Impact of Nanoclays Addition on Chickpea (Cicer arietinum L.) Flour Film Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Films Preparation
2.3. Film Thickness
2.4. Water Vapor Permeability
2.5. Dry Matter Content, Solubility, Swelling Property, and Density
2.6. Color and Opacity
2.7. Antioxidant Activity
2.8. Mechanical Properties
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Thermogravimetric Analysis (TGA)
2.11. Scanning Electron Microscopy (SEM) Analysis
2.12. Biodegradation Test
2.13. Statistical Analysis
3. Results and Discussion
3.1. Water Vapor Permeability, Thickness, Dry Matter Content, Solubility, Swelling, and Density
3.2. Color
3.3. Mechanical Properties
3.4. Antioxidant Activity
3.5. Fourier Transform Infrared Spectroscopy
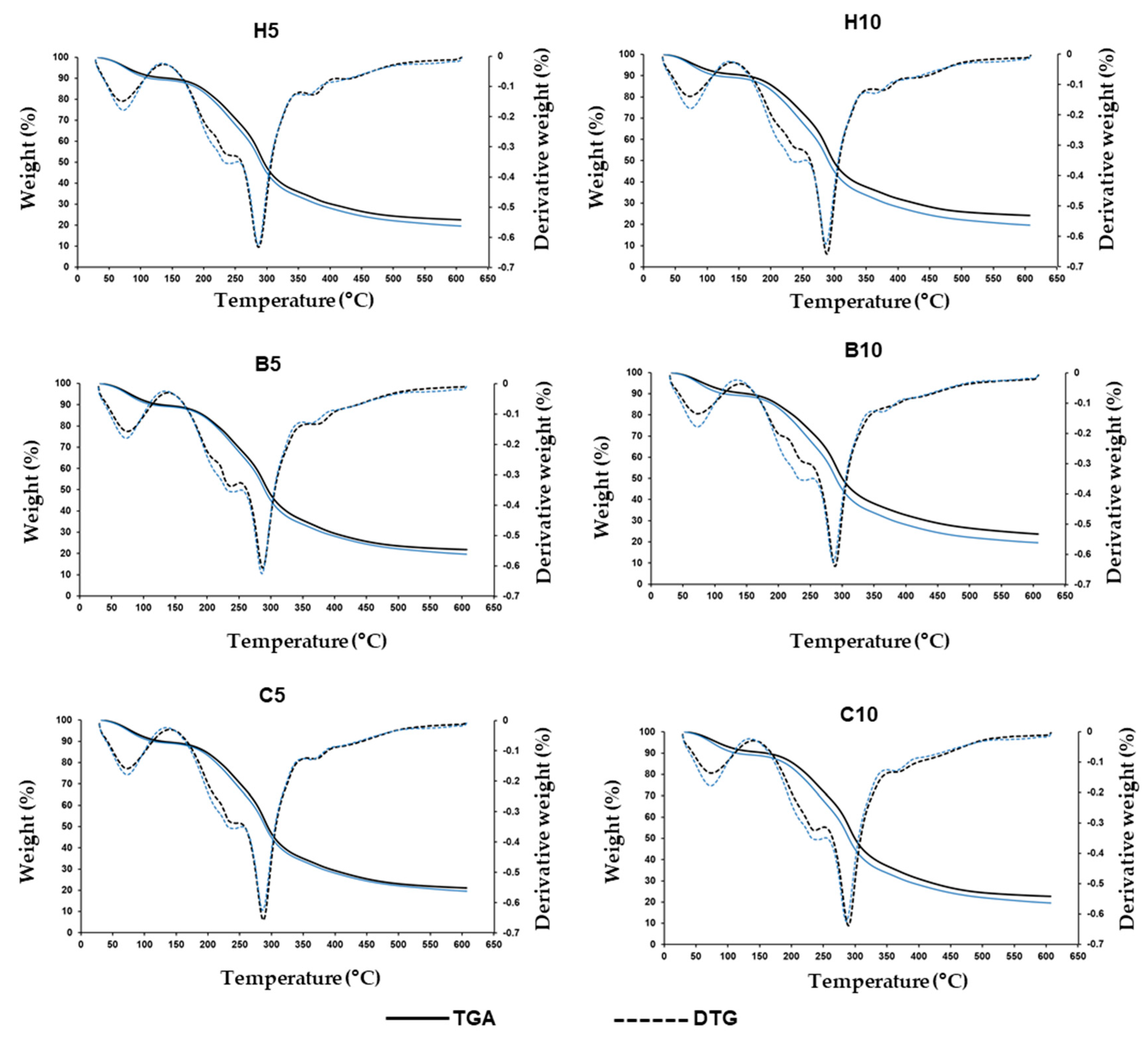
3.6. Thermogravimetric Analysis
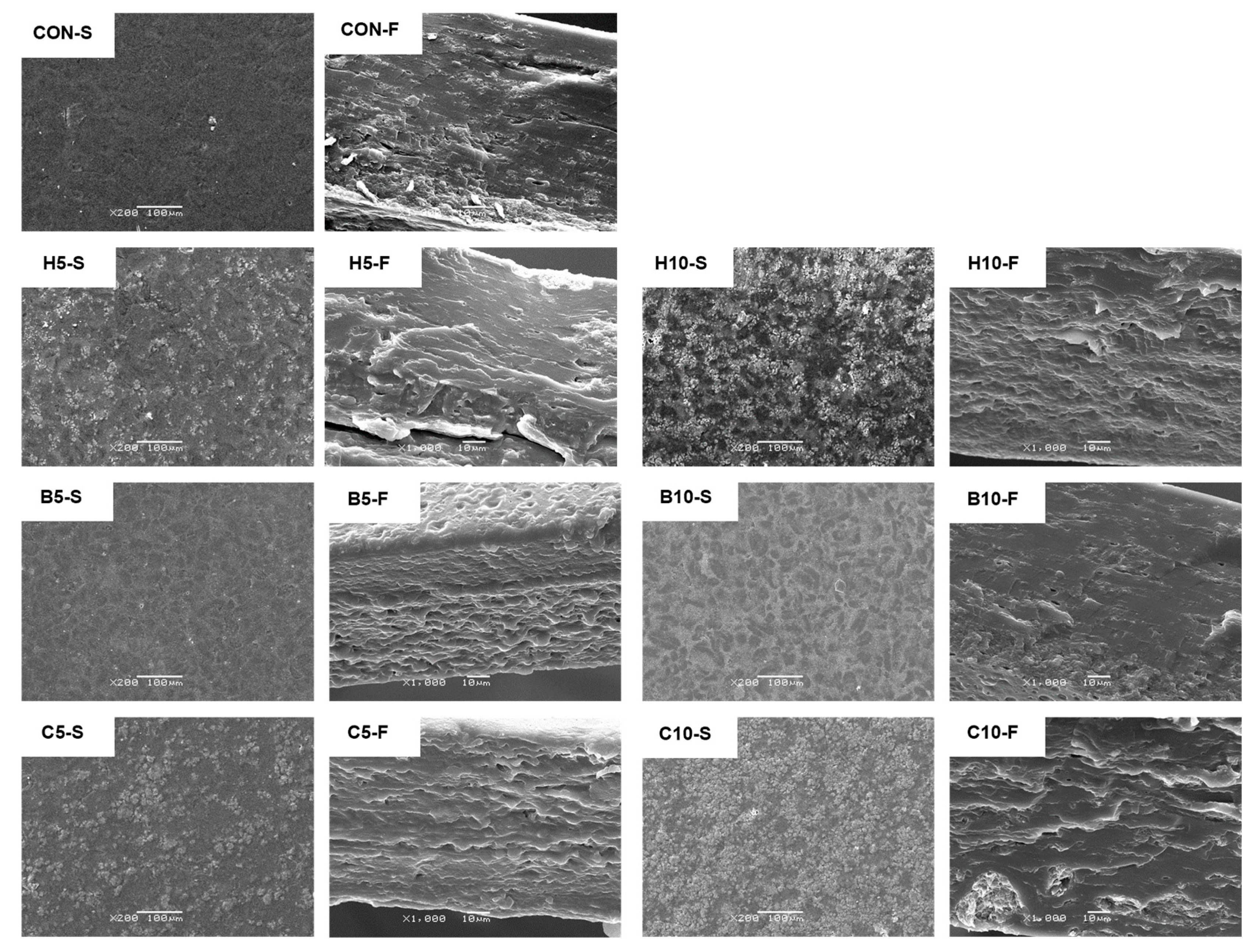
3.7. Film Microstructure
3.8. Biodegradability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarfarzadeh, S.; Forough, M.; Amjadi, S.; Kouzegaran, V.J.; Almasi, H.; Gravand, F.; Zargar, M. Plant protein-based nanocomposite films: A review on the used nanomaterials, characteristics, and food packaging applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–28. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Díaz, O.; Ferreiro, T.; Rodríguez-Otero, J.L.; Cobos, A. Characterization of chickpea (Cicer arietinum L.) flour films: Effects of pH and plasticizer concentration. Int. J. Mol. Sci. 2019, 20, 1246. [Google Scholar] [CrossRef] [PubMed]
- Kocakulak, S.; Sumnu, G.; Sahin, S. Chickpea flour-based biofilms containing gallic acid to be used as active edible films. J. Appl. Polym. Sci. 2019, 2019, 47704. [Google Scholar] [CrossRef]
