Glucoraphanin Accumulation via Glucoraphanin Synthesis Promotion during Broccoli Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Sample Collection
2.3. Analysis of Glucosinolate and Glucoraphanin Accumulation
2.4. Measurement of Glucoraphanin Biosynthesis Pathway Metabolites
2.5. Analysis of Glucoraphanin Synthesis-Related Gene Expression
2.6. RNA Isolation and Quantitative Real-Time PCR Analysis
2.7. Statistical Analysis
3. Results
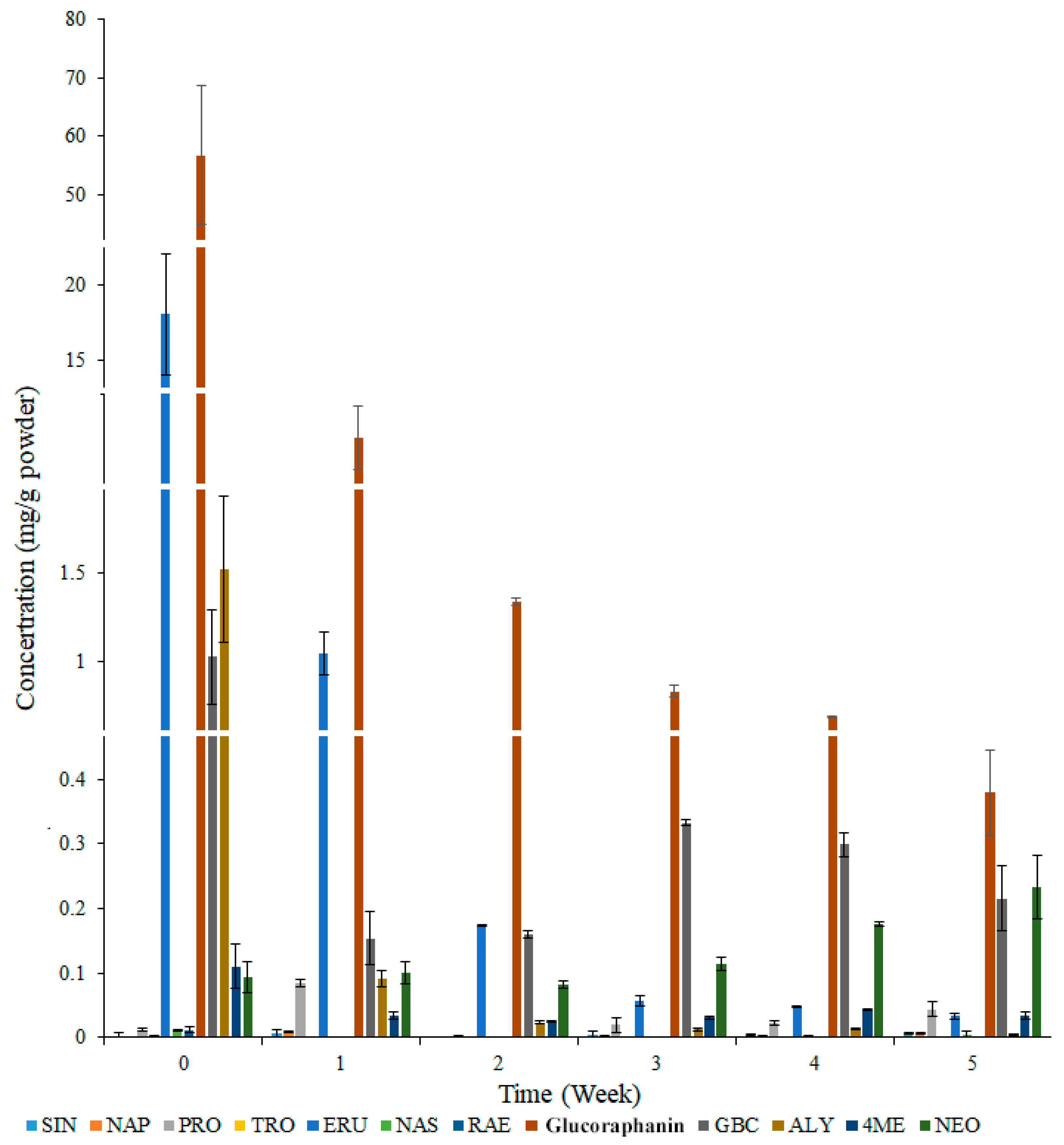
3.1. Effects of Broccoli Seedling Age on Glucosinolate Content
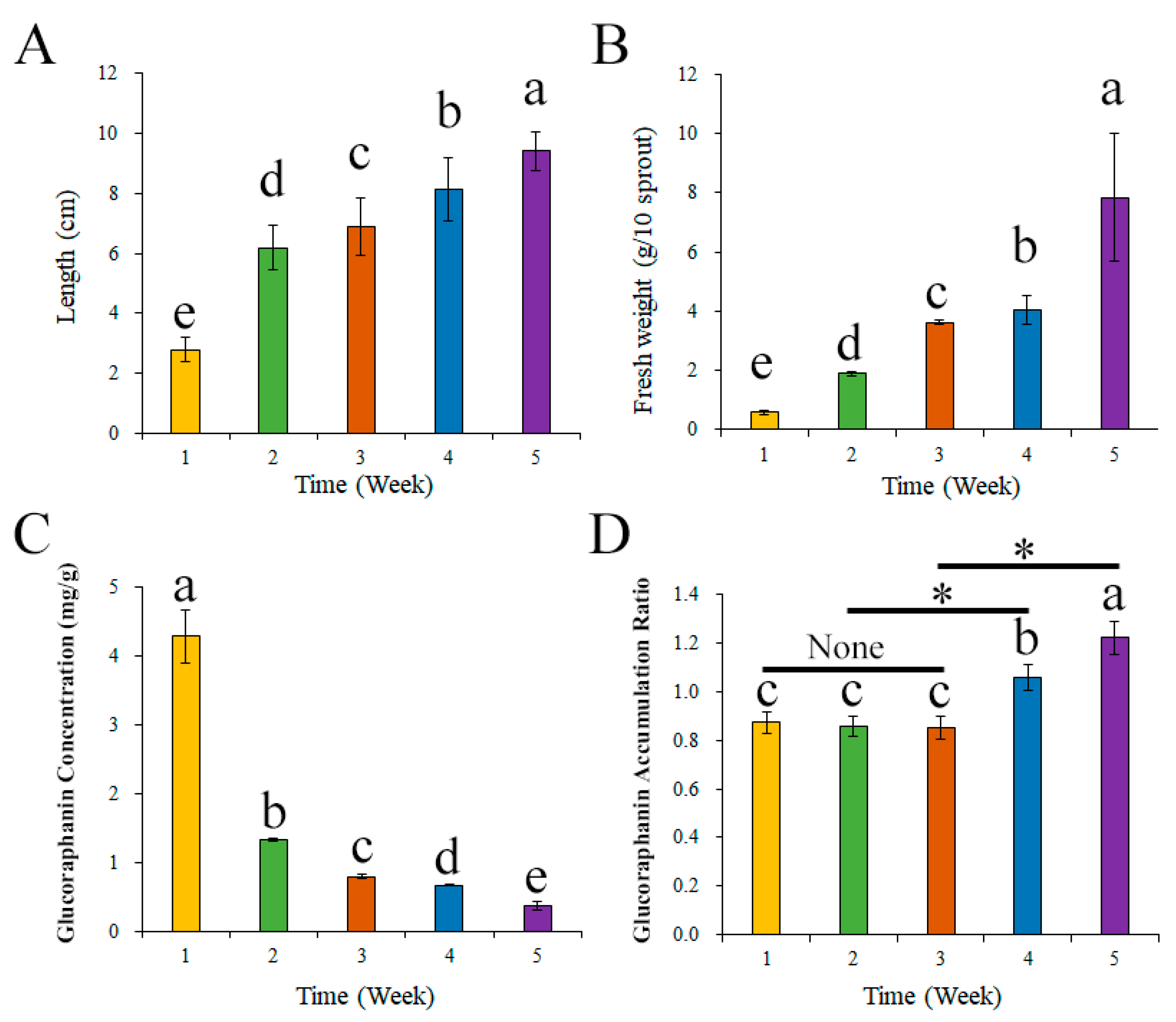
3.2. Effects of Broccoli Seedling Age on Glucoraphanin Content and the Broccoli Seedling Index
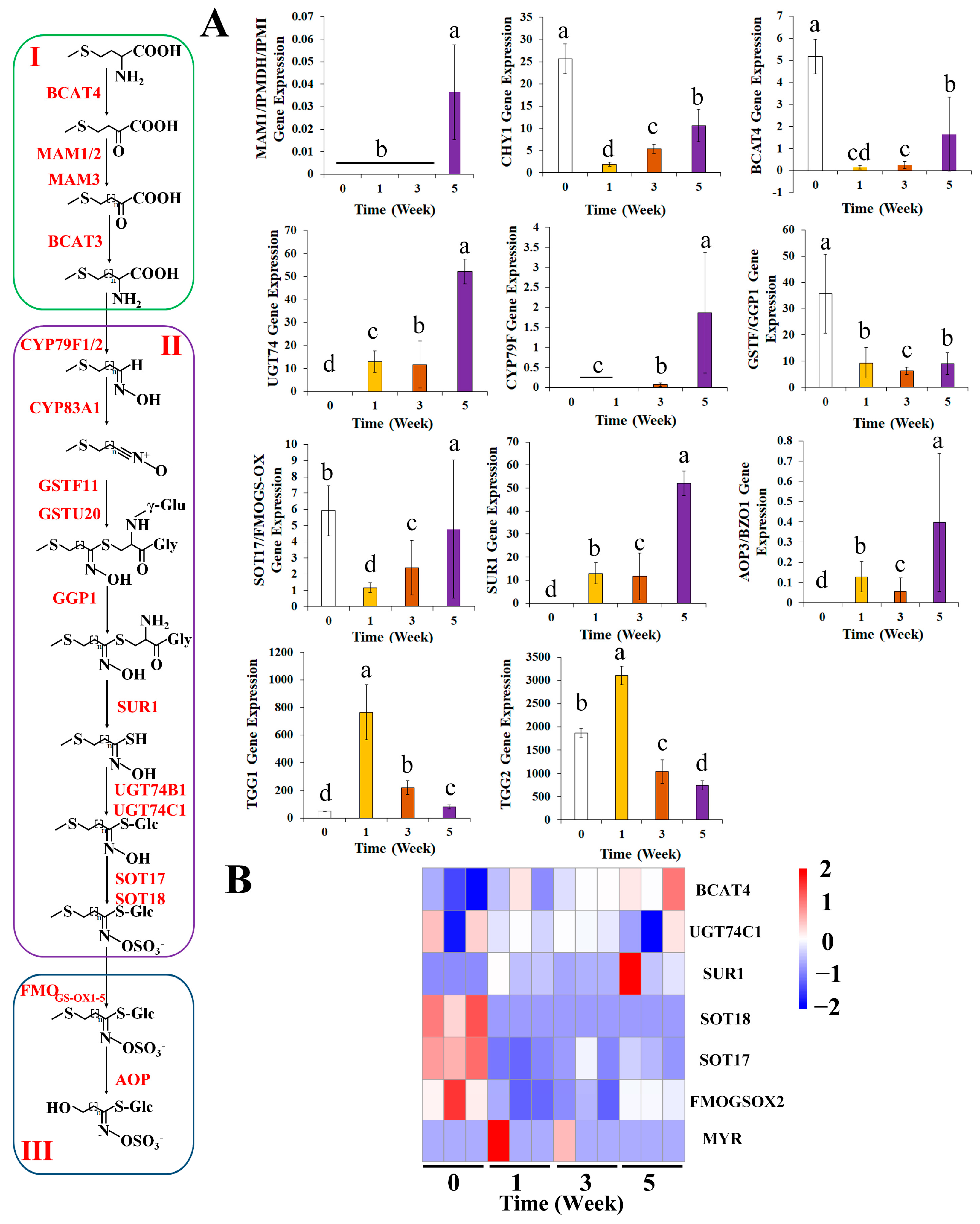
3.3. Effects of Broccoli Seedling Age on the Contents of Key Glucoraphanin Synthesis-Related Compounds and Molecular Metabolism
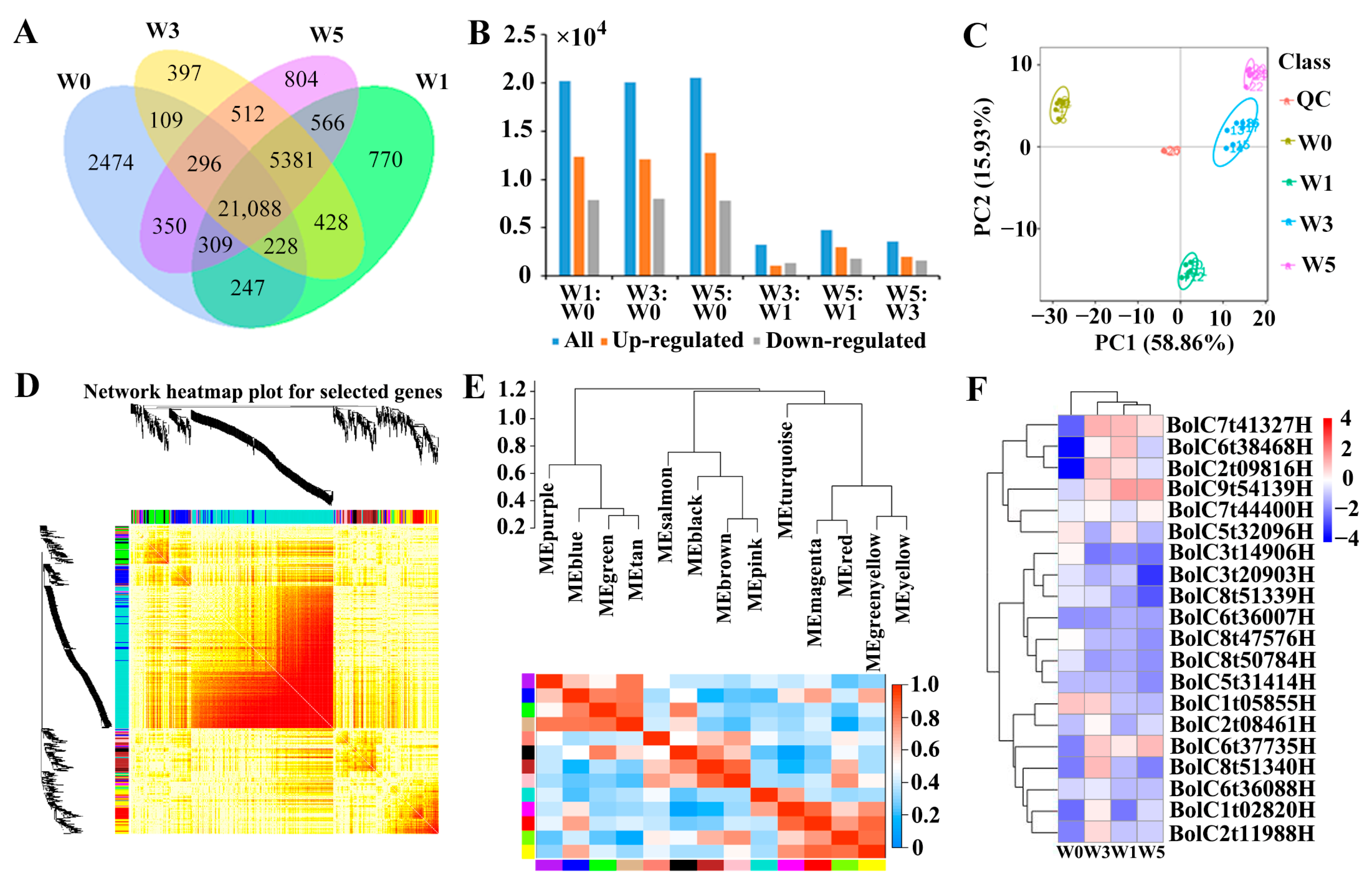
3.4. Effects of Broccoli Seedling Age on the Number of Differentially Expressed Genes
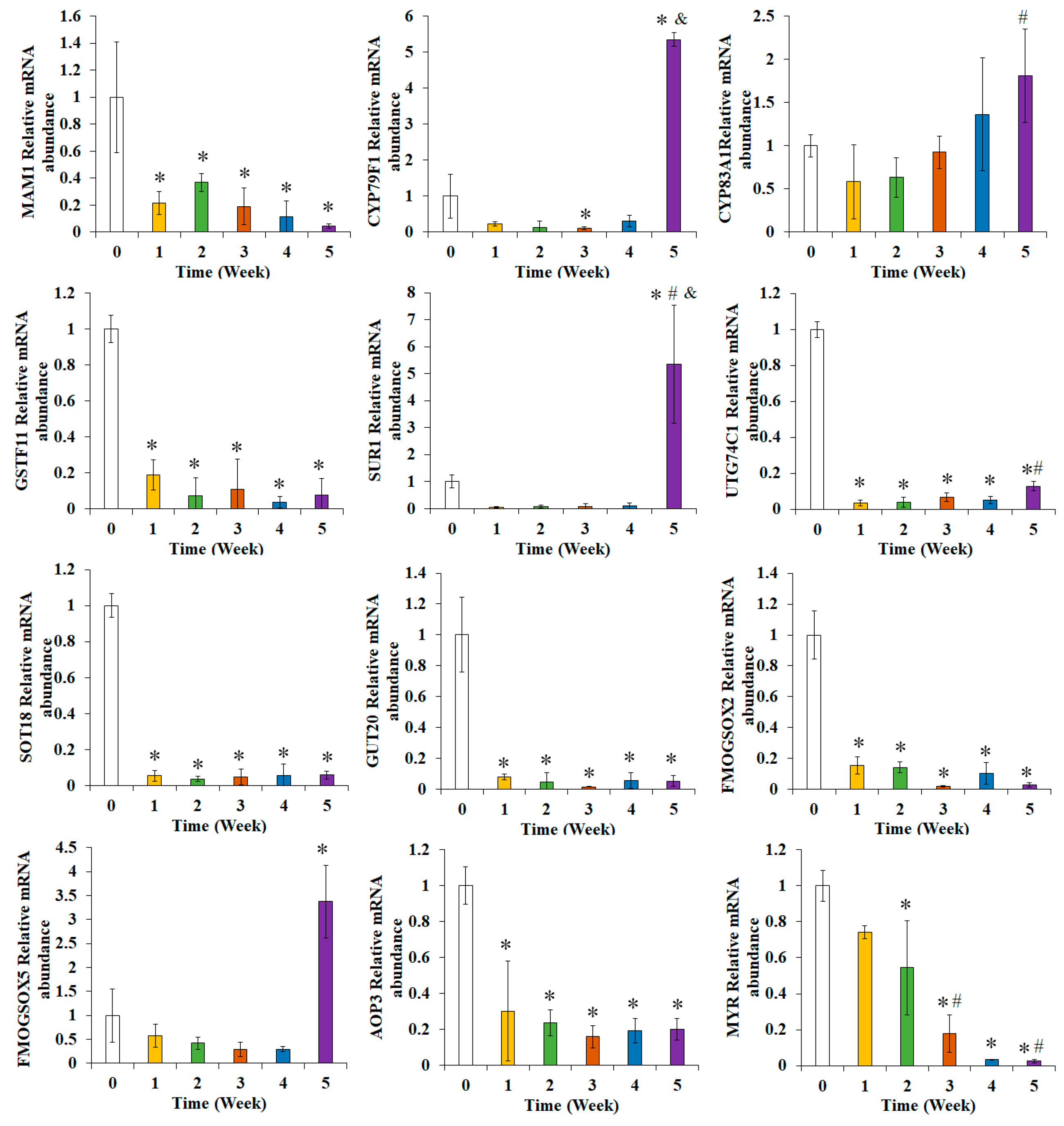
3.5. Effects of Broccoli Seedling Age on the Expression of Glucoraphanin Synthesis-Related Genes
3.6. Effects of Broccoli Seedling Age on the Relative Expression Levels of the Genes Related to Glucoraphanin Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Narae, H.; Jinwook, L.; Jongkee, K. Comparative responses of glucosinolates in Baemoochae and its parent plants, radish and Chinese cabbage, during development. Sci. Hortic. 2022, 295, 110870. [Google Scholar]
