Application of Commercial Biopreservation Starter in Combination with MAP for Shelf-Life Extension of Burrata Cheese
Abstract
1. Introduction
2. Materials and Methods
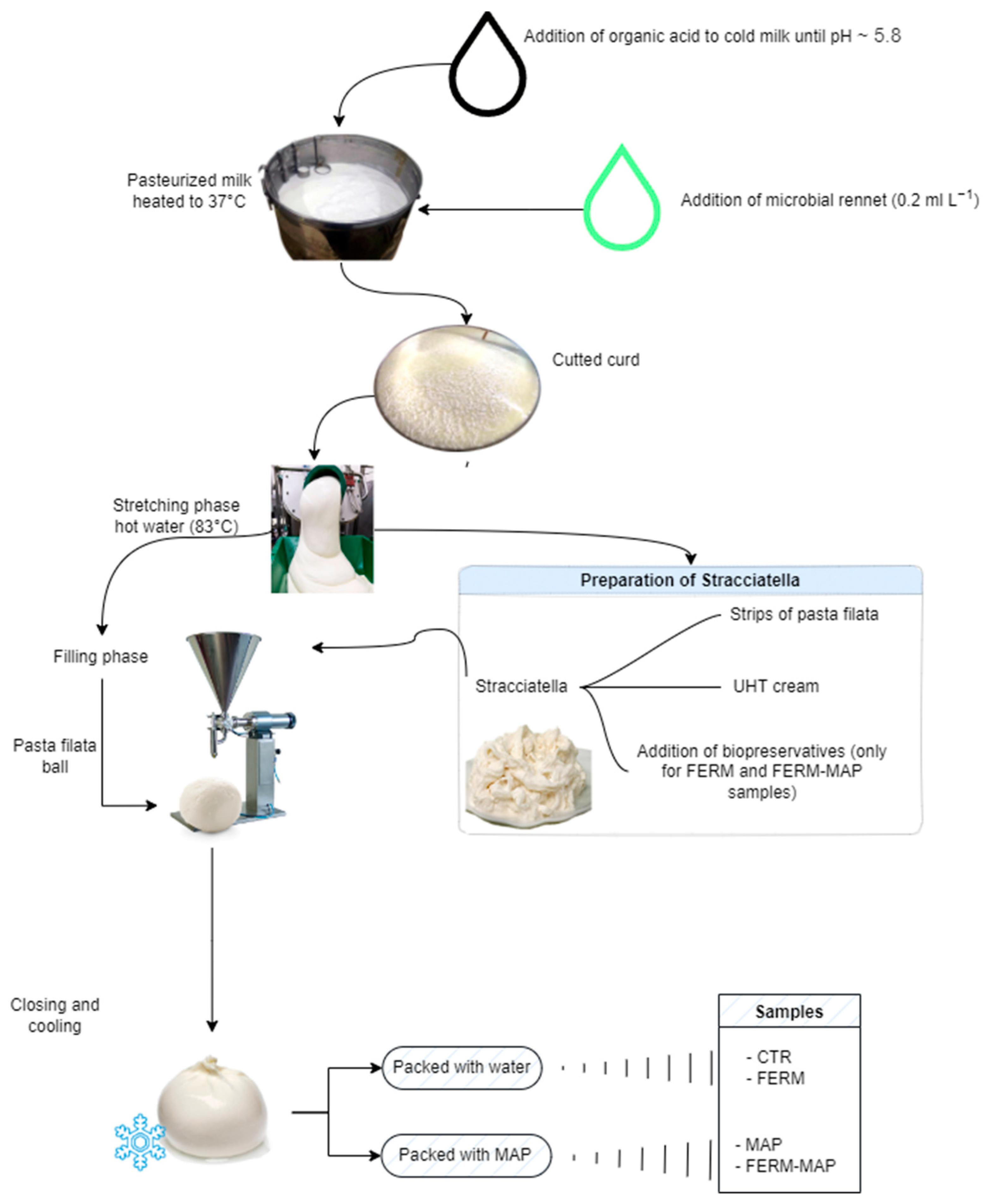
2.1. Sample Preparation
- -
- Wrapped burrata with protective starter packaged in MAP 70 N2% and 30% CO2 (coded as Ferm-MAP);
- -
- Wrapped burrata packaged in MAP 70 N2% and 30% CO2 (coded as MAP);
- -
- Water-immersed burrata with protective starter (coded as Ferm).
