Antifungal Effect of Autochthonous Aromatic Plant Extracts on Two Mycotoxigenic Strains of Aspergillus flavus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fungal Strains
2.3. Extraction of Phenolic Compounds from Plants
2.4. Determination of Total Phenolic Content and Phenolic Compound Identification
2.5. Antifungal Experimental Conditions and Sampling
2.6. Mold Growth Monitoring
2.7. Extraction and Quantification of Aflatoxins
2.8. Gene Expression Studies
2.9. Statistical Analysis
3. Results and Discussion
3.1. Quantification and Identification of Phenolic Compounds Extracted from Plants
3.2. Activity of the Plants Extracts against Growth and Aflatoxin Production of the Two Micotoxigenic Aspergillus flavus Strains
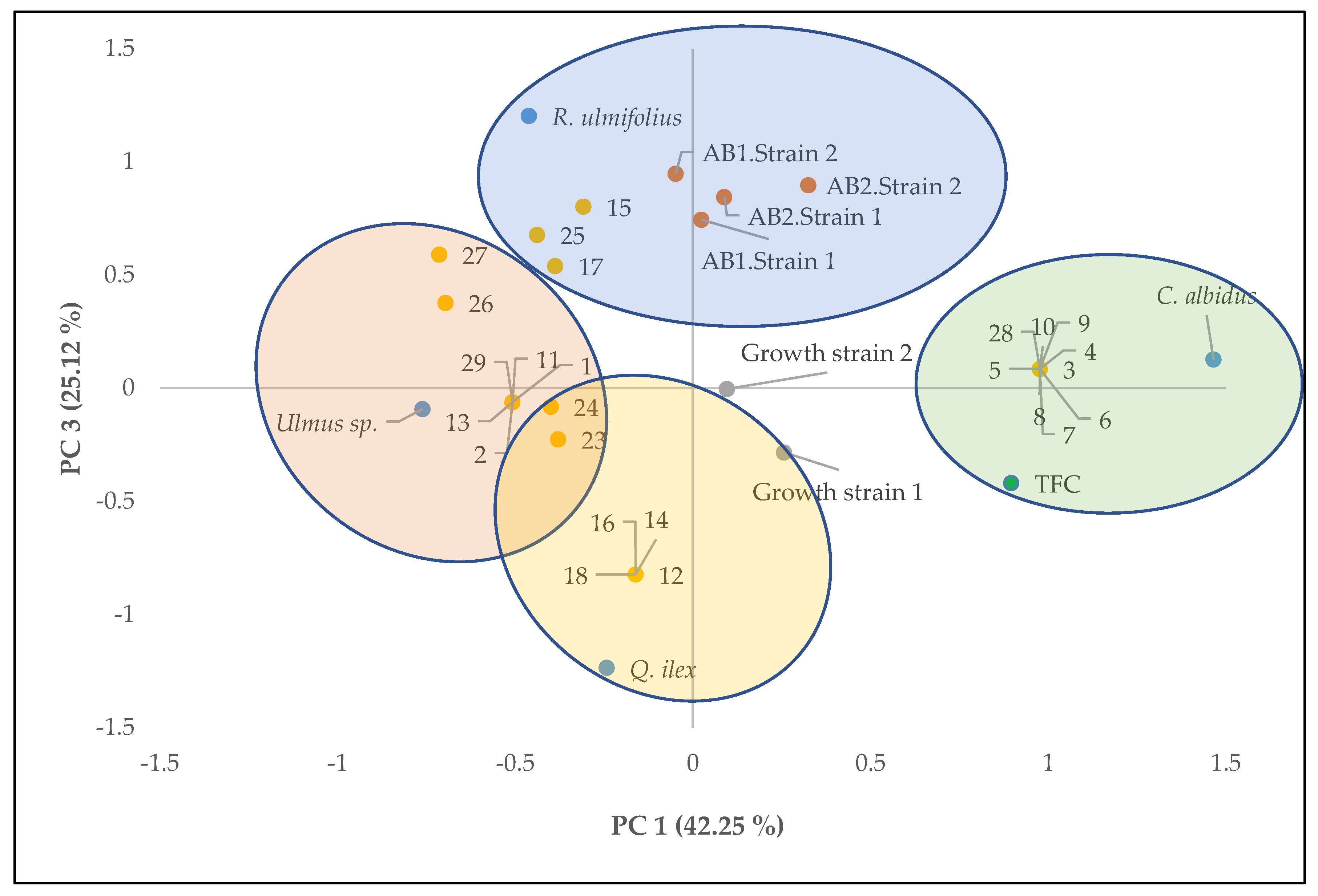
3.3. Multivariate Analysis of the Parameters Studied
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Ekren, S.; Yerlikaya, O.; Tokul, H.E.; Akpınar, A.; Açu, M. Chemical composition, antimicrobial activity and antioxidant capacity of some medicinal and aromatic plant extracts. Afr. J. Microbiol. Res. 2013, 7, 383–388. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Boy, F.R.; Casquete, R.; Martínez, A.; Córdoba, M.G.; Ruíz-Moyano, S.; Benito, M.J. Antioxidant, antihypertensive and antimicrobial properties of phenolic compounds obtained from native plants by different extraction methods. Int. J. Environ. Res. Public Health 2021, 18, 2475. [Google Scholar] [CrossRef]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal uses, phytochemistry, and pharmacological activities of Quercus species. Evid.-Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Sousa, M.J.; Morais, J.S.; Ferreira, I.C.F.R. Aromatic plants as a source of im-portant phytochemicals: Vitamins, sugars and fatty acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii leaves. Ind. Crops Prod. 2009, 30, 427–430. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; Volume 519, p. 388. [Google Scholar]
- Magan, N.; Olsen, M. Mycotoxins in Food: Detection and Control; Woodhead Publishing: Sawston, UK, 2004. [Google Scholar]
- Pereira, C.; Cunha, S.C.; Fernandes, J.O. Mycotoxins of concern in children and infant cereal food at European level: Incidence and bioaccessibility. Toxins 2022, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Bashiry, M.; Javanmardi, F.; Sadeghi, E.; Shokri, S.; Hossieni, H.; Oliveira, C.A.; Khaneghah, A.M. The prevalence of aflatoxins in commercial baby food products: A global systematic review, meta-analysis, and risk assessment study. Trends Food Sci. Technol. 2021, 114, 100–115. [Google Scholar] [CrossRef]