- Camiletti, O.F.; Bergesse, A.E.; Aleman, R.; Riveros, C.G.; Grosso, N.R. Application of chickpea-based edible coating with chickpea husk polyphenols on the preservation of sunflower seeds. J. Food Sci. 2023, 2023, 1–16. [Google Scholar] [CrossRef]
- Camiletti, O.F.; Riveros, C.G.; Aguirre, A.; Grosso, N.R. Sunflower oil preservation by using chickpea flour film as bio-packaging material. J. Food Sci. 2021, 86, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E.; Emir, A.A.; Sumnu, G.; Kahyaoglu, L.N. Citric acid cross-linked curcumin/chitosan/chickpea flour film: An active packaging for chicken breast storage. Food Biosci. 2022, 50, 102121. [Google Scholar] [CrossRef]
- Yildiz, E.; Sumnu, G.; Kahyaoglu, L.N. Assessment of curcumin incorporated chickpea flour/PEO (polyethylene oxide) based electrospun nanofiber as an antioxidant and antimicrobial food package. Food Bioprod. Process. 2022, 135, 205–216. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.d. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H. State-of-the-art nanoclay reinforcement in green polymeric nanocomposite: From design to new opportunities. Minerals 2022, 12, 1495. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Leontiou, A.A. Montmorillonite composite materials and food packaging. In Composites Materials for Food Packaging; Cirillo, G., Kozlowski, M.A., Spizzirri, U.G., Eds.; Scrivener Publishing LLC–Wiley: Hoboken, NJ, USA, 2018; pp. 1–72. [Google Scholar]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in food and beverage packaging. J. Nanomater. 