2.2. Chemical Analyses
2.3. Microbiological Analyses
2.4. Sensory Analysis
2.5. Statistical Analysis
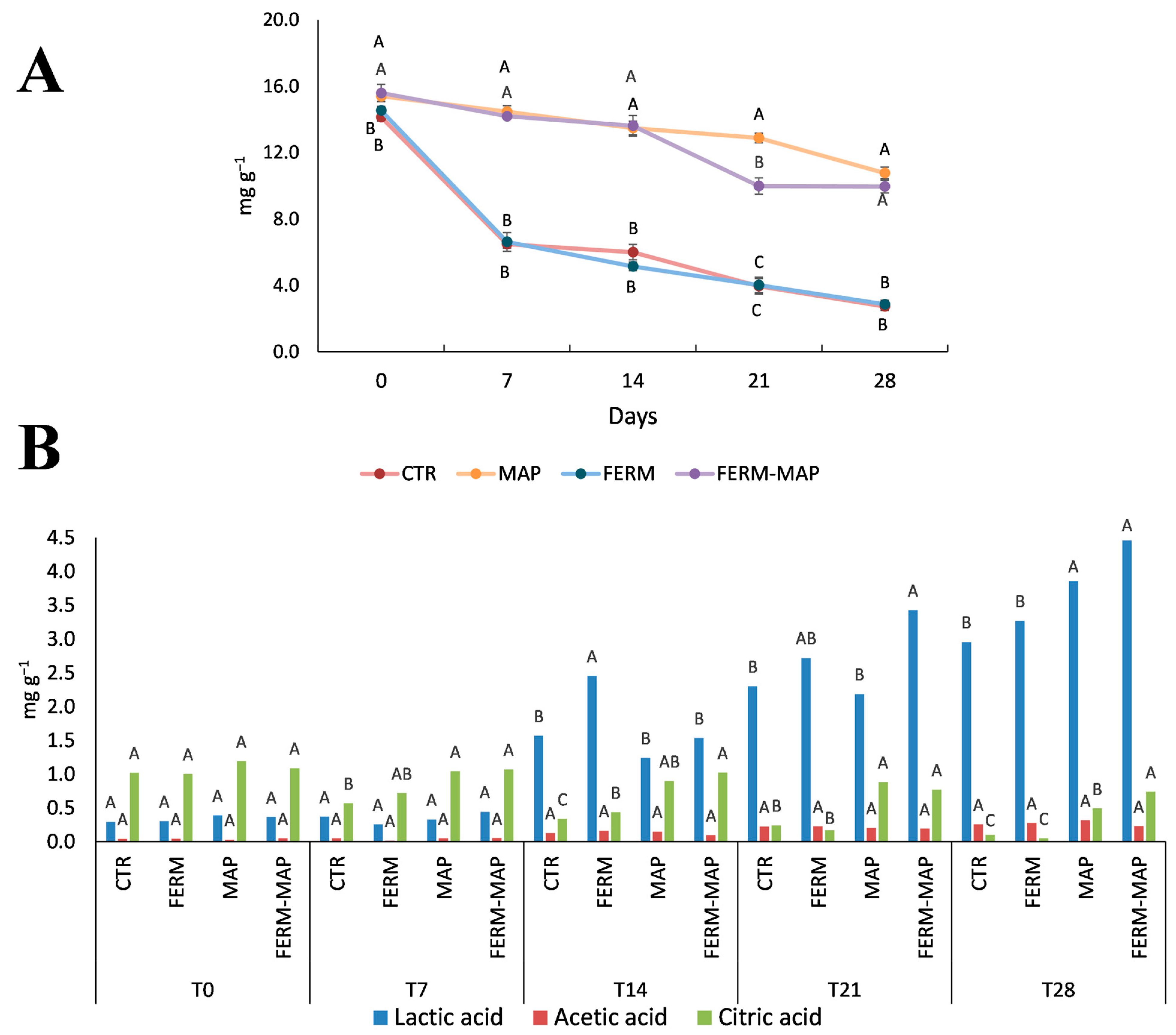
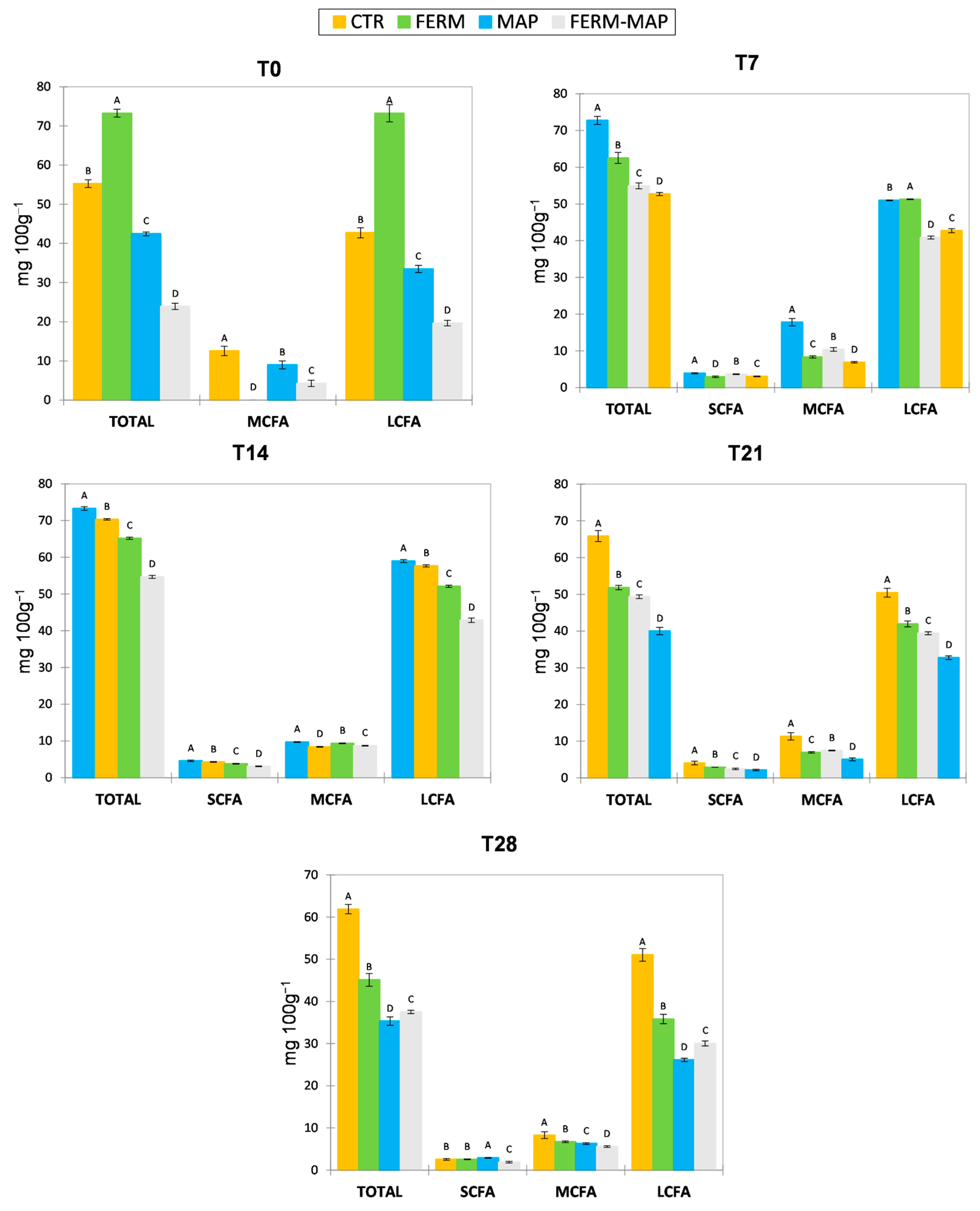
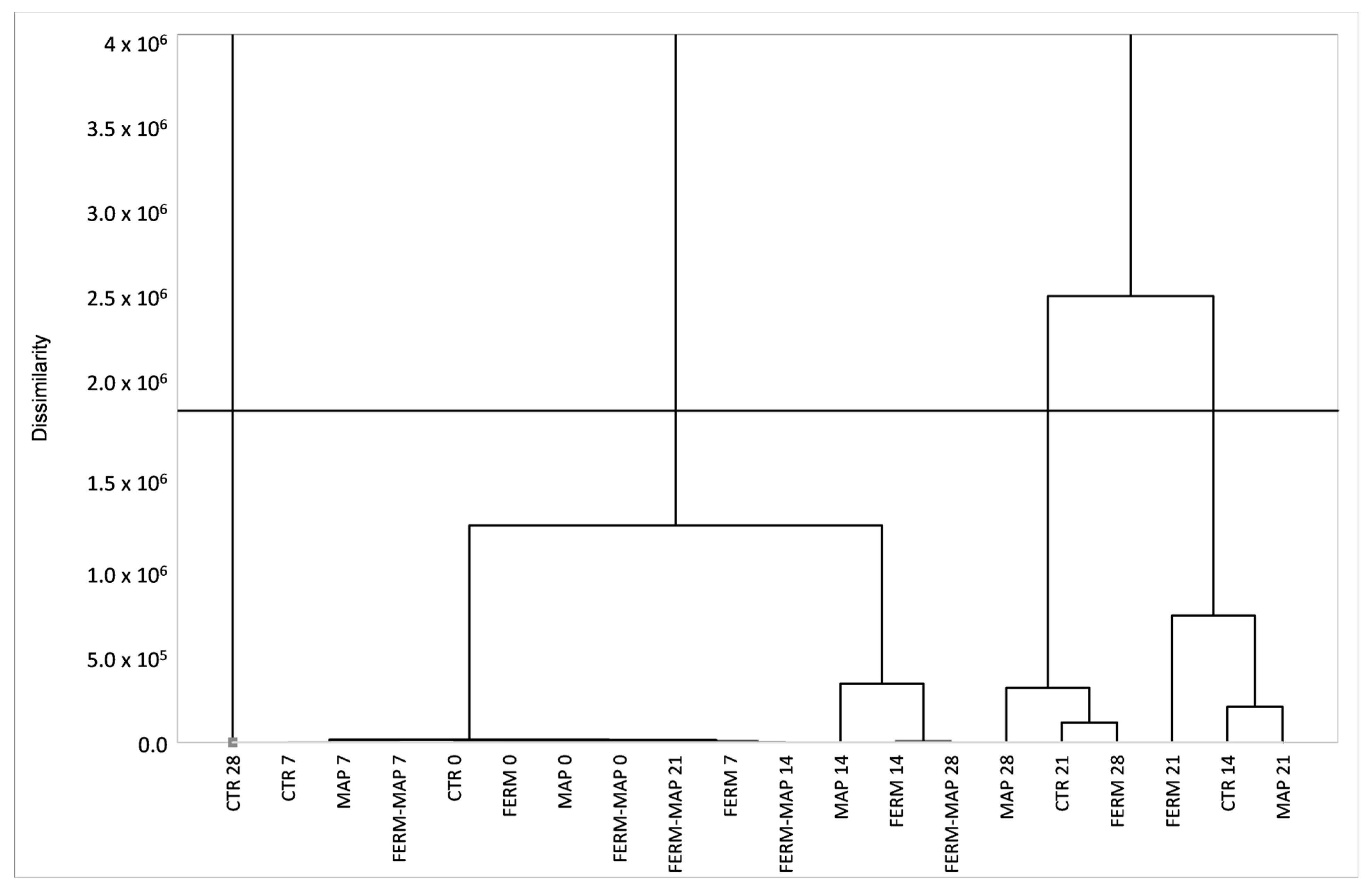
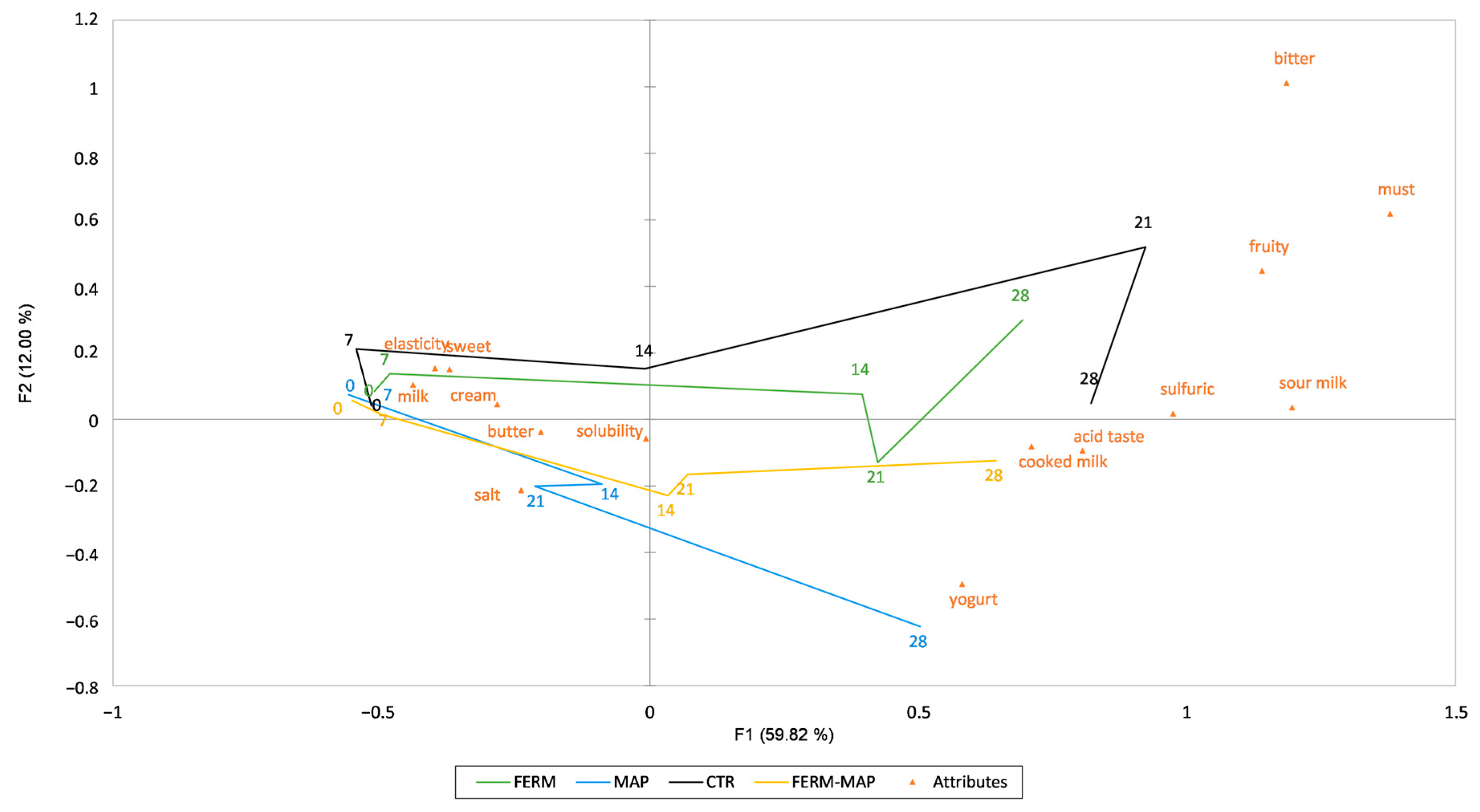
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barba, F.J.; Ahrné, L.; Xanthakis, E.; Landerslev, M.G.; Orlien, V. Innovative Technologies for Food Preservation. In Innovative Technologies for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 25–51. ISBN 9780128110324. [Google Scholar]
- Ibarra-Sánchez, L.A.; Van Tassell, M.L.; Miller, M.J. Antimicrobial behavior of phage endolysin PlyP100 and its synergy with nisin to control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2018, 72, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Natrella, G.; Difonzo, G.; Calasso, M.; Costantino, G.; Caponio, F.; Faccia, M. Evolution of VOC and Sensory Characteristics of Stracciatella Cheese as Affected by Different Preservatives. Foods 2020, 9, 1446. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Yahyaraeyat, R.; Khosravi, A.; Atefi, P.; Basti, A.A.; Akrami, F.; Bahonar, A.; Misaghi, A. Effect of Zataria multiflora Boiss. Essential Oil on Growth and Citrinin Production by Penicillium citrinum in Culture Media and Mozzarella Cheese. J. Food Saf. 2012, 32, 445–451. [Google Scholar] [CrossRef]
- Sara, G.; Nasrin, H.S.J.; Mehran, M.; Kianoush, K.D. Application of zein antimicrobial edible film incorporating Zataria multiflora boiss essential oil for preservation of Iranian ultrafiltered Feta cheese. Afr. J. Biotechnol. 2015, 14, 2014–2021. [Google Scholar] [CrossRef]