- Xing, Y.; Ouyang, Q.; Wang, S.; Zhou, X. Simultaneous determination of aflatoxins B1, B2, G1, G2, and M1 in dairy products by high-performance liquid chromatography/fluorescence. New J. Chem. 2017, 41, 9840–9846. [Google Scholar] [CrossRef]
- Kortei, N.K.; Annan, T.; Akonor, P.T.; Richard, S.A.; Annan, H.A.; Kyei-Baffour, V.; Esua-Amoafo, P. The occurrence of aflatoxins and human health risk estimations in randomly obtained maize from some markets in Ghana. Sci. Rep. 2021, 11, 4295. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Adunphatcharaphon, S.; Banerjee, K.; Elliott, C.; Petchkongkaew, A.; Kolawole, O. A Review of The Fundamental Factors and Processes Leading to The Accumulation of Aflatoxins in Cereal Crops. Preprints.org 2022, 2022010400. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Chun, H.S. Natural products for preventing and controlling aflatoxin contamination of food. In Aflatoxin-Control, Analysis, Detection and Health Risks; Abdulra’uf, L.B., Ed.; IntechOpen Limited: London, UK, 2017; pp. 13–44. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Casquete, R.; Benito, M.J.; Aranda, E.; Martín, A.; Ruiz-Moyano, S.; Córdoba, M.G. Gene expression of Aspergillus flavus strains on a cheese model system to control aflatoxin production. J. Dairy Sci. 2019, 102, 7765–7772. [Google Scholar] [CrossRef]
- Peromingo, B.; Rodríguez, M.; Delgado, J.; Andrade, M.J.; Rodríguez, A. Gene expression as a good indicator of aflatoxin contamination in dry-cured ham. Food Microbiol. 2017, 67, 31–40. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Evening primrosemeal: A source of natural antioxidants and scavenger of hydrogen peroxide and oxygen-derived free radicals. J. Agric. Food Chem. 1999, 47, 1801–1812. [Google Scholar] [CrossRef]
- Casquete, R.; Benito, M.J.; Córdoba, M.G.; Ruiz-Moyano, S.; Martín, A. The growth and aflatoxin production of Aspergillus flavus strains on a cheese model system are influenced by physicochemical factors. J. Dairy Sci. 2017, 100, 6987–6996. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T.; Al-Qudah, M.A.; Saleh, N.R.; Bataineh, T.T.; Obeidat, S.M.; Al-Sheraideh, M.S.; Lahham, J.N. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020, 13, 6256–6266. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Gómez-García, R.; Carrasco, E.; Bascón-Villegas, I.; Rodríguez, A.; Pintado, M. Quercus ilex leaf as a functional ingredient: Polyphenolic profile and antioxidant activity throughout simulated gastrointestinal digestion and antimicrobial activity. J. Funct. Foods 2022, 91, 105025. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Esoh, R.B.; Al-u’datt, M.H.; Alrababah, M.A.; Yang, W. Antioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Nat. Prod. Res. 2011, 25, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and antimicrobial preservatives: Properties, mechanism of action and applications in food—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Faustino, M.V.; Pinto, D.C.; Gonçalves, M.J.; Salgueiro, L.; Silveira, P.; Silva, A.M. Calendula L. species polyphenolic profile and in vitro antifungal activity. J. Funct. Foods 2018, 45, 254–267. [Google Scholar] [CrossRef]
- Price, M.S.; Yu, J.; Nierman, W.C.; Kim, H.; Pritchard, B.; Jacobus, C.A.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A. The aflatoxin pathway regulator AflR induces gene transcription inside and outside of theaflatoxin biosynthetic cluster. FEMS Microbiol. Lett. 2006, 255, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Bernáldez, V.; Cordoba, J.J.; Magan, N.; Peromingo, B.; Rodriguez, A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT-Food Sci. Technol. 2017, 83, 283–291. [Google Scholar] [CrossRef]
- Medina, A.; Schmidt-Heydt, M.; Rodríguez, A.; Parra, R.; Geisen, R.; Magan, N. Impacts of environmental stress on growth, secondary metabolite biosynthetic gene clusters and metabolite production of xerotolerant/xerophilic fungi. Curr. Genet. 2015, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]

| Extract | Total Phenolic Compounds | ||
|---|---|---|---|
| Mean | SD 1 | ||
| Quercus ilex | 527 | ± | 72.12 b |
| Rubus ulmifolius | 257 | ± | 45.25 c |
| Ulmus sp. | 253 | ± | 11.31 c |
| Cistus albidus | 703 | ± | 7.07 a |
| Peak | Rt (min) | [M-H]− (m/z) | MS/MS (m/z) | Compound Identified * | Quercus ilex | Rubus ulmifolius | Ulmus sp. | Cistus albidus |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.020 | 116 | 277; 365 | L-Valine | 0.00 b | 0.00 b | 47.24 a | 0.00 b |
| 2 | 4.167 | 123 | 144 | Orcinol | 0.00 b | 0.00 b | 36.63 a | 0.00 b |
| 3 | 10.529 | 179 | 133; 135; 180 | Caffeic acid | 0.00 b | 0.00 b | 0.00 b | 44,979.47 a |
| 4 | 11.026 | 179 | 105; 133; 180; 395 | Caffeic acid | 0.00 b | 0.00 b | 0.00 b | 5876.58 a |
| 5 | 11.441 | 287 | 449; 611 | Eriodictyol | 0.00 b | 0.00 b | 0.00 b | 910.52 a |
| 6 | 11.69 | 303 | Dihydroquercetin | 0.00 b | 0.00 b | 0.00 b | 384.87 a | |
| 7 | 11.839 | 287 | Eriodictyol | 0.00 b | 0.00 b | 0.00 b | 6107.77 a | |
| 8 | 12.204 | 271 | 287 | Naringenin | 0.00 b | 0.00 b | 0.00 b | 1926.97 a |
| 9 | 12.403 | 339 | 165; 323; 501 | Esculetin-O-glucoside | 0.00 b | 0.00 b | 0.00 b | 419.97 a |
| 10 | 12.850 | 353 | 127; 659 | Chlorogenic acid | 0.00 b | 0.00 b | 0.00 b | 5448.26 a |
| 11 | 13.603 | 303 | 123; 139 | Dihydroquercetin | 0.00 b | 0.00 b | 7517.37 a | 0.00 b |