- Lan, T.; Wang, J.; Yuan, Q.; Lei, Y.; Peng, W.; Zhang, M.; Li, X.; Sun, X.; Ma, T. Evaluation of the color and aroma characteristics of commercially available Chinese kiwi wines via intelligent sensory technologies and gas chromatography-mass spectrometry. Food Chem. X 2022, 15, 100427. [Google Scholar] [CrossRef] [PubMed]
- Mahn, A.; Elena Perez, C.; Zambrano, V.; Barrientos, H. Maximization of Sulforaphane Content in Broccoli Sprouts by Blanching. Foods 2022, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Mocniak, L.E.; Elkin, K.R.; Dillard, S.L.; Bryant, R.B.; Soder, K.J. Building comprehensive glucosinolate profiles for brassica varieties. Talanta 2023, 251, 123814. [Google Scholar] [CrossRef] [PubMed]
- Shakour, Z.T.; Shehab, N.G.; Gomaa, A.S.; Wessjohann, L.A.; Farag, M.A. Metabolic and biotransformation effects on dietary glucosinolates, their bioavailability, catabolism and biological effects in different organisms. Biotechnol. Adv. 2022, 54, 107784. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.W.; Oh, M.-H.; Kim, H.S.; Kim, E.O.; Chae, W.B. Glucosinolate variation among organs, growth stages and seasons suggests its dominant accumulation in sexual over asexual-reproductive organs in white radish. Sci. Hortic. 2022, 291, 110617. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, W.; Feng, X.; Liu, Q.; Ibrahim, S.A.; Liu, Y. Identification and quantification of intact glucosinolates at different vegetative growth periods in Chinese cabbage cultivars by UHPLC-Q-TOF-MS. Food Chem. 2022, 393, 133414. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; He, H.; Liu, Y.; Han, F.; Liu, W. Effects of nanocarbon solution treatment on the nutrients and glucosinolate metabolism in broccoli. Food Chem. X 2022, 15, 100429. [Google Scholar] [CrossRef]
