacy of Antibiotics Against Periodontopathogenic Bacteria within Epithelial Cells: An In Vitro Study. J. Periodontol. 2004, 75, 1327–1334. [Google Scholar] [CrossRef]
- Maezono, H.; Noiri, Y.; Asahi, Y.; Yamaguchi, M.; Yamamoto, R.; Izutani, N.; Azakami, H.; Ebisu, S. Antibiofilm Effects of Azithromycin and Erythromycin on Porphyromonas gingivalis. Antimicrob. Agents Chemother. 2011, 55, 5887–5892. [Google Scholar] [CrossRef]
- Lather, A.; Sharma, S.; Khatkar, A. Naringin Derivatives as Glucosamine-6-Phosphate Synthase Inhibitors Based Preservatives and Their Biological Evaluation. Sci. Rep. 2020, 10, 20477. [Google Scholar] [CrossRef]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol Alters Microstructure and Metabolomic Profiles of Biofilm Composed of Streptococcus gordonii and Porphyromonas gingivalis. Mol. Oral Microbiol. 2013, 28, 435–451. [Google Scholar] [CrossRef]
- Sakanaka, A.; Takeuchi, H.; Kuboniwa, M.; Amano, A. Dual Lifestyle of Porphyromonas gingivalis in Biofilm and Gingival Cells. Microb. Pathogenesis 2016, 94, 42–47. [Google Scholar] [CrossRef]
- Suci, P.A.; Mittelman, M.W.; Yu, F.P.; Geesey, G.G. Investigation of Ciprofloxacin Penetration into Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 1994, 38, 2125–2133. [Google Scholar] [CrossRef]
- Fujita, T.; Yoshimoto, T.; Kajiya, M.; Ouhara, K.; Matsuda, S.; Takemura, T.; Akutagawa, K.; Takeda, K.; Mizuno, N.; Kurihara, H. Regulation of Defensive Function on Gingival Epithelial Cells Can Prevent Periodontal Disease. Jpn. Dent. Sci. Rev. 2018, 54, 66–75. [Google Scholar] [CrossRef]
- Céliz, G.; Alfaro, F.F.; Cappellini, C.; Daz, M.; Verstraeten, S.V. Prunin- and Hesperetin Glucoside-Alkyl (C4–C18) Esters Interaction with Jurkat Cells Plasma Membrane: Consequences on Membrane Physical Properties and Antioxidant Capacity. Food Chem. Toxicol. 2013, 55, 411–423. [Google Scholar] [CrossRef]
- Bendyk, A.; Marino, V.; Zilm, P.S.; Howe, P.; Bartold, P.M. Effect of Dietary Omega-3 Polyunsaturated Fatty Acids on Experimental Periodontitis in the Mouse. J. Periodontal Res. 2009, 44, 211–216. [Google Scholar] [CrossRef]