- Barbara, S.; Milena, S.; Daniela, C.; Rosaria, C.M.; Antonio, B. Modelling the Amounts of Carbon Dioxide in the Headspace to Assess the Coliforms of Mozzarella Cheese. Ann. Microbiol. Res. 2017, 1, 4–8. [Google Scholar] [CrossRef]
- Caputo, L.; Quintieri, L.; Bianchi, D.M.; Decastelli, L.; Monaci, L.; Visconti, A.; Baruzzi, F. Pepsin-digested bovine lactoferrin prevents Mozzarella cheese blue discoloration caused by Pseudomonas fluorescens. Food Microbiol. 2015, 46, 15–24. [Google Scholar] [CrossRef]
- Huang, X.; Nzekoue, F.K.; Renzi, S.; Alesi, A.; Coman, M.M.; Pucciarelli, S.; Sagratini, G.; Silvi, S. Influence of modified governing liquid on shelf-life parameters of high-moisture mozzarella cheese. Food Res. Int. 2022, 159, 111627. [Google Scholar] [CrossRef]
- Faccia, M.; Gambacorta, G.; Natrella, G.; Caponio, F. Shelf life extension of Italian mozzarella by use of calcium lactate buffered brine. Food Control 2019, 100, 287–291. [Google Scholar] [CrossRef]
- Öztürk, M.; Güncü, B.G. Effect of Brine Calcium Concentration on the Surface Solubilization and Texture of Fresh Perline Mozzarella Cheese. Turk. J. Agric. Food Sci. Technol. 2021, 9, 650–654. [Google Scholar] [CrossRef]
- Mizuno, R.; Abe, T.; Koishihara, H.; Okawa, T. The Effect of Preservative Liquid Composition on Physicochemical Properties of Mozzarella Cheese. Food Sci. Technol. Res. 2016, 22, 261–266. [Google Scholar] [CrossRef]
- Ricciardi, F.E.; Plazzotta, S.; Conte, A.; Manzocco, L. Effect of pulsed light on microbial inactivation, sensory properties and protein structure of fresh ricotta cheese. LWT 2021, 139, 110556. [Google Scholar] [CrossRef]