- Plaszko, T.; Szucs, Z.; Vasas, G.; Gonda, S. Interactions of fungi with non-isothiocyanate products of the plant glucosinolate pathway: A review on product formation, antifungal activity, mode of action and biotransformation. Phytochemistry 2022, 200, 113245. [Google Scholar] [CrossRef]
- Karlson, D.; Mojica, J.P.; Poorten, T.J.; Lawit, S.J.; Jali, S.; Chauhan, R.D.; Pham, G.M.; Marri, P.; Guffy, S.L.; Fear, J.M.; et al. Targeted Mutagenesis of the Multicopy Myrosinase Gene Family in Allotetraploid Brassica juncea Reduces Pungency in Fresh Leaves across Environments. Plants 2022, 11, 2494. [Google Scholar] [CrossRef]
- Bousquet, J.; Le Moing, V.; Blain, H.; Czarlewski, W.; Zuberbier, T.; De La Torre, R.; Lozano, N.P.; Reynes, J.; Bedbrook, A.; Cristol, J.-P.; et al. Efficacy of broccoli and glucoraphanin in COVID-19: From hypothesis to proof-of-concept with three experimental clinical cases. World Allergy Organ. J. 2021, 14, 100498. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.K.; Preetha, R.; Haque, S.; Akhter, N.; Khan, S.; Ahmad, S.; Hussain, A. Dietary isothiocyanates inhibit cancer progression by modulation of epigenome. Semin. Cancer Biol. 2022, 83, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Sonderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates-gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Mao, P.; Li, Q.; Li, Y.; Xu, Y.; Yang, Q.; Bian, Z.; Wang, S.; He, L.; Xu, Z.; Zheng, Y.; et al. The beneficial functions of blue light supplementary on the biosynthesis of glucosinolates in pakchoi (Brassica rapa L. ssp. chinensis) under greenhouse conditions. Environ. Exp. Bot. 2022, 197, 104834. [Google Scholar] [CrossRef]
