Abstract
The objective of this investigation was to evaluate Salmonella and Yersinia enterocolitica prevalence in wild boars hunted in Sardinia and further characterize the isolates and analyse antimicrobial resistance (AMR) patterns. In order to assess slaughtering hygiene, an evaluation of carcasses microbial contamination was also carried out. Between 2020 and 2022, samples were collected from 66 wild boars hunted during two hunting seasons from the area of two provinces in northern and central Sardinia (Italy). Samples collected included colon content samples, mesenteric lymph nodes samples and carcass surface samples. Salmonella and Y. enterocolitica detection was conducted on each sample; also, on carcass surface samples, total aerobic mesophilic count and Enterobacteriaceae count were evaluated. On Salmonella and Y. enterocolitica isolates, antimicrobial susceptibility was tested and whole genome sequencing was applied. Salmonella was identified in the colon content samples of 3/66 (4.5%) wild boars; isolates were S. enterica subs. salamae, S. ser. elomrane and S. enterica subs. enterica. Y. enterocolitica was detected from 20/66 (30.3%) wild boars: in 18/66 (27.3%) colon contents, in 3/66 (4.5%) mesenteric lymph nodes and in 3/49 (6.1%) carcass surface samples. In all, 24 Y. enterocolitica isolates were analysed and 20 different sequence types were detected, with the most common being ST860. Regarding AMR, no resistance was detected in Salmonella isolates, while expected resistance towards β-lactams (blaA gene) and streptogramin (vatF gene) was observed in Y. enterocolitica isolates (91.7% and 4.2%, respectively). The low presence of AMR is probably due to the low anthropic impact in the wild areas. Regarding the surface contamination of carcasses, values (mean ± standard deviation log10 CFU/cm2) were 2.46 ± 0.97 for ACC and 1.07 ± 1.18 for Enterobacteriaceae. The results of our study confirm that wild boars can serve as reservoirs and spreaders of Salmonella and Y. enterocolitica; the finding of Y. enterocolitica presence on carcass surface highlights how meat may become superficially contaminated, especially considering that contamination is linked to the conditions related to the hunting, handling and processing of game animals.
1. Introduction
The wild boar (Sus scrofa) is an ungulate belonging to the Suidae family and is one of the most common wildlife species; its diffusion is widespread due to the broad spectrum of habitat types that this species can inhabit, ranging from semi-arid environments to marshes, forests and grasslands [1]. The endemic species in Sardinia (Italy) is Sus scrofa meridionalis, classified as a separate subspecies [2]. The wild boar population has been steadily growing in Europe for several decades, with annual increases in population that can exceed 100 percent [3]. The warming temperatures, especially in winter; the high reproductive rate and adaptability of this species; the absence of natural predators; and the rural depopulation are among the reasons given for the constant increase in the wild boars’ population [4,5,6]. Due to the growing numbers, wild boars are considered an invasive species [7], and they directly and negatively affect the ecosystem in numerous ways, including preying on a wide range of vertebrate and invertebrate species, damaging nest sites and flora, outcompeting local wildlife and serving as disease vectors [8,9,10]. In particular relation to the latter, wild boars are an omnivorous species whose feeding behaviour determines the contact and consumption of different types of foods, of both vegetable and animal origin, including mammals and reptiles, and occasionally, they act as scavengers [11]. Moreover, it is increasingly common for wild boars to approach urban areas attracted to food subsidies in areas of high human activity and therefore come into contact with scraps and waste [12]; due to these reasons, wild boars can potentially come into contact with pathogenic microorganisms. In fact, numerous studies have underlined the importance of wild boars as reservoirs and spreaders of enteric and foodborne pathogens, such as Salmonella spp. and Y. enterocolitica [13,14,15,16].
The zoonotic risk derived from infected wildlife is associated with the spread of pathogens from indirect contamination (contact with farm animals and pastures) and the direct contamination of game meat [14]. In recent years, both the demand for wild boar meat and the consumption of game meat in general are rising; this trend is due to the characteristics of this type of meat, which complies with several consumer demands: it offers good sensory and nutritional profiles and has less of an environmental effect than farmed meat since it originates from animals that were born and grown in natural environments until the moment of harvest [17,18]. However, there are certain issues with game meat; in particular, the lack of microbiological standards and process hygiene criteria makes it challenging to organize supply chain controls. According to EC Regulation 853/2004 (Section 4, Chapter 1), it is sufficient for one person among a group of hunters to have knowledge of hygiene and proper handling techniques and laws concerning the conditions of meat and public health. Moreover, as muscle tissue is considered virtually sterile, many factors can affect the microbiological conditions of meat obtained from game animals, including the types of microorganisms carried by each species, the circumstances of harvest and the conditions under which the carcass is butchered, handled and stored [19,20]. In this framework, the microbiological quality and safety of game meat is highly dependent on the sanitary status of the hunted animals and on the slaughtering and meat processing environments and procedures applied [21,22]. These factors can result in the contamination of meat with consequent possible infection for consumers.
Antimicrobial resistance (AMR) is a pressing worldwide issue: the loss of efficacy of antibiotics against common pathogens causes a significant clinical problem that has lately been compared to the challenges of climate change since it is a global-scale natural process that has been aggravated by human activity [23]. AMR has already been reported in bacteria of wildlife: although it is unlikely that wild animals come into contact with antibiotic substances, the overlap across habitats, in particular the interaction with humanized environments can cause the contact of wildlife with resistant bacteria from humans and other species [24]. In this regard, it has been reported that wildlife populations living in close proximity to humans exhibit higher levels of resistance, while populations living in natural and remote habitats show little to no resistance [25,26,27]. Wild boars, due to their ethological characteristics of being widespread and sharing habitat and distribution with other animals and humans have the potential to be reservoirs and spreaders of resistance genes, acting as a bridge between environments with a strong human influence and wild regions [28]. Numerous reviews have recently investigated the prevalence of AMR genes in wild boars in Europe, concluding that prevalence rates are highly variable and so are the antimicrobial susceptibility profiles and resistance genes detected [27,28,29,30,31].
To the best of our knowledge, very little is known regarding the microbiological quality and safety of meat and the AMR prevalence of pathogens from wild boars hunted in Sardinia; on this basis, the objective of this investigation was to evaluate the prevalence of Salmonella and Y. enterocolitica in wild boars hunted in Sardinia in order to characterize the isolates and evaluate the AMR pattern; moreover, in order to assess the slaughtering hygiene, an evaluation of the contamination of the carcasses at the end of the slaughter was carried out.
2. Materials and Methods
2.1. Sampling
Between 2020 and 2022, samples were taken from 66 wild boars hunted from the area of two provinces, Sassari and Nuoro, in northern and central Sardinia (Italy). The animals were hunted during two consecutive hunting seasons. Wild boars were hunted by authorized hunting companies in the months defined by national and regional legislation (Region of Sardinia Decree number 7602/2011 of 24 August 2020 for season 2020–2021 and Decree number 846/13 of 23 August 2021 for season 2021–2022). A total of 21 wild boars were hunted in the 2020–2021 hunting season and 45 in the following hunting season. According to the hunting area, 53 animals were hunted in the Sassari province and 13 in the Nuoro province.
Driven hunts were performed with hunting dogs during early mornings by parties of five to fifteen hunters; the hunters, armed with rifles, set up various positions, while beaters and hounds herded the boars in the direction of the guns. Carcasses were bled in the field, then the wild boars were collected in pick-up vehicles by the end of the hunting day. After harvesting, the carcasses were transported to dedicated facilities, “hunting houses”, where the slaughtering operations were carried out. Five hunting houses were utilized: three (A, B, C) were in the Sassari province and two (D, E) were in the Nuoro province. Figure 1 specifies the position of the hunting houses in Sardinia.