- Wan, Z.; Misra, N.; Li, G.; Keener, K.M. High voltage atmospheric cold plasma treatment of Listeria innocua and Escherichia coli K-12 on Queso Fresco (fresh cheese). LWT 2021, 146, 111406. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Hnosko, J.; Gonzalez, M.S.-M.; Clark, S. High-pressure processing inactivates Listeria innocua yet compromises Queso Fresco crumbling properties. J. Dairy Sci. 2012, 95, 4851–4862. [Google Scholar] [CrossRef]
- Evert-Arriagada, K.; Hernández-Herrero, M.; Guamis, B.; Trujillo, A. Commercial application of high-pressure processing for increasing starter-free fresh cheese shelf-life. LWT Food Sci. Technol. 2014, 55, 498–505. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Ekiz, H.I.; López-Rubio, A. Phytochemical-loaded electrospun nanofibers as novel active edible films: Characterization and antibacterial efficiency in cheese slices. Food Control 2020, 112, 107133. [Google Scholar] [CrossRef]
- Ordoñez, R.; Contreras, C.; González-Martínez, C.; Chiralt, A. Edible coatings controlling mass loss and Penicillium roqueforti growth during cheese ripening. J. Food Eng. 2021, 290, 110174. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, Y.; Ma, X.; Xia, M. Chitosan and procyanidin composite films with high antioxidant activity and pH responsivity for cheese packaging. Food Chem. 2020, 338, 128013. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Conte, A.; Faccia, M.; Del Nobile, M.A.; Zambrini, A.V. Combined effect of active coating and modified atmosphere packaging on prolonging the shelf life of low-moisture Mozzarella cheese. J. Dairy Sci. 2014, 97, 36–45. [Google Scholar] [CrossRef]
- Atallah, A.A.; Ismail, E.A.; Yehia, H.M.; Elkhadragy, M.F.; Khater, E.-S.G. Proteolytic Development and Volatile Compounds Profile of Domiati Cheese under Modified Atmosphere Packaging. Fermentation 2022, 8, 358. [Google Scholar] [CrossRef]