2019, 2019, 8927167. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Sadegh-Hassani, F.; Nafchi, A.M. Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int. J. Biol. Macromol. 2014, 67, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Diéguez, M.C.V.; Pelissari, F.M.; Sobral, P.J.A.; Menegalli, F.C. Effect of process conditions on the production of nanocomposite films based on amaranth flour and montmorillonite. LWT Food Sci. Technol. 2015, 61, 70–79. [Google Scholar] [CrossRef]
- Orsuwan, A.; Sothornvit, R. Development and characterization of banana flour film incorporatedwith montmorillonite and banana starch nanoparticles. Carbohydr. Polym. 2017, 174, 235–242. [Google Scholar] [CrossRef]
- Rodríguez-Marín, M.L.; Bello-Pérez, L.A.; Yee-Madeira, H.; Zhong, Q.; González-Soto, R.A. Nanocomposites of rice and banana flours blend with montmorillonite: Partial characterization. Mater. Sci. Eng. C 2013, 33, 3903–3908. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Badii, F.; Mohamed, A.; Ramaswamy, H. Evaluation of physicochemical, thermomechanical, and structural properties of chickpea flour composite films reinforced with crystalline nanocellulose. J. Appl. Polym. Sci. 2020, 2020, 48389. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Characteristics of bio-nanocomposite films from tilapia skin gelatin incorporated with hydrophilic and hydrophobic nanoclays. J. Food Eng. 2014, 143, 195–204. [Google Scholar] [CrossRef]
- ASTM E 96-93; Standard Test Method for Water Vapor Transmission of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 1993; pp. 701–708.