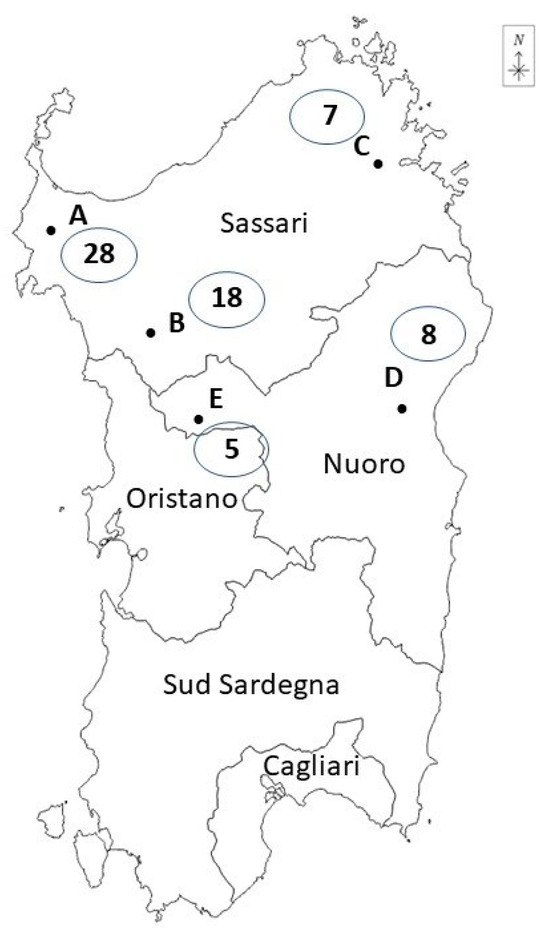
Figure 1.
The geographical distribution of the hunting houses included in the study (Sardinia, Italy) and the number of wild boars sampled.
The following samples were taken from each wild boar immediately after evisceration:
- Mesenteric lymph nodes: at least 25 g of lymph nodes in the ileo-caecal regions were cut out with a sterile, disposable scalpel and collected in a sterile plastic bag (3M Health Care, Milan).
- Colon content: the colon was incised with a sterile, disposable scalpel and at least 25 g of its contents was collected in a sterile plastic bag.
- Carcass surface: samples were taken after evisceration by means of a non-destructive method with a sterile sponge pre-moistened with 10 mL of sterile buffered peptone water (BPW, 3M Health Care, Milan) at the following points: ham, loins, abdomen and throat; these points were selected as they are indicated by European legislation for pig carcasses at the slaughterhouse (Reg. CE No. 2073/2005; ISO 17604:2015 [32]). Sampling was carried out using the same sponge for the four points, with a sterile 10 × 10 cm2 delimiter (Copan, Brescia, Italy), proceeding from the least contaminated point (ham) to the most contaminated (throat). The sponges were handled with a sterile glove and placed inside sterile sponge bags. All the samples were transported to the laboratory at +4 °C and processed within 24 h after collection. A total of 181 samples were collected, divided into 66 colon content samples, 66 mesenteric lymph node samples and 49 carcass surface samples. Carcass surface samples were collected from wild boars slaughtered in hunting houses A, B and C; in the other hunting houses (D, E) the skinning was conducted the day after harvesting, and it was therefore not possible to evaluate the surface contamination of the carcass.
2.2. Microbiological Analysis
Medium for microbiology and reagents were purchased from Biolife (Italy). On each sample, Salmonella and Y. enterocolitica detection was conducted. For the detection of Salmonella spp., the ISO 6579:2020 [33] method was used. The species confirmation of the isolates was performed via the application of simplex PCR for the search of the bcfC gene according to the described protocol [34]. On Salmonella isolates phenotypic serotyping was conducted by slide agglutination and phase inversion (ISO 6579:2020) [33].
Y. enterocolitica detection was conducted according to ISO 10273:2017 [35], with modifications [36], as previously described [37]. At least five colonies with typical appearance were collected from each CIN agar plate and subjected to preliminary characterization tests (urea, sucrose, sorbitol) and biochemical confirmation in Klinger Iron Agar (KIA, Biolife). Species identification was performed via PCR through the amplification of a 330 bp fragment of the 16S rRNA gene [38]. The biotyping and serotyping of Y. enterocolitica strains was carried out at Statens Serum Institut: isolates were serotyped in the slide agglutination test with use of somatic antigens (SSI Diagnostica, Hillerød, Denmark). The strain was classified as nonidentified (NI) in the absence of agglutination with any of the sera. The biotype was determined based on indole production, and salicin, xylose and trehalose (SSI Diagnostica, Denmark) fermentation was carried out according to the ISO standard [35].
On carcass surface samples, decimal dilutions were prepared (ISO 6887:2017) [39] and total aerobic colony count (ACC) and Enterobacteriaceae count were conducted according to ISO 4833-1:2013 [40] and ISO 21528:2017 [41], respectively. As regards the level of surface contamination of the carcasses, the results were expressed as colony-forming units per square centimetre (CFU/cm2) on a logarithmic scale (log10) and compared with the process hygiene criteria thresholds established by the Regulation Commission (EC) No. 2073/2005 for pig carcasses, as the slaughtering procedure was similar to the one used for wild boars. The thresholds reported in the Regulation have been modified to adapt to the non-destructive method, as required by the Italian State-Regions agreement (41/2016): the m and M values indicated by the regulation, intended to distinguish between “satisfactory” (mean log CFU/cm2, m), “acceptable” (mean log CFU/cm2) and “unsatisfactory” (mean log CFU/cm2, M) results, were reduced by 20%.
2.3. Antimicrobial Susceptibility Testing
The antibiotic susceptibility of the isolates was tested using the Kirby–Bauer disc-diffusion method, according to the recommendations of the European Committee on antimicrobial susceptibility testing [42]. Mueller–Hinton agar (Microbiol, Italy) and commercial antimicrobial susceptibility discs (ThermoFisher Scientific, Waltham, MA, USA) were used. Plates were incubated at 35 ± 1 °C for 18–24 h. All isolates were tested for amikacin (Ak, 30 μg), ampicillin (Amp, 10 μg), amoxicillin/clavulanic acid (Aug, 20 μg and 10 μg, respectively), azithromycin (Azm, 15 μg), cephazolin (Kz, 30 μg), cefoxitin (Fox, 30 μg), ceftriaxone (Cro, 30 μg), cefotaxime (CTX, 30 μg), ceftazidime (Caz, 5 μg), ciprofloxacin (Cip, 5 μg), doxycycline (Do, 30 μg), imipenem (Ipm, 10 μg), kanamycin (K, 30 μg), levofloxacin (Lev, 5 μg), meropenem (Mem, 10 μg), nalidixic acid (Na, 30 μg), streptomycin (S10, 10 μg), sulphonamide (S, 300 μg), tetracycline (Te, 30 μg) and trimethoprim/sulfamethoxazole (Sxt, 1:19, 25 μg). According to the test results, isolates were categorized as susceptible or resistant according to the EUCAST recommendations, and intermediate isolates were considered susceptible.
2.4. Whole Genome Sequencing
Whole genome sequencing (WGS) was carried out at Statens Serum Institut on 3 Salmonella isolates and 25 Y. enterocolitica isolates. Sequencing data were uploaded to NCBI under BioProject PRJNA1043856. Bioproject accession number for all the isolates are listed in Supplemental Table S1. Genomic DNA was extracted from an enzymatic pre-lysis step prior to automated purification using the MagNA Pure 96 DNA and Viral NA Small Volume Kit and DNA Blood ds SV 2.0 protocol (Roche Diagnostics). Genomic libraries were constructed, and sequencing was performed using the Nextera XT Kit (Illumina) and 300-cycle kits on the NextSeq® 550 (Illumina, San Diego, CA, USA) platform according to the manufacturer’s instructions. The quality control of the obtained sequencing data was conducted using Bifrost software (https://github.com/ssi-dk/bifrost, accessed on 31 July 2023) to ensure adequate sequencing depth, species verification and the identification of contamination issues.
Salmonella serovars were detected from raw reads using SeqSero ver. 1.0 (for reference see http://denglab.info/SeqSero2, accessed on 31 July 2023) and subspecies were predicted using an in-house script based on the seven-gene MLST (multi-locus sequence type). MLST was determined using read mapping and named according to the Achtman seven-genes MLST scheme for Salmonella [43].
Salmonella and Y. enterocolitica genomes were uploaded to Enterobase [44,45]; on Y. enterocolitica genomes, seven-genes MLST and core-genome MLST (cgMLST) were applied [46]. The seven-gene MLST on Yersinia isolates was performed according to the McNally scheme [41]. The minimum spanning tree was generated with the minimal spanning tree algorithm MSTree V2 in EnteroBase. Salmonella and Y. enterocolitica genomes were subsequently compared to the publicly available genomes using the hierarchical clustering of cgMLST (HierCC clustering) at different levels of resolution [45].
On all samples, resistance, virulence and plasmid-associated genes were obtained from BioNumerics 8.1 plugin (Applied Maths, Sint Martems Latem, Belgium), AMRFinder [47], ResFinder [48], PlasmidFinder [49] and VirulenceFinder [50].