- Spanu, C.; Piras, F.; Mocci, A.; Nieddu, G.; De Santis, E.; Scarano, C. Use of Carnobacterium spp protective culture in MAP packed Ricotta fresca cheese to control Pseudomonas spp. Food Microbiol. 2018, 74, 50–56. [Google Scholar] [CrossRef]
- Cosentino, S.; Viale, S.; Deplano, M.; Fadda, M.E.; Pisano, M.B. Application of Autochthonous Lactobacillus Strains as Biopreservatives to Control Fungal Spoilage in Caciotta Cheese. BioMed. Res. Int. 2018, 2018, 3915615. [Google Scholar] [CrossRef] [PubMed]
- Trani, A.; Gambacorta, G.; Gomes, T.F.; Loizzo, P.; Cassone, A.; Faccia, M. Production and characterisation of reduced-fat and PUFA-enriched Burrata cheese. J. Dairy Res. 2016, 83, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Natrella, G.; Gambacorta, G.; Faccia, M. Use of dry ice as innovative technology to preserve the chemical and microbial characteristics of burrata cheese. J. Food Process. Preserv. 2022, 46, e16908. [Google Scholar] [CrossRef]
- Minervini, F.; Conte, A.; Del Nobile, M.A.; Gobbetti, M.; De Angelis, M. Dietary Fibers and Protective Lactobacilli Drive Burrata Cheese Microbiome. Appl. Environ. Microbiol. 2017, 83, e01494-17. [Google Scholar] [CrossRef]
- Conte, A.; Brescia, I.; Del Nobile, M. Lysozyme/EDTA disodium salt and modified-atmosphere packaging to prolong the shelf life of burrata cheese. J. Dairy Sci. 2011, 94, 5289–5297. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Conte, A.; Zambrini, A.V.; Del Nobile, M.A. Technological Strategies to Preserve Burrata Cheese Quality. Coatings 2017, 7, 97. [Google Scholar] [CrossRef]