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Díaz, O.; Candia, D.; Cobos, A. Effects of ultraviolet radiation on properties of films from whey protein concentrate treated before or after film formation. Food Hydrocoll. 2016, 55, 189–199. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Voung, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crop. Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Márquez-Reyes, J.M.; Rodríguez-Quiroz, R.E.; Hernández-Rodríguez, J.P.; Rodríguez-Romero, B.A.; Flores-Breceda, H.; Napoles-Armenta, J.; Romero-Soto, I.C.; Galindo-Rodríguez, S.A.; Báez-González, J.G.; Treviño-Garza, M.Z. Production and characterization of biocomposite films of bacterial cellulose from kombucha and coated with chitosan. Polymers 2022, 14, 3632. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.V.; Costa, T.M.H.; Rios, A.O.; Flores, S.H. Comparative study on the properties of films based on red rice (Oryza glaberrima) flour and starch. Food Hydrocoll. 2017, 65, 96–106. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: Philadelphia, PA, USA, 2000; pp. 160–168.
- Dias, L.D.; Bertolo, M.R.V.; Alves, F.; de Faria, C.M.G.; Rodrigues, M.A.V.; Lopes, L.K.B.C.; Plepis, A.M.G.; Mattoso, L.H.C.; Bogusz Junior, S.; Bagnato, V.S. Preparation and characterization of curcumin and pomegranate peel extract chitosan/gelatin-based films and their photoinactivation of bacteria. Mater. Today Commun. 2022, 31, 103791. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, D.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, J.-H.; Kwak, H.-S. Mechanical and water barrier properties of soy protein and clay mineral composite films. Food Sci. Biotechnol. 2005, 14, 112–116. [Google Scholar]
- Follain, N.; Ren, J.; Pollet, E.; Avérous, L. Study of the water sorption and barrier performances of potato starch nano-biocomposites based on halloysite nanotubes. Carbohydr. Polym. 2022, 277, 118805. [Google Scholar] [CrossRef]
- Frangopoulos, T.; Marinopoulou, A.; Goulas, A.; Likotrafiti, E.; Rhoades, J.; Petridis, D.; Kannidou, E.; Stamelos, A.; Theodoridou, M.; Arampatzidou, A.; et al. Optimizing the functional properties of starch-based biodegradable films. Foods 2023, 12, 2812. [Google Scholar] [CrossRef]
- Camani, P.H.; Tobuchi, J.P.M.; Fiori, A.P.S.M.; Rosa, D.S. Impact of unmodified (PGV) and modified (Cloisite20A) nanoclays into biodegradability and other properties of (bio)nanocomposites. Appl. Clay Sci. 2020, 186, 105453. [Google Scholar] [CrossRef]