The results of the present study were compared with a similar previous investigation conducted on wild boars from the Asinara National Park [51].
2.5. Statistical Analysis
Differences in the prevalence of Salmonella and Y. enterocolitica between samples (lymph nodes, colon content and carcass surface), hunting houses and hunting days were evaluated using one-way ANOVA with Tukey’s post hoc HSD. The significance level was defined as p < 0.05.
3. Results
3.1. Prevalence of Salmonella and Y. enterocolitica
Salmonella was identified in 3/66 wild boars, with a prevalence of 4.5%. The samples that tested positive were colon content samples only, with a prevalence of 4.5% (3/66) of the total colon content samples and a prevalence of 1.6% (3/181) of the total samples collected from wild boars (colon contents, lymph nodes, carcass surface). Y. enterocolitica had an overall prevalence of 30.3% (20/66) in wild boars. Among the Y. enterocolitica-positive animals, 2/20 (10%) were positive in colon content and carcass surface samples and 2 more animals tested positive in colon content and lymph nodes samples, while 1/20 (5%) were positive for Y. enterocolitica in carcass surface samples only. In particular, Y. enterocolitica was identified in 18/66 (27.3%) colon content samples, in 3/66 (4.5%) mesenteric lymph nodes samples and 3/49 (6.1%) carcass surface samples. Table 1 shows the results regarding the prevalence of Salmonella and Y. enterocolitica in wild boars and in the samples.

Table 1.
Prevalence of Salmonella and Y. enterocolitica in wild boars and samples of colon content, mesenteric lymph nodes and carcass surface (positive/total, prevalence %).
Among the wild boars that tested positive for Salmonella, 2/3 were also positive for Y. enterocolitica in the intestine or lymph nodes. The prevalence of wild boars carrying Salmonella and Y. enterocolitica in relation to hunting houses is reported in Table 2; the highest number of wild boars positive for the two pathogens was detected in hunting house A, with a total of 12/28 positive individuals and a prevalence of 42.8%. However, no statistically significant difference (p > 0.05) was observed between the prevalence recorded in the hunting houses under investigation.

Table 2.
Prevalence of wild boar carriers of Salmonella and Y. enterocolitica in terms of the hunting houses.
Overall, 3 Salmonella isolates and 24 Y. enterocolitica isolates were collected and submitted to further analysis.
3.2. Antimicrobial Susceptibility Testing
Salmonella isolates (3/3, 100%) were susceptible to all antimicrobials tested. Regarding Y. enterocolitica isolates, three different AMR profiles were identified: 10/24 (41.7%) showed resistance to amoxicillin-clavulanic acid, ampicillin and cefoxitin (AugAmpFox); 11/24 (48.8%) showed resistance to amoxicillin-clavulanic acid and ampicillin (AugAmp); 1/24 (4.2%) showed resistance to ampicillin (Amp) and 2/24 (8.3%) were sensible to all antimicrobials tested. Overall, 22/24 (91.7%) of Y. enterocolitica isolates showed phenotypic resistance to at least one beta-lactam compound. The AMR profile of Salmonella and Y. enterocolitica isolates is reported in Table 3.

Table 3.
AMR profile of Salmonella and Y. enterocolitica isolates.
3.3. Salmonella Characterization
The three Salmonella isolates we found were classified as follows: (i) S. subsp. salamae (antigenic formula 48:h,z:1,5), (ii) S. ser. elomrane (9:z38:-) and (iii) a novel serotype of S. subs. enterica (28:e,h:z6). The third Salmonella strain identified is not currently referable to any known serotype and the strain has been sent to the European Union Reference Laboratory for Salmonella (EURL-Salmonella) for further study. Multilocus sequence types (ST) of the strains were, respectively, ST10546, ST7139 and ST10597, as reported in Table 3. The genetic characterization of genes in the virulence factors database showed that a total of 104 virulence genes were detected in the three Salmonella isolates. The Salmonella operons bcfABCDEFG, csg(agf)ABCDEFG, fimCDFHI, invABEFGHJ, and sipABCD were identified in all the isolates; moreover, S. elomrane also had the lpfABCDE operon. The following plasmids were detected: IncFIB(S) in ST 7138 strain, IncFII(S) in the novel serotypes. Salmonella isolates virulence genes detected are reported in Figure 2.

Figure 2.
Virulence genes of Salmonella strains.
Regarding AMR genes, no resistance genes were detected.
3.4. Yersinia enterocolitica Characterization
Among Y. enterocolitica isolates, 13/24 (54.2%) were biotype 1A and 11/24 (45.8%) were biotype 2. Biotype 2 strains were serotype O:3 (6/11, 54.5%) and O:5 (3/11, 27.3%); in 2/11 (18.2%), it was not possible to detect the serotype phenotypically. Among the isolates, 23 STs were detected. The single linkage tree in Figure 2 shows the genetic linkage between the isolates. The most common MLST sequence type was ST860, detected in five samples isolated from four wild boars hunted on two different days but in the same hunting house (A): the single linkage tree based on cgMLST (Figure 3) shows the genetic linkage between the isolates; regarding ST860 strains, isolates were genetically closely related with <7 SNP.
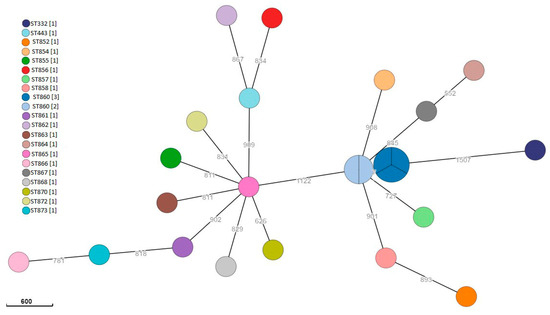
Figure 3.
Minimum spanning tree of Y. enterocolitica isolates based on cgMLST. Branch labels indicate allelic distances. Numbers in square brackets indicate the number of isolates for each ST.
Regarding virulence genes (Figure 4), all isolates had two or more virulence genes, namely arsB (24/24, 100%), arsR (23/24, 95.8%), inv (23/24, 95.8%), myfA (1/24, 4.2%), yfeB (24/24, 100%), ymoA (23/24, 95.8%) and ystB (1/24, 4.2%). Regarding AMR genes, two genes were detected, namely blaA and vat(F). The blaA gene was detected in 100% (24/24) of the isolates. The vat(F) gene was found in the ST332 isolate (1/24, 4.2%). WGS also allowed the detection of five Yersinia aleksiciae isolates, which were detected in 5/66 (7.6%) wild boars. The isolates were found from colon content samples (3/66, 4.5%) and carcass surface samples (2/49, 4.1%) collected from hunting houses B and D. Y. aleksiciae isolates (5/5, 100%) had arsB and ymoA genes. No other typical Y. enterocolitica virulence or AMR genes were detected.
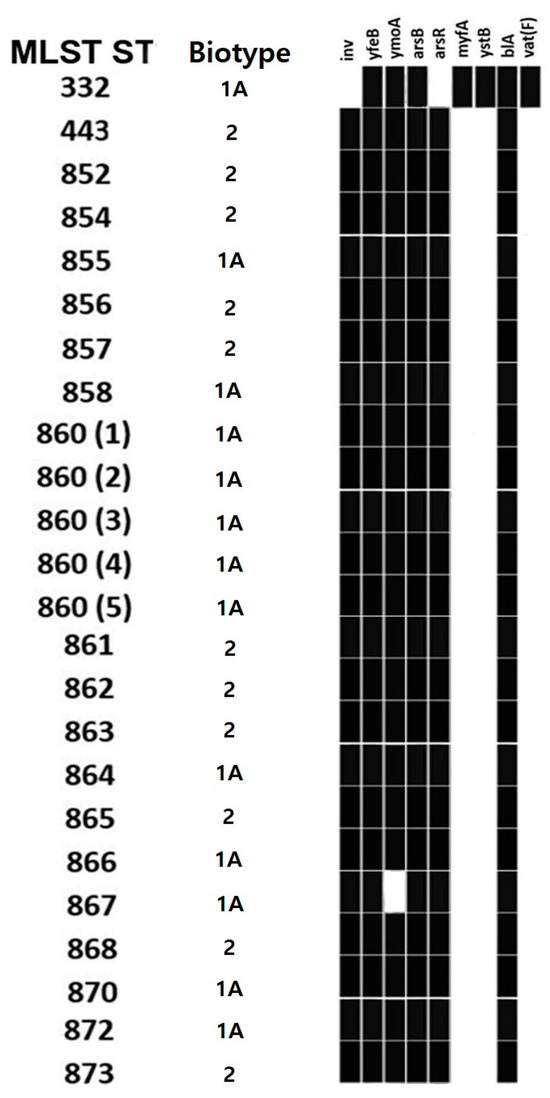
Figure 4.