- Costantino, G.; Calasso, M.; Minervini, F.; De Angelis, M. Use of Exopolysaccharide-Synthesizing Lactic Acid Bacteria and Fat Replacers for Manufacturing Reduced-Fat Burrata Cheese: Microbiological Aspects and Sensory Evaluation. Microorganisms 2020, 8, 1618. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Kelly, P.M.; Wilkinson, M.G.; Guinee, T.P. Effect of fat and salt reduction on the changes in the concentrations of free amino acids and free fatty acids in Cheddar-style cheeses during maturation. J. Food Compos. Anal. 2017, 59, 132–140. [Google Scholar] [CrossRef]
- Natrella, G.; Gambacorta, G.; Faccia, M. Volatile organic compounds throughout the manufacturing process of Mozzarella di Gioia del Colle PDO cheese. Czech J. Food Sci. 2020, 38, 215–222. [Google Scholar] [CrossRef]
- ISO 21528-2:2017; Microbiology of the Food Chain Horizontal Method for the Detection and Enumeration of Enterobacteriaceae Part 2: Colonycount Technique. ISO (International Organization for Standardization): Geneva, Switzerland, 2017.
- ISO 11059:2009 (IDF/RM 225:2009); Milk and Milk Products. Method for the Enumeration of Pseudomonas spp. ISO (International Organization for Standardization): Geneva, Switzerland, 2017.
- ISO 16649-3:2015; Microbiology of the Food Chain-Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli—Part 3: Detection and Most Probable Number Technique Using 5-bromo-4-chloro-3-indolyl-ß-D-glucuronide. ISO (International Organization for Standardization): Geneva, Switzerland, 2015.
- ISO 6888-1:2018; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Par. ISO (International Organization for Standardization): Geneva, Switzerland, 2018.
- ISO 4832:2007; Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Enumeration of Coliforms: Colony-Count Technique. ISO (International Organization for Standardization): Geneva, Switzerland, 2007.
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO (International Organization for Standardization): Geneva, Switzerland, 2012.