- Harun, S.; Abdullah-Zawawi, M.R.; Goh, H.H.; Mohamed-Hussein, Z.A. A Comprehensive Gene Inventory for Glucosinolate Biosynthetic Pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020, 68, 7281–7297. [Google Scholar] [CrossRef]
- Kang, H.; Nugroho AB, D.; Park, M.; Kim, J.A.; Lee, S.W.; Moon, H.; Choi, D.; Kim, S.; Kim, D.-H. Vernalization Regulates Flowering Genes and Modulates Glucosinolates Biosynthesis in Chinese Cabbage. J. Plant Biol. 2022, 65, 157–173. [Google Scholar] [CrossRef]
- Qian, H.; Sun, B.; Miao, H.; Cai, C.; Xu, C.; Wang, Q. Variation of glucosinolates and quinone reductase activity among different varieties of Chinese kale and improvement of glucoraphanin by metabolic engineering. Food Chem. 2015, 168, 321–326. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Yuan, S.; Han, F.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; et al. Fine mapping of the major QTLs for biochemical variation of sulforaphane in broccoli florets using a DH population. Sci. Rep. 2021, 11, 9004. [Google Scholar] [CrossRef]
- Men, X.; Han, X.; Lee, S.-J.; Oh, G.; Park, K.-T.; Han, J.-K.; Choi, S.-I.; Lee, O.-H. Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo. Nutrients 2022, 14, 3814. [Google Scholar] [CrossRef]
- Jo, J.S.; Bhandari, S.R.; Kang, G.H.; Shin, Y.K.; Lee, J.G. Selection of broccoli (Brassica oleracea var. italica) on composition and content of glucosinolates and hydrolysates. Sci. Hortic. 2022, 298, 110984. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Abdelgawad, H.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.; Khamis, G. Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, S.; He, Y.; Zhang, X.; Liu, H. CaCl2-HCl electrolyzed water affects glucosinolate metabolism and improves the quality of broccoli sprouts. Food Res. Int. 2021, 150 Pt B, 110807. [Google Scholar] [CrossRef]