Virulence genes of Y. enterocolitica isolates based on MLST sequence type and biotype.
3.5. Bacterial Contamination of Carcasses
As shown in Table 4, samples were taken over 12 hunting days and in three hunting houses (A, B and C). The ACC median value (log10 CFU/cm2) in hunting house A was 1.98, with a minimum of 1.98 and a maximum of 4.35; in hunting house B, the value was 2.47, with a minimum of 1.47 and a maximum of 3.91; and in hunting house C, the median was 3.37 (minimum of 2.93 and maximum of 4.00). Regarding Enterobacteriaceae, in hunting house A, the median value (log10 CFU/cm2) was 1.57, with the minimum value below the detection limit and the maximum value of 4.35; in hunting house B, the median value was 0.58 (minimum below the detection limit and maximum of 2.16); hunting house C had a median value equal to 2.48 (minimum of 1.60 and maximum of 3.48). In relation to the compliance with process hygiene criteria, the mean (log10 CFU/cm2 ± standard deviation) values for ACC and Enterobacteriaceae were 2.46 ± 0.97 and 1.07 ± 1.18, respectively. In particular, 37/49 (75.5%) of the samples taken from the surface of the carcasses showed CCA values <3.2 log10 CFU/cm2 and were considered satisfactory, 8/49 (16.3%) samples had values between 3.2 and 4 log10 CFU/cm2 were considered acceptable and 4/49 (8.2%) samples had values >4 log10 CFU/cm2 and were considered unsatisfactory. For the Enterobacteriaceae count, 30/49 (61.2%) samples showed values <1.6 log10 CFU/cm2 and were considered satisfactory, 9/49 (18.4%) samples had values between 1.6 and 2.4 log10 CFU/cm2 were considered acceptable and 10/49 (2%) samples had values >2.4 log10 CFU/cm2 and were considered unsatisfactory.

Table 4.
Bacterial contamination of carcasses during the sampling days (mean log10 CFU/cm2) in hunting houses A–C.
4. Discussion
The prevalence of carrier wild boars (positive in the colon content and/or mesenteric lymph nodes) was higher for Y. enterocolitica (30.3%) than for Salmonella (4.5%). Two cases of co-infection were recorded: Y. enterocolitica isolates were also found in 2/3 of wild boars testing positive for Salmonella. Overall, 33.4% (22/66) of the wild boars carried at least one of the considered pathogens.
In this investigation, Salmonella was isolated in 4.5% (3/66) of wild boars sampled; a similar prevalence has been identified in surveys conducted in central and northern Italy: 4.2% in Tuscany and 6% in Liguria [52,53]. Other authors in various Italian regions have identified a higher prevalence, with a range from 10.8% to 17% [30,54,55,56]. Similar surveys in European countries have shown prevalence ranging from 7.7% to 22% [13,57,58,59,60]. However, prevalence studies conducted on hunted wildlife have limitations because the animals sampled may not be representative of the entire population. This is due to hunting strategies, which tend to select older and heavier animals that are less likely to be Salmonella carriers and shedders than younger ones. In fact, significant differences regarding Salmonella prevalence were observed in pigs of different ages, with pathogen elimination rates decreasing with age [61,62,63]; this may depend on a greater susceptibility to infection in young pigs [64], associated with a greater ability of the pathogen to establish infection in the early stages of life [63]. In this framework, the prevalence of the pathogen in wild populations can be underestimated [56].
In a previous investigation [51] conducted on wild boars coming from the Asinara Island National Park (northwestern end of Sardinia), the prevalence of Salmonella was 46.7%, notably much higher than the findings of the present study. A possible explanation for this difference may depend on the fact that the wild boars were periodically captured within the Asinara Park, as part of the specific plan for the numerical control of the wild boar population on the island. The animals were transported to a slaughterhouse in Sardinia and were therefore possibly affected by the same stressors (capture, handling and transport), which are known to increase the susceptibility and probability of spreading Salmonella in pigs subjected to slaughter [65]. On the other hand, the wild boars in the present study did not undergo the cited stressors before the moment of harvest.
Two Salmonella species and three different serotypes were identified: (i) S. subsp. salamae, (ii) S. elomrane and (iii) a novel serotype of S. subs. enterica (28:e,h:z6). S. salamae and S. elomrane strains are not common in human outbreaks; comparisons with public databases (Enterobase) give few results and also highlights the limited diffusion of these serotypes. The novel serotype did not yield any results regarding publicly available similar isolates. The genome of S. salamae ST10546 was compared to other Salmonella genomes using the hierarchical clustering of cgMLST (HierCC clustering) on Enterobase; the isolate belonged to HC400_202057, which also included six genomes from strains isolated from wild boars in Sardinia in 2016 and 2019 [51]. No other genomes were assigned to cluster HC200_359455, which means that no genome in EnteroBase has less than 200 allele differences, indicating that the S. salamae ST10546 is a rather rare type and, to the best of our knowledge, currently identified only in wild boars in Sardinia. Interestingly, also S. elomrane isolates have been previously isolated in Sardinia from wild boars of the Asinara National Park and in both investigations possessed the same ST 7139. This finding is an indication of the circulation of these strains in wild environments. S. salamae and S. elomrane have also been linked to wild boars in other parts of Italy and Spain [30,51,60]. S. enterica and “non-enterica” subspecies are typically found in the environment and cold-blooded animals: subs. salamae strains have been detected in reptiles [64,66,67,68]; elomrane is a rare serotype and spread by reptiles or migratory birds is also suggested [69,70], although little information regarding this serotype is available. In the wild, the ingestion of contaminated food or water is considered the most typical transmission path of Salmonella [66]; therefore, the observation of Salmonella at colon content level and not in lymph node samples suggests that the infection of wild boars with Salmonella is linked to the diet. The main route of infection for humans is the consumption of the meat of infected animals or the contact (direct or indirect) with reptiles, particularly when they are kept as pets [71,72,73].
The genetic characterization of genes showed that the Salmonella operons bcfABCDEFG, csg(agf)ABCDEFG, fimCDFHI, invABEFGHJ, and sipABCD were identified in all the isolates; moreover S. elomrane also had the lpfABCDE operon. These cited operons are invasion-related genes, widespread in Salmonella isolates and typical of S. Typhimurium and are implicated in the colonization of intestinal tissues [74,75,76,77]. Also, the mig-14 and mgtC genes, related to Salmonella survival and proliferation in macrophages and host [78], were detected in S. elomrane and in the novel Salmonella strain. On the other hand, the spv operon, involved in the modulation of the host immune response to infection [74], was not detected in any strain. The novel Salmonella strains also showed the ratB gene, which is involved in long-term intestinal persistence encoding a Peyer’s patch and cecum colonization factor, and the orgA gene, which is involved in promoting cellular invasion of the pathogen [79,80,81]. S. elomrane and S. subsp. salamae had the cdtB gene, which encodes a variant of the cytolethal distending toxin (CDT), an important virulence factor for S. Typhi but is commonly found also in non-typhoidal serovars [82].
As regards Y. enterocolitica, it was isolated from 30.3% (20/66) of the wild boars sampled in our investigation; this prevalence is higher than observed in similar surveys in Italy that reported values between 2.9% and 17.8% [31,52,83]. Other authors have also reported a highly variable prevalence in wild boars in Europe, ranging from 1.3% to 33.3% [58,84,85,86]. The high prevalence of this microorganism in wild boars probably depends on the contact with other infected wild species and/or livestock reared in extensive grazing systems [9]; in particular, sheep have been described as a reservoir of Y. enterocolitica strains [87,88]. However, there is little information regarding Yersinia infections in sheep in Sardinia or regarding their relationship with species identified in wild boars, although this zoonotic pathogen has been already identified in raw sheep milk and cheese-making plants in Sardinia [37,89]. It is also noteworthy that the wild boar sampling took place over two hunting seasons conducted from November to January over the course of two consecutive seasons (2020–2021 and 2021–2022). In this regard, some authors have observed a seasonality in the prevalence of pathogenic microorganisms, including Yersinia, in wild animals, with an increase in cases concentrated in the winter months [87,90]. Latent infection can, in fact, manifest itself in stressful conditions, such as those observed in the cold season in which animals, especially wild species, are exposed to low temperatures and food shortage [91]. The sequence types identified in Y. enterocolitica strains were very diverse. The most common sequence type was ST860, detected in five genetically closely related isolates found in four wild boars, processed in the same hunting house (A) and less than one month apart. As shown in Figure 2, overall, the ST860 strains had less than 10 SNP between each other. Although these data cannot distinguish between contaminations linked to animal-to-animal transmission or common environmental sources, genetic relatedness points to epidemiological connections among the strains.