- Ares, G.; Jaeger, S. Check-all-that-apply (CATA) questions with consumers in practice: Experimental considerations and impact on outcome. Rapid Sens. Profiling Tech. 2023, 257–280. [Google Scholar] [CrossRef]
- Ares, G.; Barreiro, C.; Deliza, R.; Giménez, A.; Gámbaro, A. Application of a check-all-that-apply question to the development of chocolate milk desserts. J. Sens. Stud. 2010, 25, 67–86. [Google Scholar] [CrossRef]
- Dooley, L.; Lee, Y.-S.; Meullenet, J.-F. The application of check-all-that-apply (CATA) consumer profiling to preference mapping of vanilla ice cream and its comparison to classical external preference mapping. Food Qual. Prefer. 2010, 21, 394–401. [Google Scholar] [CrossRef]
- Adda, J.; Gripon, J.; Vassal, L. The chemistry of flavour and texture generation in cheese. Food Chem. 1982, 9, 115–129. [Google Scholar] [CrossRef]
- Lombardi, A.; Bevilacqua, A.; Califano, A. Variation in organic acids content during ripening of Reggianito cheese in air-tight sealed bags. Food Chem. 1994, 51, 221–226. [Google Scholar] [CrossRef]
- Lues, J.; Botha, W. Relationships amongst South African processed, young and matured Cheddar cheese pertaining to organic acid content and non-starter population. Food Res. Int. 1998, 31, 449–457. [Google Scholar] [CrossRef]
- Califano, A.N.; Bevilacqua, A.E. Freezing low moisture Mozzarella cheese: Changes in organic acid content. Food Chem. 1999, 64, 193–198. [Google Scholar] [CrossRef]
- Tirloni, E.; Bernardi, C.; Ghelardi, E.; Celandroni, F.; Andrighetto, C.; Rota, N.; Stella, S. Biopreservation as a potential hurdle for Bacillus cereus growth in fresh cheese. J. Dairy Sci. 2020, 103, 150–160. [Google Scholar] [CrossRef]
- Passerini, D.; Laroute, V.; Coddeville, M.; Le Bourgeois, P.; Loubière, P.; Ritzenthaler, P.; Cocaign-Bousquet, M.; Daveran-Mingot, M.-L. New insights into Lactococcus lactis diacetyl- and acetoin-producing strains isolated from diverse origins. Int. J. Food Microbiol. 2013, 160, 329–336. [Google Scholar] [CrossRef]
- Alegría, Á.; González, P.; Delgado, S.; Flórez, A.B.; Hernández-Barranco, A.M.; Rodríguez, A.; Mayo, B. Characterisation of the technological behaviour of mixtures of mesophilic lactic acid bacteria isolated from traditional cheeses made of raw milk without added starters. Int. J. Dairy Technol. 2016, 69, 507–519. [Google Scholar] [CrossRef]
- Faccia, M.; Natrella, G.; Gambacorta, G. Analysis of the water-soluble compounds as a tool for discriminating traditional and industrial high moisture mozzarella made with citric acid. Int. J. Food Sci. Technol. 2021, 56, 5352–5361. [Google Scholar] [CrossRef]
- Tirloni, E.; Bernardi, C.; Rosshaug, P.; Stella, S. Potential growth of Listeria monocytogenes in Italian mozzarella cheese as affected by microbiological and chemical-physical environment. J. Dairy Sci. 2019, 102, 4913–4924. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Collins, Y.F.; Mukdsi, M.C.A.; McSweeney, P.L.H.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and Metabolism of Fatty Acids in Cheese. In Cheese; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 423–444. ISBN 9780124170124. [Google Scholar]
- Cadwallader, K.R.; Singh, T.K. Flavours and Off-Flavours in Milk and Dairy Products. In Advanced Dairy Chemistry: Volume 3: Lactose, Water, Salts and Minor Constituents; McSweeney, P., Fox, P.F., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 634–690. ISBN 978-0-387-84864-8. [Google Scholar]
- Tarakci, Z.; Temiz, H.; Aykut, U.; Turhan, S. Influence of Wild Garlic on Color, Free Fatty Acids, and Chemical and Sensory Properties of Herby Pickled Cheese. Int. J. Food Prop. 2011, 14, 287–299. [Google Scholar] [CrossRef]
- Akın, N.; Aydemir, S.; Koçak, C.; Yıldız, M.A. Changes of free fatty acid contents and sensory properties of white pickled cheese during ripening. Food Chem. 2003, 80, 77–83. [Google Scholar] [CrossRef]
- Kaminarides, S.; Stamou, P.; Massouras, T. Changes of organic acids, volatile aroma compounds and sensory characteristics of Halloumi cheese kept in brine. Food Chem. 2007, 100, 219–225. [Google Scholar] [CrossRef]