- Rao, S.; Gou, Y.; Yu, T.; Cong, X.; Gui, J.; Zhu, Z.; Zhang, W.; Liao, Y.; Ye, Z.; Cheng, S.; et al. Effects of selenate on Se, flavonoid, and glucosinolate in broccoli florets by combined transcriptome and metabolome analyses. Food Res. Int. 2021, 146, 110463. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, G.; Li, X.; Xiao, J.; Guo, L. Effect of different LED lights on aliphatic glucosinolates metabolism and biochemical characteristics in broccoli sprouts. Food Res. Int. 2022, 154, 111015. [Google Scholar]
- Demir, K.; Sarikamis, G.; Seyrek, G.C. Effect of LED lights on the growth, nutritional quality and glucosinolate content of broccoli, cabbage and radish microgreens. Food Chem. 2023, 401, 134088. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Gómez-Jodar i Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hortic. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Yu, X.; He, H.; Zhao, X.; Liu, G.; Hu, L.; Cheng, B.; Wang, Y. Determination of 18 Intact Glucosinolates in Brassicaceae Vegetables by UHPLC-MS/MS: Comparing Tissue Disruption Methods for Sample Preparation. Molecules 2022, 27, 231. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.E.; Robin, A.H.; Yang, K.; Park, J.I.; Hwang, B.H.; Nou, I.S. Exogenous Methyl Jasmonate and Salicylic Acid Induce Subspecies-Specific Patterns of Glucosinolate Accumulation and Gene Expression in Brassica oleracea L. Molecules 2016, 21, 1417. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.A.; Hellin, P.; Perez, B.; Viadel, B.; Alapont, A.; Agudelo, A. Fresh Brassicaceae sprouting broccoli (Bimi) glucosinolates profile characterization and bioaccessibility through an in vitro dynamic digestion study. J. Food Compos. Anal. 2023, 115, 104941. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, T.L.; Saha, S.; Bernuzzi, F.; Savva, G.M.; Troncoso-Rey, P.; Traka, M.H.; Mills, R.D.; Ball, R.Y.; Mithen, R.F. Accumulation of Sulforaphane and Alliin in Human Prostate Tissue. Nutrients 2022, 14, 3263. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, K.; Zhu, Y.; Wang, F.; Xiao, J.; Guo, L. Calcium affects glucoraphanin metabolism in broccoli sprouts under ZnSO4 stress. Food Chem. 2021, 334, 127520. [Google Scholar]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of light-emitting diodes on the accumulation of phenolic compounds and glucosinolates in Brassica juncea sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, J.; Purevdorj, E.; Son, Y.J.; Nho, C.W.; Yoo, G. Effects of long light exposure and drought stress on plant growth and glucosinolate production in pak choi (Brassica rapa subsp. chinensis). Food Chem. 2021, 340, 128167. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhao, G.; Liu, G.; Wang, P.; Li, J. Microorganisms-An Effective Tool to Intensify the Utilization of Sulforaphane. Foods 2022, 11, 3775. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Chen, J.; Chen, B.; Tang, W.; Chen, X.; Lai, Z.; Guo, R. Comparative transcriptomic analyses of glucosinolate metabolic genes during the formation of Chinese kale seeds. BMC Plant Biol. 2021, 21, 394. [Google Scholar] [CrossRef]