Regarding the virulence genes detected in the isolates, inv, myfA and ystB are chromosomal virulence genes that encode for the internalization factor invasin invA and the mucoid Yersiniae factor myfA, respectively [87]. The heat-stable enterotoxin gene ystB was detected in 1/24 (4.2%) of the strains, while the heat-stable enterotoxin gene ystA was never detected. Moreover, the ymoA gene was also detected in 23/24 (95.8%) of the strains, which negatively modulate the expression and transcription of virulence factors, inv and yst genes in particular [92,93]. The ars operon confers virulence and resistance to arsenite, arsenate and antimonite in Yersinia species; yfe is a transport system that accumulates both iron and manganese [94]. YstB gene is usually carried by 1A biotype strains, while ystA is detected in pathogenic 1B strains [43,87,90]; therefore, our findings suggest that the Y. enterocolitica strains detected in the present investigation are not particularly pathogenic.
Five Y. aleksiciae strains were isolated among wild boars. Y. aleksiciae is categorized among the Y. enterocolitica-like species [95,96]. Strains appear to have scarce pathogenicity, due to the lack of typical Y. enterocolitica virulence genes. The species seem to be well adapted to warm-blooded animals, and it has been isolated from the faeces of humans, rats, reindeer and pigs, as well as from dairy products [97]. However, the importance of this species must be studied further.
As reported in Table 3, in our study, no AMR profile was detected in Salmonella isolates. Y. enterocolitica strains (24/24, 100%) possessed the blaA gene, which encodes for the production the β-lactamase BlaA (a constitutive class A enzyme) [98]. The presence of the gene was reflected in phenotypical resistance to at least one β-lactam compound in 91.7% (22/24) of the strains. This result was expected as the blaA gene has been reported widely in Y. enterocolitica isolates, regardless of biovars or the geographical origin of the strains, and intrinsic resistance to β-lactam compounds has been suggested by EUCAST [42]. In 1/24 (4.7%) Y. enterocolitica isolate (ST332), the vat(F) gene was detected; it is a chromosomal gene that encodes resistance towards streptogramin and is also widespread in Y. enterocolitica strains [99,100]. The detection of resistance patterns and genes are typical of the species and are widespread in the isolates, suggesting that these are inherent resistances, and most likely not acquired from the environment or from contact with other resistant strains. The low prevalence of AMR in both Salmonella and Y. enterocolitica isolates is probably due to the low selective pressure given by the low level of exposure to antimicrobial substances of resistant microbial populations. This positive result could also indicate the scarce anthropic impact in the areas where wild boars live [27].
Regarding surface contamination of carcasses, the mean values (log10 CFU/cm2 ± standard deviation) of the ACC were 2.46 ± 0.97, while the mean values of Enterobacteriaceae were 1.07 ± 1.18. In particular, based on the limits established for surface samples of pig carcasses by EC Regulation no. 2073/2005 and the Italian State-Regions Agreement 41/2016, 8.2% of the samples (4/49) and 2% (10/49) showed values higher than the maximum limit established for CCA and Enterobacteriaceae, respectively. The average values observed in our investigation are, however, lower than those reported in similar studies [22,55,101,102]. The average values identified in the present study indicate an overall correct application of hygiene practices (GHP) and slaughtering practices (GMP) during game meat handling. However, the values differed in the sampling days, particularly for the levels of Enterobacteriaceae (p < 0.05), where the range was between a minimum mean value of 0.10 ± 0.22 and a maximum mean value of 3.68 ± 0.52. The production of meat from hunted wildlife might occasionally reveal some deficiencies in the GHP and GMP application process. Particularly, the harvesting and processing of meat frequently take place in conditions that are unsuitable for meat production [22]. In this regard, in our investigation, after killing, the wild boars were collected on pick-up vehicles, and therefore exposed to environmental conditions, until the end of the hunting day; subsequently, the carcasses were transported to dedicated facilities where the slaughter operations were carried out. At these stages, the time and conditions between the moment of death and processing were highly variable among animals [20]. Some authors have observed the higher contamination of carcasses on hunting days characterized by adverse weather conditions, which provide settings for faecal and environmental contamination or the spread of pre-existing contamination [22]. In the present investigation, 6.1% (3/49) of the carcass surface samples tested positive for Y. enterocolitica and one wild boar was positive only in carcass surface samples. This is probably due to cross-contamination and poor hygiene in the processing phases or incorrect evisceration practices. The presence of wild boars excreting Salmonella and Y. enterocolitica strains poses a concern to consumers since it is feasible that meat and carcasses become superficially contaminated, particularly since the conditions during the hunting, handling and processing of game carcasses are crucial to microbiological contamination. Even though game meat is usually consumed cooked, wild boar meat products are frequently only dry-cured (such as traditional dry, fermented sausages), and it is therefore possible for pathogenic bacteria to cause foodborne infections [101]. The education of hunters and the use of appropriate hygienic procedures are essential in this circumstance.
5. Conclusions
Monitoring enteric pathogens in wildlife is crucial to trace the evolution and factors contributing to selection and spread. The results of our investigation confirm that wild boars in Sardinia can act as reservoirs and spreaders of both Salmonella and Y. enterocolitica strains. The wild boars in this study hosted uncommon strains, due to the wild environment. The data show overall good carcass hygiene status, with generally acceptable contamination levels. However, the presence of wild boars carrying enteric pathogens represents a risk for the consumer as it is possible that the superficial contamination of carcasses and meat may occur, especially throughout the processing of game meat. In this context, in particular, the training of hunters and the application of good hygiene practices is fundamental. Regarding antimicrobial resistance, an overall low prevalence of resistant strains in the isolates identified from wild boars hunted in Sardinia, with the detection of only intrinsic and expected resistance profiles. This positive result could indicate the low level of exposure to antimicrobial compounds and scarce contact with resistant strains in wild areas. However, the constant numerical increase of the wild boar population and the possible contacts between humans and farmed and wild animals due to the expansion of urban areas make it necessary to constantly monitor the spread of antimicrobial resistance in wild species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13010065/s1, Table S1: Bioproject accession number for all the isolates.