- Dharini, V.; Selvam, S.P.; Jayaramudu, J.; Emmanuel, R.S. Functional properties of clay nanofillers used in the biopolymer-based composite films for active food packaging applications—Review. Appl. Clay Sci. 2022, 226, 106555. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Spychaj, T. Novel hydrophilic carboxymethyl starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2015, 128, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Calambas, H.L.; Fonseca, A.; Adames, D.; Aguirre-Loredo, Y.; Caicedo, C. Physical-mechanical behavior and water-barrier properties of biopolymers-clay nanocomposites. Molecules 2021, 26, 6734. [Google Scholar] [CrossRef] [PubMed]
- Shanmathy, M.; Mohanta, M.; Thirugnanam, A. Development of biodegradable bioplastic films from Taro starch reinforced with bentonite. Carbohydr. Polym. Technol. Appl. 2021, 2, 100173. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, S.-B.; Hong, S.-I. Preparation and characterization of agar/clay nanocomposite films: The effect of clay type. J. Food Sci. 2011, 76, N40–N48. [Google Scholar] [CrossRef] [PubMed]
- Salarbashi, D.; Noghabi, M.S.; Bazzaz, B.S.F.; Shahabi-Ghahfarrokhi, I.; Jafari, B.; Ahmadi, R. Eco-friendly soluble soybean polysaccharide/nanoclay Na+ bionanocomposite: Properties and characterization. Carbohydr. Polym. 2017, 169, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.S.; Tatol, M. Colour difference ΔE—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- López, O.V.; Castillo, L.A.; Barbosa, S.E.; Villar, M.A.; García, M.A. Processing–properties–applications relationship of nanocomposites based on thermoplastic corn starch and talc. Polym. Compos. 2018, 39, 1331–1338. [Google Scholar] [CrossRef]
- Rivadeneira-Velasco, K.E.; Utreras-Silva, C.A.; Díaz-Barrios, A.; Sommer-Márquez, A.E.; Tafur, J.P.; Michell, R.M. Green nanocomposites based on thermoplastic starch: A review. Polymers 2021, 13, 3227. [Google Scholar] [CrossRef]
- Hong, S.-I.; Wang, L.-F.; Rhim, J.-W. Preparation and characterization of nanoclays-incorporated polyethylene/thermoplastic starch composite films with antimicrobial activity. Food Packag. Shelf Life 2022, 31, 100784. [Google Scholar] [CrossRef]
- Sahabi, N.; Soleimani, S.; Ghorbani, M. Investigating functional properties of halloysite nanotubes and propolis used in reinforced composite film based on soy protein/basil seed gum for food packaging application. Int. J. Biol. Macromol. 2023, 231, 123350. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic effect of chitosan and halloysite nanotubes on improving agar film properties. Food Hydrocoll. 2020, 101, 105471. [Google Scholar] [CrossRef]
- Soheilmoghaddam, M.; Wahit, M.U.; Mahmoudian, S.; Hanid, N.A. Regenerated cellulose/halloysite nanotube nanocomposite films prepared with an ionic liquid. Mater. Chem. Phys. 2013, 141, 936–943. [Google Scholar] [CrossRef]
- Neji, A.B.; Jridi, M.; Kchaou, H.; Nasri, M.; Sahnoun, R.D. Preparation, characterization, mechanical and barrier properties investigation of chitosan-kaolinite nanocomposite. Polym. Test. 2020, 84, 106380. [Google Scholar] [CrossRef]