- Wang, J.; Mao, S.; Xu, H.; Wu, Q.; Liang, M.; Yuan, Y.; Liu, M.; Huang, K.; Wu, Q. Effects of Sulfur and Selenium on Glucosinolate Biosynthesis in Cabbage. Plant Mol. Biol. Report. 2019, 38, 62–74. [Google Scholar] [CrossRef]
- Borpatragohain, P.; Rose, T.J.; King, G.J. Fire and Brimstone: Molecular Interactions between Sulfur and Glucosinolate Biosynthesis in Model and Crop Brassicaceae. Front. Plant Sci. 2016, 7, 1735. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Wang, J.; Wang, H.; Liu, T.; Zhang, X.; Song, J.; Yang, W.; Wu, C.; Yang, H.; et al. A Comparative Transcriptome and Metabolome Combined Analysis Reveals the Key Genes and Their Regulatory Model Responsible for Glucoraphasatin Accumulation in Radish Fleshy Taproots. Int. J. Mol. Sci. 2022, 23, 2953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, J.; Lu, X.; Tian, P.; Yang, J.; Wei, Y.; Li, S.; Ma, S. Transcriptome analysis of melatonin regulating the transformation of glucoraphanin to sulforaphane in broccoli hairy roots. Physiol. Mol. Biol. Plants 2022, 28, 51–64. [Google Scholar] [CrossRef]

| Gene Name | Sequence (5′-3′) | Expected Size (bp) |
|---|---|---|
| UTG74C1 | Forward: CCTGACCGATTTCATCTCTAGTGC | 24 |
| Reverse: TGGCTATGTCCAATGCAAAGGG | 22 | |
| GSTF11 | Forward: CTTTGGAGGGACGAGCCATT | 20 |
| Reverse: TGTGAATTCATCACCGCCCA | 20 | |
| FMO-GS-OX2 | Forward: GACCGTGGTTACGGGAGACTTG | 22 |
| Reverse: GTAGCCATTGTATAACAAGCAACCC | 25 | |
| FMO-GS-OX5 | Forward: CGAACATGTCTTTCCGCCTG | 20 |
| Reverse: TCTTAGGTTGCCCGAAAGCC | 20 | |
| AOP3 | Forward: TTGATGCGGAGTTGGGCTTA | 20 |