Author Contributions
C.S., F.P. and E.P.L.D.S. conceived, validated, and supervised the research; methodology was created by C.S., F.P., G.S. and M.T.; G.S., F.P., M.P.M., P.G., M.M., D.C. and M.C. carried out the formal analysis; G.S., F.P., P.G., M.T., M.F.-A. and M.P.M. contributed to writing, reviewing and editing the manuscript; funding acquisition was carried out by C.S. and E.P.L.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondo di Ateneo per la Ricerca 2020, University of Sassari, Resp. Christian Scarano.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequencing data were uploaded to NCBI under BioProject PRJNA1043856. Bioproject accession number for all the isolates are listed in Supplemental Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Powell, D.M. Pigs. In Grzimek’s Animal Life Encyclopedia, 2nd ed.; Kleiman, D.G., Geist, V., McDade, M., Eds.; Mammals IV; Michael Hutching, Ed.; Gale Research Inc.: Farmington Hills, MI, USA, 2003; Volume 15, pp. 275–290. [Google Scholar]
- Scandura, M.; Iacolina, L.; Cossu, A.; Apollonio, M. Effects of human perturbation on the genetic make-up of an island population: The case of the Sardinian Wild Boar. Heredity 2010, 106, 1012–1020. [Google Scholar] [CrossRef][Green Version]
- Náhlik, A.; Cahill, S.; Cellina, S.; Gál, J.; Jánoska, F.; Rosell, C.; Massei, G. Wild Boar Management in Europe: Knowledge and Practice. In Ecology, Conservation and Management of Wild Pigs and Peccaries; Melletti, M., Meijaard, E., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 339–353. [Google Scholar] [CrossRef]
- Bieber, C.; Ruf, T. Population dynamics in wild boar Sus scrofa: Ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. J. Appl. Ecol. 2005, 42, 1203–1213. [Google Scholar] [CrossRef]
- Sales, J.; Kotrba, R. Meat from wild boar (Sus scrofa L.): A Review. Meat Sci. 2013, 94, 187–201. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Lowe, S.M.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000; Volume 12. [Google Scholar]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Gortázar, C.; Ferroglio, E.; Höfle, U.; Frölich, K.; Vicente, J. Diseases shared between wildlife and livestock: A European perspective. Eur. J. Wildl. Res. 2007, 53, 241. [Google Scholar] [CrossRef]
- Risch, D.R.; Ringma, J.; Price, M.R. The global impact of wild pigs (Sus scrofa) on terrestrial biodiversity. Sci. Rep. 2021, 11, 13256. [Google Scholar] [CrossRef]
- Chiari, M.; Zanoni, M.; Tagliabue, S.; Lavazza, A.; Alborali, L.G. Salmonella serotypes in wild boars (Sus scrofa) hunted in northern Italy. Acta Vet. Scand. 2013, 55, 42. [Google Scholar] [CrossRef]
- Davidson, A.; Malkinson, D.; Shanas, U. Wild boar foraging and risk perception—Variation among urban, natural, and agricultural areas. J. Mammal. 2022, 103, 945–955. [Google Scholar] [CrossRef]
- Wacheck, S.; Fredriksson-Ahomaa, M.; König, M.; Stolle, A.; Stephan, R. Wild boars as an important reservoir for foodborne pathogens. Foodborne Pathog. Dis. 2010, 7, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, F.; Smulders, F.J.M.; Chopra-Dewasthaly, R.; Paulsen, P. Salmonella in the wildlife-human interface. Food Res. Int. 2012, 45, 603–608. [Google Scholar] [CrossRef]
- Brown, V.R.; Bowen, R.A.; Bosco-Lauth, A.M. Zoonotic pathogens from feral swine that pose a significant threat to public health. Transbound. Emerg. Dis. 2018, 65, 649–659. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M. Wild boar: A reservoir of Foodborne Zoonoses. Foodborne Pathog. Dis. 2019, 16, 153–165. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D. Editorial for the Special Issue: Game Meat and Game Meat Products: Safety, Quality and Consumer Perception. Foods 2022, 11, 2073. [Google Scholar] [CrossRef]
- Corradini, A.; Marescotti, M.E.; Demartini, E.; Gaviglio, A. Consumers’ perceptions and attitudes toward hunted wild game meat in the modern world: A literature review. Meat Sci. 2022, 194, 108955. [Google Scholar] [CrossRef]
- Gill, C.O. Microbiological conditions of meats from large game animals and birds. Meat Sci. 2007, 77, 149–160. [Google Scholar] [CrossRef]
- Gomes-Neves, E.; Abrantes, A.C.; Vieira-Pinto, M.; Müller, A. Wild game meat—A microbiological safety and hygiene challenge? Curr. Clin. Microbiol. Rep. 2021, 8, 31–39. [Google Scholar] [CrossRef]
- Giuggioli, G.; Olivastri, A.; Pennisi, L.; Paludi, D.; Ianieri, A.; Vergara, A. The hygiene-sanitary control in the wild game meats. Ital. J. Food Saf. 2018, 6, 222–224. [Google Scholar] [CrossRef][Green Version]
- Ranucci, D.; Roila, R.; Onofri, A.; Cambiotti, F.; Primavilla, S.; Miraglia, D.; Andoni, E.; Di Cerbo, A.; Branciari, R. Improving Hunted wild boar carcass hygiene: Roles of different factors involved in the harvest phase. Foods 2021, 10, 1548. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 1670. [Google Scholar] [CrossRef] [PubMed]
- Vittecoq, M.; Godreuil, S.; Prugnolle, F.; Durand, P.; Brazier, L.; Renaud, N.; Arnal, A.; Aberkane, S.; Jean-Pierre, H.; Gauthier-Clerc, M.; et al. Antimicrobial resistance in wildlife. J. Appl. Ecol. 2016, 53, 519–529. [Google Scholar] [CrossRef]
- Gilliver, M.A.; Bennett, M.; Begon, M.; Hazel, S.M.; Hart, C.A. Antibiotic resistance found in wild rodents. Nature 1999, 401, 233–234. [Google Scholar] [CrossRef]
- Österblad, M.; Norrdahl, K.; Korpimäki, E.; Huovinen, P. How wild are wild mammals? Nature 2001, 409, 37–38. [Google Scholar] [CrossRef]
- Tinoco Torres, R.; Fernandes, J.; Carvalho, J.; Cunha, M.V.; Caetano, T.; Mendo, S.; Serrano, E.; Fonseca, C. Wild boar as a reservoir of antimicrobial resistance. Sci. Total Environ. 2020, 717, 135001. [Google Scholar] [CrossRef]
- Plaza-Rodríguez, C.; Alt, K.; Grobbel, M.; Hammerl, J.A.; Irrgang, A.; Szabo, I.; Stingl, K.; Schuh, E.; Wiehle, L.; Pfefferkorn, B.; et al. Wildlife as sentinels of antimicrobial resistance in Germany? Front. Vet. Sci. 2021, 7, 627821. [Google Scholar] [CrossRef]
- Literak, I.; Dolejska, M.; Radimersky, T.; Klimes, J.; Friedman, M.; Aarestrup, F.M.; Hasman, H.; Cizek, A. Antimicrobial-resistant faecal Escherichia coli in wild mammals in Central Europe: Multiresistant Escherichia coli producing extended-spectrum beta-lactamases in wild boars. J. Appl. Microbiol. 2010, 108, 1702–1711. [Google Scholar] [CrossRef]
- Zottola, T.; Montagnaro, S.; Magnapera, C.; Sasso, S.; De Martino, L.; Bragagnolo, A.; D’Amici, L.; Condoleo, R.; Pisanelli, G.; Iovane, G.; et al. Prevalence and antimicrobial susceptibility of Salmonella in European wild boar (Sus scrofa); Latium region–Italy. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 161–168. [Google Scholar] [CrossRef]
- Modesto, P.; De Ciucis, C.G.; Vencia, W.; Pugliano, M.C.; Mignone, W.; Berio, E.; Masotti, C.; Ercolini, C.; Serracca, L.; Andreoli, T.; et al. Evidence of antimicrobial resistance and presence of pathogenicity genes in Yersinia enterocolitica isolate from wild boars. Pathogens 2021, 10, 398. [Google Scholar] [CrossRef]
- ISO 17604; Microbiology of the Food Chain. Carcass sampling for microbiological analysis. The International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 6579; Microbiology of the Food Chain. Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. The International Organization for Standardization: Geneva, Switzerland, 2020.
- Kiskároly, F.; Morić, I.; Dokić, L.; Vasiljević, B.; Šenerović, L.; Mišić, D. Development of PCR-based identification of Salmonella enterica serovars. Acta Vet.-Beograd 2017, 67, 271–284. [Google Scholar] [CrossRef]
- ISO 10273; Microbiology of the Food Chain. Horizontal Method for the Detection of Pathogenic Yersinia Enterocolitica. The International Organization for Standardization: Geneva, Switzerland, 2017.
- Van Damme, I.; Berkvens, D.; Baré, J.; De Zutter, L. Influence of isolation methods on the occurrence of plasmid-carrying Yersinia enterocolitica serotype O:3 in slaughter pig tonsils, faeces and carcass surface swabs. Int. J. Food Microbiol. 2013, 164, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Piras, F.; Spanu, C.; Sanna, R.; Siddi, G.; Mocci, A.M.; Demontis, M.; Meloni, M.P.; Spanu, V.; De Santis, E.P.L.; Scarano, C. Detection, virulence genes and antimicrobial resistance of Yersinia enterocolitica in sheep and goat raw milk. Int. Dairy. J. 2021, 117, 105011. [Google Scholar] [CrossRef]
- Garzetti, D.; Susen, R.; Fruth, A.; Tietze, E.; Heesemann, J.; Rakin, A. A molecular scheme for Yersinia enterocolitica patho-serotyping derived from genome-wide analysis. Int. J. Med. Microbiol. 2014, 304, 275–283. [Google Scholar] [CrossRef] [PubMed]
- ISO 6887; Microbiology of the Food Chain. Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. The International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4833; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. The International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21528; Microbiology of the Food Chain. Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. The International Organization for Standardization: Geneva, Switzerland, 2017.
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters 2023; Version 13.1. 2023. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 8 November 2023).