- Kim, H.M.; Oh, J.-M. Physico–chemical interaction between clay minerals and albumin protein according to the type of clay. Minerals 2019, 9, 396. [Google Scholar] [CrossRef]
- Issa, A.T.; Schimmel, K.A.; Worku, M.; Shahbazi, A.; Ibrahim, S.A.; Tahergorabi, R. Sweet potato starch-based nanocomposites: Development, characterization, and biodegradability. Starch 2018, 70, 1700273. [Google Scholar] [CrossRef]
- Jha, P. Functional properties of starch-chitosan blend bionanocomposite films for food packaging: The influence of amylose-amylopectin ratios. J. Food Sci. Technol. 2021, 58, 3368–3378. [Google Scholar] [CrossRef]
- Guarás, M.P.; Menossi, M.; Torres Nicolini, A.; Alvarez, V.A.; Ludueña, L.N. Bio-nanocomposites films based on unmodified and modified thermoplastic starch reinforced with chemically modified nanoclays. J. Mater. Sci. 2023, 58, 5456–5476. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Capron, I.; Robert, P.; Colonna, P.; Brogly, M.; Planchot, V. Starch in rubbery and glassy states by FTIR spectroscopy. Carbohydr. Polym. 2007, 68, 249–259. [Google Scholar] [CrossRef]
- Smits, A.L.M.; Ruhnau, F.C.; Vliegenthart, J.F.G.; van Soest, J.J.G. Ageing of starch based systems as observed with FT-IR and solid state NMR spectroscopy. Starch 1999, 50, 478–483. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q. Retrogradation characteristics of pulse starches. Food Res. Int. 2013, 54, 203–212. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Liang, T.; Wang, L. Preparation and characterization of a novel edible film based on Artemisia sphaerocephala Krasch. gum: Effects of type and concentration of plasticizers. Food Hydrocoll. 2018, 77, 502–508. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Fonseca-García, A.; Calambas, H.L.; Salazar-Arango, A.; Caicedo, C. Improvements of thermal and mechanical properties of achira starch/chitosan/clay nanocomposite films. Helyon 2023, 9, e16782. [Google Scholar] [CrossRef]
- Ren, U.; Dang, K.M.; Pollet, E.; Avérous, L. Preparation and characterization of thermoplastic potato starch/halloysite nano-biocomposites: Effect of plasticizer nature and nanoclay content. Polymers 2018, 10, 808. [Google Scholar] [CrossRef]
- Romero-Bastida, C.A.; Tapia-Blácido, D.R.; Méndez-Montealvo, G.; Bello-Pérez, L.A.; Velázquez, G.; Alvarez-Ramirez, J. Effect of amylose content and nanoclay incorporation order in physicochemical properties of starch/montmorillonite composites. Carbohydr. Polym. 2016, 152, 351–360. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A.; Armada, M. Water barrier properties of starch-clay nanocomposite films. Braz. J. Food Technol. 2012, 15, 208–218. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, W.; Zhang, H.; Dai, Y.; Hou, H.; Dong, H. Effects of organic modification of montmorillonite on the properties of hydroxypropyl di-starch phosphate films prepared by extrusion blowing. Materials 2018, 11, 1064. [Google Scholar] [CrossRef]