| Reverse: CTCGGTGATACGGTGAAGGG | 20 | |
| MYR | Forward: GATGGGCGAACTCAATGCTAC | 21 |
| Reverse: CACTCCCCTACTCACCTTTCCTT | 23 | |
| MAM1 | Forward: AAATTCTGGCATTGCTCGTGG | 21 |
| Reverse: ATCACCAGATTCACCGCACG | 20 | |
| GSTU20 | Forward: GCTAGAGTTGCGTTGCGAGA | 20 |
| Reverse: CCCGTAAGGATCGGAAGGGA | 20 | |
| CYP83A1 | Forward: TTCATATCCTACGGCAGGCG | 20 |
| Reverse: TTATCCGCGGCCTTGTTGAT | 20 | |
| SOT18 | Forward: CAAGGCTACGATCACGACCA | 20 |
| Reverse: GACGGTAGCCACCAGTAACC | 20 | |
| SUR1 | Forward: CCAAGCCGTGTGATTGTACG | 20 |
| Reverse: TTGTGGCCCTAGACACTGGA | 20 | |
| CYP79F1 | Forward: ATCAATCAGTTTGCTTGGCCG | 21 |
| Reverse: CCTTTCAAGGCGATGTCGAT | 20 | |
| Actin | Forward: CTGTTCCAATCTACGAGGGTTTC | 23 |
| Reverse: GCTCGGCTGTGGTGGTGAA | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; He, H.; Wang, P.; Zhao, X.; Ren, F. Glucoraphanin Accumulation via Glucoraphanin Synthesis Promotion during Broccoli Germination. Foods 2024, 13, 41. https://doi.org/10.3390/foods13010041
Liu G, He H, Wang P, Zhao X, Ren F. Glucoraphanin Accumulation via Glucoraphanin Synthesis Promotion during Broccoli Germination. Foods. 2024; 13(1):41. https://doi.org/10.3390/foods13010041
Chicago/Turabian StyleLiu, Guangmin, Hongju He, Pengjie Wang, Xirui Zhao, and Fazheng Ren. 2024. "Glucoraphanin Accumulation via Glucoraphanin Synthesis Promotion during Broccoli Germination" Foods 13, no. 1: 41. https://doi.org/10.3390/foods13010041
APA StyleLiu, G., He, H., Wang, P., Zhao, X., & Ren, F. (2024). Glucoraphanin Accumulation via Glucoraphanin Synthesis Promotion during Broccoli Germination. Foods, 13(1), 41. https://doi.org/10.3390/foods13010041