- Achtman, M.; Wain, J.; Weill, F.-X.; Nair, S.; Zhou, Z.; Sangal, V.; Krauland, M.G.; Hale, J.L.; Harbottle, H.; Uesbeck, A.; et al. Correction: Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 2020, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Enterobase. Available online: http://enterobase.warwick.ac.uk/ (accessed on 8 November 2023).
- Zhou, Z.; Alikhan, N.-F.; Mohamed, K.; Fan, Y.; Achtman, M. The user’s guide to comparative genomics with EnteroBase, including case studies on transmissions of micro-clades of Salmonella, the phylogeny of ancient and modern Yersinia pestis genomes, and the core genomic diversity of all Escherichia. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Chattaway, M.A.; Reuter, S.; Savin, C.; Strauch, E.; Carniel, E.; Connor, T.; VanDamme, I.; Rajakaruna, L.; Rajendram, D.; et al. Use of whole-genus genome sequence data to develop a multilocus sequence typing tool that accurately identifies Yersinia isolates to the species and subspecies levels. J. Clin. Microbiol. 2015, 53, 35–42. [Google Scholar] [CrossRef] [PubMed]
- AMR Finder. Available online: https://github.com/ncbi/amr (accessed on 8 November 2023).
- ResFinder. Available online: https://cge.food.dtu.dk/services/ResFinder/ (accessed on 8 November 2023).
- PlasmidFinder. Available online: https://cge.cbs.dtu.dk/services/PlasmidFinder/ (accessed on 8 November 2023).
- VirulenceFinder. Available online: https://cge.food.dtu.dk/services/VirulenceFinder/ (accessed on 8 November 2023).
- Piras, F.; Spanu, V.; Siddi, G.; Gymoese, P.; Spanu, C.; Cibin, V.; Schjørring, S.; De Santis, E.P.L.; Scarano, C. Whole-genome sequencing analysis of highly prevalent Salmonella serovars in wild boars from a national park in Sardinia. Food Control 2021, 130, 108247. [Google Scholar] [CrossRef]
- Cilia, G.; Turchi, B.; Fratini, F.; Bilei, S.; Bossù, T.; De Marchis, M.L.; Cerri, D.; Pacini, M.I.; Bertelloni, F. Prevalence, virulence and antimicrobial susceptibility of Salmonella spp., Yersinia enterocolitica and Listeria monocytogenes in European Wild Boar (Sus scrofa) hunted in Tuscany (Central Italy). Pathogens 2021, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Razzuoli, E.; Listorti, V.; Martini, I.; Migone, L.; Decastelli, L.; Mignone, W.; Berio, E.; Battistini, R.; Ercolini, C.; Serracca, L.; et al. Prevalence and antimicrobial resistances of Salmonella spp. isolated from Wild Boars in Liguria Region, Italy. Pathogens 2021, 10, 568. [Google Scholar] [CrossRef]
- Botti, V.; Navillod, F.V.; Domenis, L.; Orusa, R.; Pepe, E.; Robetto Guidetti, C. Salmonella spp. and antibiotic-resistant strains in wild mammals and birds in north-western Italy from 2002 to 2010. Vet. Ital. 2013, 49, 195–202. [Google Scholar]
- Stella, S.; Tirloni, E.; Castelli, E.; Colombo, F.; Bernardi, C. Microbiological evaluation of carcasses of Wild Boar hunted in a hill area of Northern Italy. J. Food Prot. 2018, 81, 1519–1525. [Google Scholar] [CrossRef]
- Bonardi, S.; Bolzoni, L.; Zanoni, R.G.; Morganti, M.; Corradi, M.; Gilioli, S.; Pongolini, S. Limited exchange of Salmonella among domestic pigs and wild boars in Italy. Ecohealth 2019, 16, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Pinto, M.; Morais, L.; Caleja, C.; Themudo, P.; Aranha, J.; Torres, C.; Igrejas, G.; Poeta, P.; Martins, C. Salmonella spp. in wild boar (Sus scrofa): A public and animal health concern. In Game Meat Hygiene in Focus; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 131–136. [Google Scholar] [CrossRef]
- Sannö, A.; Aspán, A.; Hestvik, G.; Jacobson, M. Presence of Salmonella spp.; Yersinia enterocolitica, Yersinia pseudotuberculosis and Escherichia coli O157:H7 in wild boars. Epidemiol. Infect. 2014, 142, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Sannö, A.; Rosendal, T.; Aspán, A.; Backhans, A.; Jacobson, M. Distribution of enteropathogenic Yersinia spp. and Salmonella spp. in the Swedish wild boar population, and assessment of risk factors that may affect their prevalence. Acta Vet. Scand. 2018, 60, 40. [Google Scholar] [CrossRef] [PubMed]
- Gil Molino, M.; García Sánchez, A.; Risco Pérez, D.; Gonçalves Blanco, P.; Quesada Molina, A.; Rey Pérez, J.; Martín Cano, F.E.; Cerrato Horrillo, R.; Hermoso-de-Mendoza Salcedo, J.; Fernández Llario, P. Prevalence of Salmonella spp. in tonsils, mandibular lymph nodes and faeces of wild boar from Spain and genetic relationship between isolates. Transbound. Emerg. Dis. 2019, 66, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Vigo, G.B.; Cappuccio, J.A.; Piñeyro, P.E.; Salve, A.; Machuca, M.A.; Quiroga, M.A.; Moredo, F.; Giacoboni, G.; Cancer, J.L.; Caffer, I.G.; et al. Salmonella enterica subclinical infection: Bacteriological, serological, pulsed-field gel electrophoresis, and antimicrobial resistance profiles-longitudinal study in a three-site farrow-to-finish farm. Foodborne Pathog. Dis. 2009, 6, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Molla, B.; Sterman, A.; Mathews, J.; Artuso-Ponte, V.; Abley, M.; Farmer, W.; Rajala-Schultz, P.; Morrow, M.W.E.; Gebreyes, W.A. Salmonella enterica in commercial swine feed and subsequent isolation of phenotypically and genotypically related strains from fecal samples. Appl. Environ. Microbiol. 2010, 76, 21. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Funk, J.A.; Bolin, C. Risk factors associated with persistence of Salmonella shedding in finishing pigs. Prev. Vet. Med. 2014, 116, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A.; Rodriguez-Zas, S.L.; Ellis, M.; Salak-Johnson, J.L. Breed and age affect baseline immune traits, cortisol, and performance in growing pigs. Sci. J. Anim. Sci. 2005, 83, 2087–2095. [Google Scholar] [CrossRef]
- Pesciaroli, M.; Cucco, L.; De Luca, S.; Massacci, F.R.; Maresca, C.; Medici, L.; Paniccià, M.; Scoccia, E.; Staffolani, M.; Pezzotti, G.; et al. Association between pigs with high caecal Salmonella loads and carcass contamination. Int. J. Food Microbiol. 2017, 242, 82–86. [Google Scholar] [CrossRef]
- Hidalgo-Vila, J.; Díaz-Paniagua, C.; de Frutos-Escobar, C.; Jiménez-Martínez, C.; Pérez-Santigosa, N. Salmonella in free living terrestrial and aquatic turtles. Vet. Microbiol. 2007, 119, 311–315. [Google Scholar] [CrossRef]
- Scheelings, T.F.; Lightfoot, D.; Holz, P. Prevalence of Salmonella in Australian reptiles. J. Wildl. Dis. 2011, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, A.M.; Sandvik, L.M.; Skarstein, M.M.; Svendal, L.; Debenham, J.J. Prevalence of Salmonella serovars isolated from reptiles in Norwegian zoos. Acta Vet. Scand. 2020, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Orlandella, B.M. Migrating birds and Infectious Diseases: Research on the role of the migrating birds in the spread of Salmonella. A First Report. Acta Med. Vet. 1986, 32, 73–87. [Google Scholar]
- Orlandella, V.; Alosi, C.; Campagna, A.; Conti, F.; Ilacqua, G.; Coppola, L. Studies on the epidemic-epizootologic role of Lacertilia in the diffusion of Salmonella. II. Isolation of S. elomrane, S. elomrane, var. 01, S. minnesota, S. siegburg, S. siegburg, var. sandinia, S. uphill and S. rhône from chalcides ocellatus tiligugu, Gmelin (syn. Gongyllus ocellatus, Bedriaga) of the family Scincidae. G. Di Batteriol. Virol. Immunol. Ann. Dell’ospedale Maria Vittor. Torino 1971, 64, 300–315. [Google Scholar]