- Oleyaie, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef]
- Kumar, P.; Sandeep, K.P.; Alavi, S.; Truong, V.D.; Gorga, R.E. Effect of type and content of modified montmorillonite on the structure and properties of bio-nanocomposite films based on soy protein isolate and montmorillonite. J. Food Sci. 2010, 75, N46–N56. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Váquez-Torres, H.; Garfias-Mesías, L.F.; Paul, D.R. Thermal degradation of commercially available organoclays studied by TGA–FTIR. Thermochim. Acta 2007, 457, 92–102. [Google Scholar] [CrossRef]
- Li, Q.; Ren, T.; Perkins, P.; Hu, X.; Wang, X. Applications of halloysite nanotubes in food packaging for improving film performance and food preservation. Food Control 2021, 124, 107876. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Han, N.; Bai, S. Effect of citric acid and processing on the performance of thermoplastic starch/montmorillonite nanocomposites. Carbohydr. Polym. 2009, 76, 68–73. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Montazer, M.; Rashidi, A.; Rahimi, M.K. Antibacterial properties of clay layers silicate: A special study of montmorillonite on cotton fiber. Asian J. Chem. 2013, 25, 2889–2892. [Google Scholar] [CrossRef]


| Film | WVP 1 | Thickness μm | Dry Matter (g/100 g) | Solubility (% D.M. 2) | Swelling (%) | Density (%) |
|---|---|---|---|---|---|---|
| Control | 0.036 ± 0.001 a | 114.08 ± 1.33 b | 78.88 ± 1.30 | 30.77 ± 1.10 a | 98.55 ± 2.28 c | 1.58 ± 0.02 b |
| H5 3 | 0.032 ± 0.003 ab | 119.03 ± 2.41 ab | 79.68 ± 1.35 | 29.37 ± 0.63 b | 102.04 ± 1.0 c | 1.65 ± 0.01 a |
| H10 4 | 0.031 ± 0.002 ab | 118.68 ± 6.67 ab | 80.37 ± 1.46 | 27.93 ± 0.49 cd | 93.28 ± 0.83 d | 1.65 ± 0.02 a |
| B5 5 | 0.030 ± 0.003 b | 117.76 ± 0.57 ab | 80.15 ± 0.27 | 28.87 ± 0.74 bc | 120.59 ± 0.27 a | 1.56 ± 0.02 bc |
| B10 6 | 0.021 ± 0.001 c | 114.33 ± 0.50 b | 80.13 ± 1.30 | 28.23 ± 0.58 bcd | 107.51 ± 4.07 b | 1.65 ± 0.04 a |
| C5 7 | 0.033 ± 0.002 ab | 123.42 ± 8.07 a | 79.73 ± 1.12 | 28.77 ± 0.77 bc | 91.42 ± 4.06 d | 1.53 ± 0.03 c |
| C10 8 | 0.034 ± 0.002 ab | 124.67 ± 1.92 a | 79.24 ± 0.75 | 27.49 ± 0.42 d | 82.91 ± 3.86 e | 1.56 ± 0.02 bc |
| Film | L* | a* | b* | ΔE | YI | WI | Opacity |
|---|---|---|---|---|---|---|---|
| Control | 91.41 ± 0.21 bd | −1.66 ± 0.11 c | 16.65 ± 0.72 b | 0.00 ± 0.00 d | 26.03 ± 1.19 b | 81.19 ± 0.73 b | 14.99 ± 0.29 c |
| H5 1 | 92.12 ± 0.13 a | −1.70 ± 0.06 c | 14.42 ± 0.40 c | 2.18 ± 0.34 b | 22.39 ± 0.69 c | 83.43 ± 0.50 a | 16.74 ± 0.10 b |
| H10 2 | 91.53 ± 0.20 b | −1.73 ± 0.00 c | 16.63 ± 0.91 b | 2.00 ± 0.48 bc | 25.99 ± 1.47 b | 81.25 ± 0.89 b | 17.65 ± 0.07 a |
| B5 3 | 91.23 ± 0.14 bd | −1.33 ± 0.08 b | 16.82 ± 0.71 b | 1.40 ± 0.32 c | 26.36 ± 1.13 b | 80.98 ± 0.69 b | 16.72 ± 0.31 b |