- Polak, D.; Wilensky, A.; Shapira, L.; Halabi, A.; Goldstein, D.; Weiss, E.I.; Houri-Haddad, Y. Mouse Model of Experimental Periodontitis Induced by Porphyromonas gingivalis/Fusobacterium Nucleatum Infection: Bone Loss and Host Response. J. Clin. Periodontol. 2009, 36, 406–410. [Google Scholar] [CrossRef]
- Shinohara, M.; Maetani, M.; Kitada, C.; Nishigami, Y.; Yazawa, A.; Kamitani, S. Analysis of the Effects of Food Additives on Porphyromonas gingivalis. Pathogens 2022, 11, 65. [Google Scholar] [CrossRef]


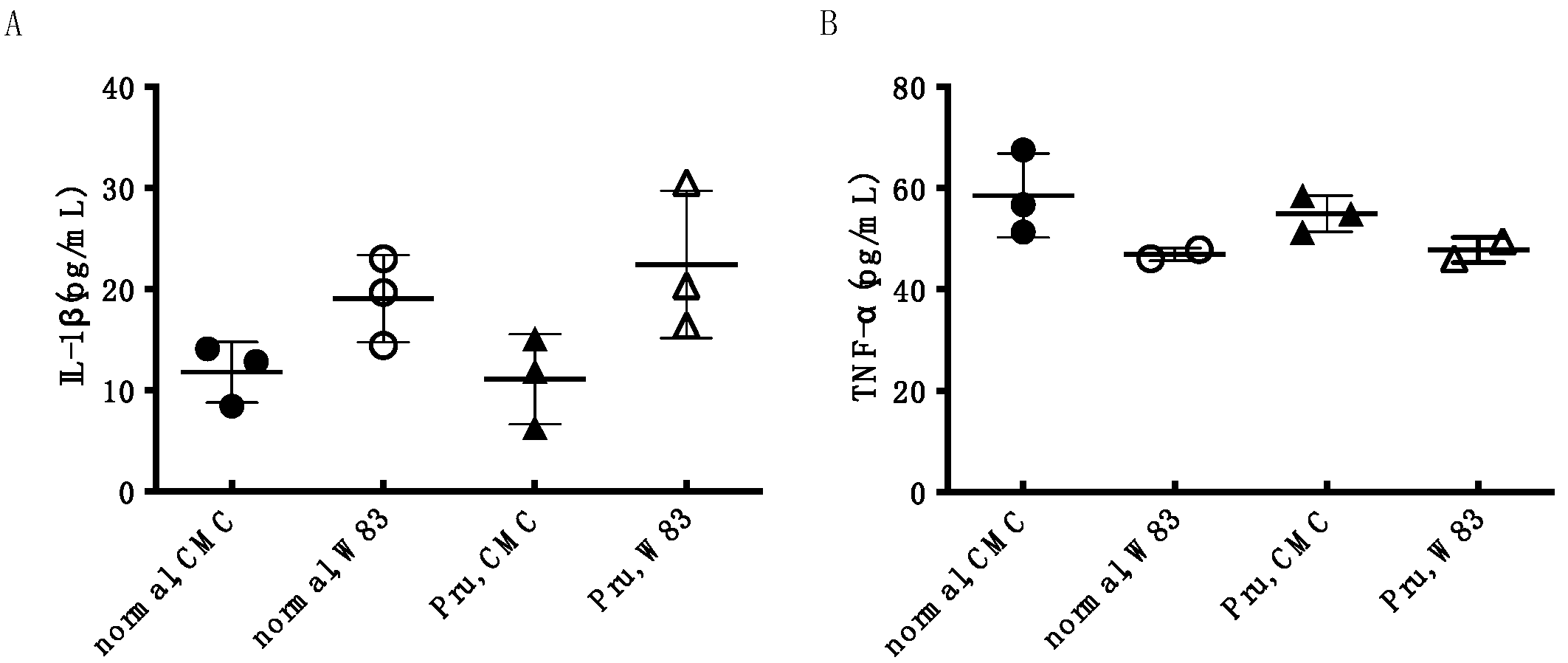
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, E.; Ito, C.; Shinohara, M.; Handa, S.; Maetani, M.; Yasugi, M.; Miyake, M.; Sakamoto, T.; Yazawa, A.; Kamitani, S. Prunin Laurate Derived from Natural Substances Shows Antibacterial Activity against the Periodontal Pathogen Porphyromonas gingivalis. Foods 2024, 13, 1917. https://doi.org/10.3390/foods13121917
Wada E, Ito C, Shinohara M, Handa S, Maetani M, Yasugi M, Miyake M, Sakamoto T, Yazawa A, Kamitani S. Prunin Laurate Derived from Natural Substances Shows Antibacterial Activity against the Periodontal Pathogen Porphyromonas gingivalis. Foods. 2024; 13(12):1917. https://doi.org/10.3390/foods13121917
Chicago/Turabian StyleWada, Erika, Chiharu Ito, Mai Shinohara, Satoshi Handa, Miki Maetani, Mayo Yasugi, Masami Miyake, Tatsuji Sakamoto, Ayaka Yazawa, and Shigeki Kamitani. 2024. "Prunin Laurate Derived from Natural Substances Shows Antibacterial Activity against the Periodontal Pathogen Porphyromonas gingivalis" Foods 13, no. 12: 1917. https://doi.org/10.3390/foods13121917
APA StyleWada, E., Ito, C., Shinohara, M., Handa, S., Maetani, M., Yasugi, M., Miyake, M., Sakamoto, T., Yazawa, A., & Kamitani, S. (2024). Prunin Laurate Derived from Natural Substances Shows Antibacterial Activity against the Periodontal Pathogen Porphyromonas gingivalis. Foods, 13(12), 1917. https://doi.org/10.3390/foods13121917