- Bertrand, S.; Rimhanen-Finne, R.; Weill, F.X.; Rabsch, W.; Thornton, L.; Perevoščikovs, J.; van Pelt, W.; Heck, M. Salmonella infections associated with reptiles: The current situation in Europe. Euro Surveill. 2008, 13, 18902. [Google Scholar] [CrossRef] [PubMed]
- Wikström, V.O.; Fernström, L.; Melin, L.; Boqvist, S. Salmonella isolated from individual reptiles and environmental samples from terraria in private households in Sweden. Acta Vet. Scand. 2014, 56, 7. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Miranda, J.M.; Regal, P.; V’azquez, B.; Franco, C.M.; Cepeda, A. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol. Res. 2018, 206, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Galán, J.E. Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol. 1996, 20, 263–271. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Tsolis, R.M.; Valentine, P.J.; Ficht, T.A.; Heffron, F. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella Typhimurium to cause murine typhoid. Infect. Immun. 1997, 65, 2254–2259. [Google Scholar] [CrossRef]
- van der Velden, A.W.M.; Bäumler, A.J.; Tsolis, R.M.; Heffron, F. Multiple fimbrial adhesins are required for full virulence of Salmonella Typhimurium in mice. Infect. Immun. 1998, 66, 2803–2808. [Google Scholar] [CrossRef]
- Weening, E.H.; Barker, J.D.; Laarakker, M.C.; Humphries, A.D.; Tsolis, R.M.; Bäumler, A.J. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 2005, 73, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, I.E.; Ghori, N.; Falkow, S.; Monack, D. Mig-14 is an inner membrane-associated protein that promotes Salmonella typhimurium resistance to CRAMP, survival within activated macrophages and persistent infection. Mol. Microbiol. 2005, 55, 954–972. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Puente, J.L.; Calva, E. Salmonella virulence plasmid: Pathogenesis and ecology. Pathog. Dis. 2017, 75, ftx070. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, R.A.; Humphries, A.D.; Weening, E.H.; De Zoete, M.R.; Winter, S.; Papaconstantinopoulou, A.; Dougan, G.; Bäumler, A.J. Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype Typhimurium: Identification of intestinal colonization and persistence determinants. Infect. Immun. 2003, 71, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.D.; Schmieder, R.; Silva, G.G.; Busch, J.; Cassman, N.; Dutilh, B.E.; Green, D.; Matlock, B.; Heffernan, B.; Olsen, G.J. Genomic comparison of the closely-related Salmonella enterica serovars Enteritidis, Dublin and Gallinarum. PLoS ONE 2015, 10, 0126883. [Google Scholar] [CrossRef]
- Miller, R.; Wiedmann, M. Dynamic Duo-The Salmonella cytolethal distending toxin combines adp-ribosyltransferase and nuclease activities in a novel form of the cytolethal distending toxin. Toxins 2016, 8, 121. [Google Scholar] [CrossRef]
- Bonardi, S.; Brémont, S.; Vismarra, A.; Poli, I.; Diegoli, G.; Bolzoni, L.; Corradi, M.; Gilioli, S.; Le Guern, A.S. Is Yersinia bercovieri surpassing Yersinia enterocolitica in Wild Boars (Sus scrofa)? Ecohealth 2020, 17, 388–392. [Google Scholar] [CrossRef]
- Bancerz-Kisiel, A.; Platt-Samoraj, A.; Szczerba-Turek, A.; Syczyło, K.; Szweda, W. The first pathogenic Yersinia enterocolitica bioserotype 4/O:3 strain isolated from a hunted wild boar (Sus scrofa) in Poland. Epidemiol. Infect. 2015, 143, 2758–2765. [Google Scholar] [CrossRef]
- von Altrock, A.; Seinige, D.; Kehrenberg, C. Yersinia enterocolitica Isolates from Wild Boars Hunted in Lower Saxony, Germany. Appl. Environ. Microbiol. 2015, 81, 4835–4840. [Google Scholar] [CrossRef]
- Arrausi-Subiza, M.; Gerrikagoitia, X.; Alvarez, V.; Ibabe, J.C.; Barral, M. Prevalence of Yersinia enterocolitica and Yersinia pseudotuberculosis in wild boars in the Basque Country, northern Spain. Acta Vet. Scand. 2016, 58, 4. [Google Scholar] [CrossRef]
- Slee, K.J.; Skilbeck, N.W. Epidemiology of Yersinia pseudotuberculosis and Y. enterocolitica infections in sheep in Australia. J. Clin. Microbiol. 1992, 30, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Söderqvist, K.; Boqvist, S.; Wauters, G.; Vågsholm, I.; Thisted-Lambertz, S. Yersinia enterocolitica in sheep-a high frequency of biotype 1A. Acta Vet. Scand. 2012, 54, 39. [Google Scholar] [CrossRef] [PubMed]
- Piras, F.; Siddi, G.; Le Guern, A.S.; Brémont, S.; Fredriksson-Ahomaa, M.; Sanna, R.; Meloni, M.P.; De Santis, E.P.L.; Scarano, C. Traceability, virulence and antimicrobial resistance of Yersinia enterocolitica in two industrial cheese-making plants. Int. J. Food Microbiol. 2023, 398, 110225. [Google Scholar] [CrossRef] [PubMed]
- Milnes, A.S.; Sayers, A.R.; Stewart, I.; Clifton-Hadley, F.A.; Davies, R.H.; Newell, D.G.; Cook, A.J.; Evans, S.J.; Smith, R.P.; Paiba, G.A. Factors related to the carriage of Verocytotoxigenic E. coli, Salmonella, thermophilic Campylobacter and Yersinia enterocolitica in cattle, sheep and pigs at slaughter. Epidemiol. Infect. 2009, 137, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Mair, N.S. Yersiniosis in wildlife and its public health implications. J. Wildl. Dis. 1973, 9, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bancerz-Kisiel, A.; Pieczywek, M.; Łada, P.; Szweda, W. The most important virulence markers of Yersinia enterocolitica and their role during infection. Genes 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.W.; Young, B.; Nelson, K.; Miller, V.L. YMOA negatively regulates expression of invasin from Yersinia enterocolitica. J. Bacteriol. 2003, 185, 7153–7159. [Google Scholar] [CrossRef] [PubMed]
- Fetherston, J.D.; Mier, I.; Truszczynska, H.; Perry, R.D. The YFE and feo transporters are involved in microaerobic growth and virulence of Yersinia pestis in bubonic plague. Infect. Immun. 2012, 80, 3880–3891. [Google Scholar] [CrossRef]
- Howard, S.L.; Gaunt, M.W.; Hinds, J.; Witney, A.A.; Stabler, R.; Wren, B.W. Application of comparative phylogenomics to study the evolution of Yersinia enterocolitica and to identify genetic differences relating to pathogenicity. J. Bacteriol. 2006, 188, 3645–3653. [Google Scholar] [CrossRef]
- Falcão, J.P.Y.; Batt, C.A.; Tortorello, M.L. Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-384733-1. [Google Scholar]
- Sprague, L.D.; Neubauer, H. Yersinia aleksiciae sp. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 831–835. [Google Scholar] [CrossRef]
- Stock, I.; Heisig, P.; Wiedemann, B. β-Lactamase expression in Yersinia enterocolitica biovars 1A, 1B and 3. J. Med. Microbiol. 2000, 49, 403–408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seoane, A.; García Lobo, J.M. Identification of a streptogramin, an acetyltransferase gene in the chromosome of Yersinia enterocolitica. Antimicrob. Agents Chemother. 2000, 44, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Kuhn, M.L.; Evdokimova, E.; Courvalin, P.; Anderson, W.F.; Savchenko, A. Potential for reduction of streptogramin A resistance revealed by structural analysis of acetyltransferase VatA. Antimicrob. Agents Chemother. 2014, 58, 7083–7092. [Google Scholar] [CrossRef] [PubMed]
- Mirceta, J.; Petrovic, J.; Malesevic, M.; BlaAgojevic, B.; Antic, D. Assessment of microbial carcass contamination of hunted wild boars. Eur. J. Wildl. Res. 2017, 63, 37–44. [Google Scholar] [CrossRef]
- Bonardi, S.; Tansini, C.; Cacchioli, A.; Soliani, L.; Poli, L.; Lamperti, L.; Corradi, M.; Gilioli, S. Enterobacteriaceae and Salmonella contamination of wild boar (Sus scrofa) carcasses: Comparison between different sampling strategies. Eur. J. Wildl. Res. 2021, 67, 88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).