| 12 | 13.664 | 163 | 164 | Coumaric acid | 473.95 a | 0.00 b | 0.00 b | 0.00 b |
| 13 | 13.835 | 287 | Eriodictyol | 0.00 b | 0.00 b | 5914.22 a | 0.00 b | |
| 14 | 13.846 | 153 | 171 | Protocatechuic acid | 255.62 a | 0.00 b | 0.00 b | 0.00 b |
| 15 | 13.969 | 303 | Dihydroquercetin | 0.00 b | 761.21 a | 0.00 b | 0.00 b | |
| 16 | 14.062 | 303 | Dihydroquercetin | 7605.68 a | 0.00 b | 0.00 b | 0.00 b | |
| 17 | 14.317 | 317 | 318 | Myricetin | 309.19 b | 867.96 a | 0.00 c | 0.00 c |
| 18 | 14.443 | 163 | 163 | Coumaric acid | 1890.40 a | 0.00 b | 0.00 b | 0.00 b |
| 19 | 15.173 | 147 | 148 | Cynnamic acid | 7496.65 a | 3785.26 b | 0.00 c | 0.00 c |
| 20 | 16.208 | 147 | 148 | Cynnamic acid | 1726.29 b | 4078.55 a | 0.00 c | 0.00 c |
| 21 | 17.550 | 147 | 148 | Cynnamic acid | 784.53 b | 3021.26 a | 0.00 c | 0.00 c |
| 22 | 17.750 | 147 | 148; 283; 847 | Cynnamic acid | 806.54 a | 870.59 a | 0.00 b | 0.00 b |
| 23 | 19.862 | 537 | 261; 511 | Ligstroside glucuronide | 660.18 a | 487.32 a | 0.00 b | 0.00 b |
| 24 | 19.945 | 353 | 354 | 5-O-Caffeoylquinic acid | 653.41 a | 597.34 a | 0.00 b | 0.00 b |
| 25 | 20.138 | 315 | 316 | 5.7-Dihydroxy-3′.4′-dimethoxyflavanone | 362.77 b | 1663.85 a | 181.54 b | 0.00 c |
| 26 | 21.149 | 593 | 533; 534; 594 | Kaempferol 3-O-rutinoside | 0.00 c | 8806.99 b | 16,605.12 a | 0.00 c |
| 27 | 23.173 | 533 | 534; 593; 594 | Kaempferol 3-O-malonyl-glucoside | 0.00 c | 1163.51 b | 1323.69 a | 0.00 c |
| 28 | 23.497 | 593 | 533 | Kaempferol diglycoside | 0.00 b | 0.00 b | 0.00 b | 846.83 a |
| 29 | 24.536 | 635 | 575; 576; 636 | Trigalloyl-hexoside | 0.00 b | 0.00 b | 200.31 a | 0.00 b |
| Extract | Concentration (mg/L) | Cq103 Strain | Cq8 Strain | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD 1 | Mean | SD | ||||
| Control | 0 | 7.04 | ± | 0.51 a,b | 7.69 | ± | 0.21 a |
| Quercus ilex | 150 | 6.40 | ± | 0.07 a,b | 7.19 | ± | 0.13 a |
| 250 | 6.63 | ± | 0.77 a,b | 6.94 | ± | 0.33 a,b | |
| Rubus ulmifolius | 150 | 6.50 | ± | 0.20 a,b | 7.01 | ± | 0.48 a,b |
| 250 | 6.01 | ± | 0.19 a,b | 6.87 | ± | 0.07 a,b | |
| Ulmus sp. | 150 | 7.88 | ± | 0.01 a | 7.05 | ± | 0.03 a,b |
| 250 | 4.75 | ± | 0.66 b | 4.96 | ± | 0.58 c | |
| Cistus albidus | 150 | 6.93 | ± | 0.69 a,b | 7.17 | ± | 0.50 a |
| 250 | 5.87 | ± | 0.34 a,b | 6.07 | ± | 0.51 b | |
| Extract | Concentration (mg/L) | Cq103 A. flavus Strain | Cq8 A. flavus Strain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aflatoxin B1 (ppb) | Aflatoxin B2 (ppb) | Aflatoxin B1 (ppb) | Aflatoxin B2 (ppb) | ||||||||||
| Mean | SD 1 | Mean | SD | Mean | SD | Mean | SD | ||||||
| Control | 0 | 210.82 | ± | 24.74 a | 14.12 | ± | 0.83 a | 621.31 | ± | 164.20 a | 37.05 | ± | 13.99 a,b |