| B10 4 | 90.77 ± 0.18 c | −1.02 ± 0.03 a | 17.26 ± 0.29 ab | 1.75 ± 0.28 bc | 27.18 ± 0.50 ab | 80.40 ± 0.34 bc | 17.70 ± 0.10 a |
| C5 5 | 91.13 ± 0.13 d | −1.62 ± 0.05 c | 17.34 ± 0.53 ab | 1.55 ± 0.20 c | 27.21 ± 0.84 ab | 80.45 ± 0.48 bc | 17.07 ± 0.48 b |
| C10 6 | 90.56 ± 0.24 c | −1.42 ± 0.05 b | 18.24 ± 0.68 a | 3.06 ± 0.58 a | 28.79 ± 1.14 a | 79.42 ± 0.71 c | 17.53 ± 0.12 a |
| Film | Tensile Strength at Maximum (MPa) | Elongation at Break (%) | Elastic Modulus (N/mm) | Puncture Strength (MPa) | Puncture Deformation (%) |
|---|---|---|---|---|---|
| Control | 3.88 ± 0.12 e | 26.75 ± 1.16 b | 1.12 ± 0.03 d | 4.14 ± 0.38 cd | 9.53 ± 0.86 ab |
| H5 1 | 4.24 ± 0.24 ce | 32.07 ± 0.35 a | 1.28 ± 0.10 d | 4.52 ± 0.05 bc | 10.01 ± 0.21 a |
| H10 2 | 4.42 ± 0.16 c | 31.02 ± 1.48 a | 1.29 ± 0.17 d | 4.19 ± 0.15 bcd | 9.04 ± 0.26 bc |
| B5 3 | 5.13 ± 0.21 b | 25.40 ± 1.07 b | 1.90 ± 0.03 b | 4.58 ± 0.21 b | 7.96 ± 0.62 de |
| B10 4 | 6.26 ± 0.38 a | 21.32 ± 1.02 c | 2.85 ± 0.20 a | 5.10 ± 0.14 a | 6.14 ± 0.22 f |
| C5 5 | 3.96 ± 0.17 de | 25.46 ± 0.98 b | 1.10 ± 0.07 d | 3.53 ± 0.15 e | 8.39 ± 0.32 cd |
| C10 6 | 4.32 ± 0.19 cd | 21.09 ± 0.58 c | 1.58 ± 0.21 c | 3.98 ± 0.34 d | 7.54 ± 0.23 e |
| Film | DPPH (mg/g Film) | 993/1020 cm−1 Ratio | 1043/1020 cm−1 Ratio | TGA Third Stage Temperature Peak 1 (°C) | TGA Residual Weight (%) |
|---|---|---|---|---|---|
| Control | 0.73 ± 0.02 be | 1.77 ± 0.13 b | 0.90 ± 0.05 a | 287.03 ± 0.80 c | 19.59 ± 0.84 d |
| H5 2 | 0.65 ± 0.03 cd | 1.63 ± 0.11 b | 0.55 ± 0.07 d | 288.84 ± 0.92 b | 22.46 ± 0.30 b |
| H10 3 | 0.59 ± 0.02 d | 2.51 ± 0.12 a | 0.76 ± 0.11 bc | 289.19 ± 0.39 b | 24.30 ± 0.15 a |
| B5 4 | 0.92 ± 0.07 a | 1.73 ± 0.24 b | 0.69 ± 0.08 c | 288.79 ± 0.51 b | 21.85 ± 0.22 bc |
| B10 5 | 0.67 ± 0.04 ce | 2.31 ± 0.05 a | 0.88 ± 0.03 ab | 290.04 ± 0.24 ab | 23.64 ± 0.97 a |
| C5 6 | 0.78 ± 0.01 b | 1.63 ± 0.27 b | 0.79 ± 0.09 abc | 288.95 ± 0.93 b | 21.08 ± 0.38 c |
| C10 7 | 0.76 ± 0.03 b | 1.34 ± 0.12 c | 0.74 ± 0.09 bc | 290.81 ± 1.11 a | 22.64 ± 0.54 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobos, Á.; Díaz, O. Impact of Nanoclays Addition on Chickpea (Cicer arietinum L.) Flour Film Properties. Foods 2024, 13, 75. https://doi.org/10.3390/foods13010075
Cobos Á, Díaz O. Impact of Nanoclays Addition on Chickpea (Cicer arietinum L.) Flour Film Properties. Foods. 2024; 13(1):75. https://doi.org/10.3390/foods13010075
Chicago/Turabian StyleCobos, Ángel, and Olga Díaz. 2024. "Impact of Nanoclays Addition on Chickpea (Cicer arietinum L.) Flour Film Properties" Foods 13, no. 1: 75. https://doi.org/10.3390/foods13010075
APA StyleCobos, Á., & Díaz, O. (2024). Impact of Nanoclays Addition on Chickpea (Cicer arietinum L.) Flour Film Properties. Foods, 13(1), 75. https://doi.org/10.3390/foods13010075