- Faccia, M.; Gambacorta, G.; Pasqualone, A.; Summo, C.; Caponio, F. Quality Characteristics and Consumer Acceptance of High-Moisture Mozzarella Obtained from Heat-Treated Goat Milk. Foods 2021, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Mateo, J.; Quinto, E.J.; Caro, I. Changes in quality of nonaged pasta filata Mexican cheese during refrigerated vacuum storage. J. Dairy Sci. 2015, 98, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; Marino, L.; Stocchi, R.; Branciari, R.; Loschi, A.R.; Miraglia, D.; Ranucci, D. Differences in chemical, physical and microbiological characteristics of Italian Burrata cheeses made in artisanal and industrial plants of the Apulia Region. Ital. J. Food Saf. 2016, 5, 5879. [Google Scholar] [CrossRef] [PubMed]
- Abee, T.; Krockel, L.; Hill, C. Bacteriocins: Modes of Action and Potentials in Food Preservation and Control of Food Poisoning. Int. J. Food Microbiol. 1995, 28, 169–185. [Google Scholar] [CrossRef]
- Akhtar, S.; Nawaz, S.K. Antimicrobial efficacy of Lactobacillus plantarum strain against the B. cereus, B. subtilis, S. aureus and E.coli strains. Biosci. J. 2023, 39, e39061. [Google Scholar] [CrossRef]
- Milioni, C.; Martínez, B.; Degl’innocenti, S.; Turchi, B.; Fratini, F.; Cerri, D.; Fischetti, R. A novel bacteriocin produced by Lactobacillus plantarum LpU4 as a valuable candidate for biopreservation in artisanal raw milk cheese. Dairy Sci. Technol. 2015, 95, 479–494. [Google Scholar] [CrossRef]
- Natrella, G.; Gambacorta, G.; De Palo, P.; Maggiolino, A.; Faccia, M. Volatile organic compounds in milk and mozzarella: Comparison between two different farming systems. Int. J. Food Sci. Technol. 2020, 55, 3403–3411. [Google Scholar] [CrossRef]
| LOG CFU g−1 | Storage (Days) | Enterobacteriaceae | Coliforms | E. Coli | Staphylococcus Coagulase + | Pseudomonas |
|---|---|---|---|---|---|---|
| Ctr | T0 | 2.2 ± 0.1 A | 2.1 ± 0.1 A | <1 A | <1 A | 2.3 ± 0.1 A |
| Ferm | T0 | 1.9 ± 0.1 B | 1.9 ± 0.1 B | <1 A | <1 A | 2.0 ± 0.1 BC |
| MAP | T0 | 2.0 ± 0.1 AB | 2.0 ± 0.1 AB | <1 A | <1 A | 2.1 ± 0.1 AB |
| Ferm MAP | T0 | 1.7 ± 0.2 C | 1.5 ± 0.1 C | <1 A | <1 A | 1.9 ± 0.1 C |
| Ctr | T7 | 1.0 ± 0.1 B | 1.3 ± 0.2 C | <1 A | <1 A | 3.3 ± 0.1 A |
| Ferm | T7 | 2.7 ± 0.3 A | 2.6 ± 0.2 AB | <1 A | <1 A | 3.0 ± 0.1 B |
| MAP | T7 | 2.8 ± 0.2 A | 2.7 ± 0.1 A | <1 A | <1 A | 2.7 ± 0.1 C |
| Ferm MAP | T7 | 2.6 ± 0.2 A | 2.5 ± 0.1 B | <1 A | <1 A | 2.5 ± 0.1 D |
| Ctr | T14 | 1.0 ± 0.1 A | 1.0 ± 0.1 B | <2 A | <2 A | 3.5 ± 0.4 A |
| Ferm | T14 | 0.7 ± 0.1 B | 2.9 ± 0.3 A | <2 A | <2 A | 3.0 ± 0.2 A |
| MAP | T14 | 0.7 ± 0.1 B | 2.9 ± 0.3 A | <2 A | <2 A | 3.0 ± 0.3 A |
| Ferm MAP | T14 | 0.3 ± 0.1 C | 2.9 ± 0.4 A | <2 A | <2 A | 2.9 ± 0.2 A |
| Ctr | T21 | 1.7 ± 0.3 A | 1.7 ± 0.2 A | <2 A | <2 A | 3.6 ± 0.2 A |
| Ferm | T21 | 0.9 ± 0.1 B | 0.8 ± 0.1 B | <2 A | <2 A | 3.3 ± 0.1 B |
| MAP | T21 | 0.8 ± 0.2 BC | 0.7 ± 0.1 BC | <2 A | <2 A | 3.0 ± 0.2 B |
| Ferm MAP | T21 | 0.6 ± 0.1 C | 0.6 ± 0.1 C | <2 A | <2 A | 2.9 ± 0.3 B |
| Ctr | T28 | 2.7 ± 0.3 A | 2.6 ± 0.2 A | <3 A | <2 A | 2.0 ± 0.2 A |
| Ferm | T28 | 1.2 ± 0.1 B | 1.1 ± 0.1 B | <2 B | <2 A | 1.4 ± 0.2 B |
| MAP | T28 | 1.1 ± 0.1 BC | 1.0 ± 0.1 BC | <2 B | <2 A | 1.2 ± 0.1 B |
| Ferm MAP | T28 | 0.9 ± 0.1 C | 0.9 ± 0.1 C | <2 B | <2 A | 1.0 ± 0.1 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natrella, G.; Gambacorta, G.; Faccia, M. Application of Commercial Biopreservation Starter in Combination with MAP for Shelf-Life Extension of Burrata Cheese. Foods 2023, 12, 1867. https://doi.org/10.3390/foods12091867
Natrella G, Gambacorta G, Faccia M. Application of Commercial Biopreservation Starter in Combination with MAP for Shelf-Life Extension of Burrata Cheese. Foods. 2023; 12(9):1867. https://doi.org/10.3390/foods12091867
Chicago/Turabian StyleNatrella, Giuseppe, Giuseppe Gambacorta, and Michele Faccia. 2023. "Application of Commercial Biopreservation Starter in Combination with MAP for Shelf-Life Extension of Burrata Cheese" Foods 12, no. 9: 1867. https://doi.org/10.3390/foods12091867
APA StyleNatrella, G., Gambacorta, G., & Faccia, M. (2023). Application of Commercial Biopreservation Starter in Combination with MAP for Shelf-Life Extension of Burrata Cheese. Foods, 12(9), 1867. https://doi.org/10.3390/foods12091867