| Quercus ilex | 150 | 64.37 | ± | 11.71 b | 2.74 | ± | 0.27 b | 199.00 | ± | 12.73 b,c,d | 8.07 | ± | 2.22 b |
| 250 | 53.22 | ± | 6.90 b | 3.33 | ± | 0.48 b | 105.06 | ± | 21.26 d | 3.51 | ± | 1.19 b | |
| Rubus ulmifolius | 150 | 106.01 | ± | 24.37 b | 8.93 | ± | 0.27 b | 446.05 | ± | 60.43 a,b | 60.86 | ± | 1.53 a |
| 250 | 96.01 | ± | 74.37 b | 6.93 | ± | 5.27 b | 411.05 | ± | 30.43 a,b,c | 30.86 | ± | 1.53 b | |
| Ulmus sp. | 150 | 69.86 | ± | 13.11 b | 4.42 | ± | 0.90 b | 217.38 | ± | 42.71 b,c,d | 9.51 | ± | 1.77 b |
| 250 | 39.66 | ± | 24.05 b | 2.99 | ± | 2.00 b | 163.44 | ± | 74.01 c,d | 6.44 | ± | 4.15 b | |
| Cistus albidus | 150 | 94.00 | ± | 19.30 b | 6.97 | ± | 2.23 b | 402.93 | ± | 76.71 b,c | 65.73 | ± | 15.21 a |
| 250 | 60.84 | ± | 12.11 b | 4.61 | ± | 1.20 b | 218.68 | ± | 25.26 b,c,d | 22.31 | ± | 4.77 b | |
| Extract | Concentration (mg/L) | Cq103 A. flavus Strain | Cq8 A. flavus Strain | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD 1 | Mean | SD | ||||
| Control | 0 | 0.00 | ± | 0.000 | 0.00 | ± | 0.000 |
| Quercus ilex | 150 | −0.46 | ± | 0.337 | −0.12 | ± | 1.061 |
| 250 | −3.04 | ± | 0.001 | −0.26 | ± | 0.742 | |
| Rubus ulmifolius | 150 | −2.37 | ± | 0.004 | 0.21 | ± | 0.531 |
| 250 | −2.49 | ± | 0.004 | 0.21 | ± | 0.531 | |
| Ulmus sp. | 150 | −0.40 | ± | 0.519 | −2.00 | ± | 0.012 |
| 250 | −0.58 | ± | 0.321 | 0.00 | ± | 1.087 | |
| Cistus albidus | 150 | −0.27 | ± | 0.593 | −2.17 | ± | 0.000 |
| 250 | −0.29 | ± | 0.647 | −1.79 | ± | 0.023 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boy, F.R.; Casquete, R.; Gudiño, I.; Merchán, A.V.; Peromingo, B.; Benito, M.J. Antifungal Effect of Autochthonous Aromatic Plant Extracts on Two Mycotoxigenic Strains of Aspergillus flavus. Foods 2023, 12, 1821. https://doi.org/10.3390/foods12091821
Boy FR, Casquete R, Gudiño I, Merchán AV, Peromingo B, Benito MJ. Antifungal Effect of Autochthonous Aromatic Plant Extracts on Two Mycotoxigenic Strains of Aspergillus flavus. Foods. 2023; 12(9):1821. https://doi.org/10.3390/foods12091821
Chicago/Turabian StyleBoy, Francisco Ramiro, Rocío Casquete, Iris Gudiño, Almudena V. Merchán, Belén Peromingo, and María José Benito. 2023. "Antifungal Effect of Autochthonous Aromatic Plant Extracts on Two Mycotoxigenic Strains of Aspergillus flavus" Foods 12, no. 9: 1821. https://doi.org/10.3390/foods12091821
APA StyleBoy, F. R., Casquete, R., Gudiño, I., Merchán, A. V., Peromingo, B., & Benito, M. J. (2023). Antifungal Effect of Autochthonous Aromatic Plant Extracts on Two Mycotoxigenic Strains of Aspergillus flavus. Foods, 12(9), 1821. https://doi.org/10.3390/foods12091821








