Breadsticks Flavoured with Olives and Onions: One-Year Shelf Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Analyses of Treccine
2.2.1. Water Activity (aw)
2.2.2. Moisture Content Determination
2.2.3. Textural Profile Analysis
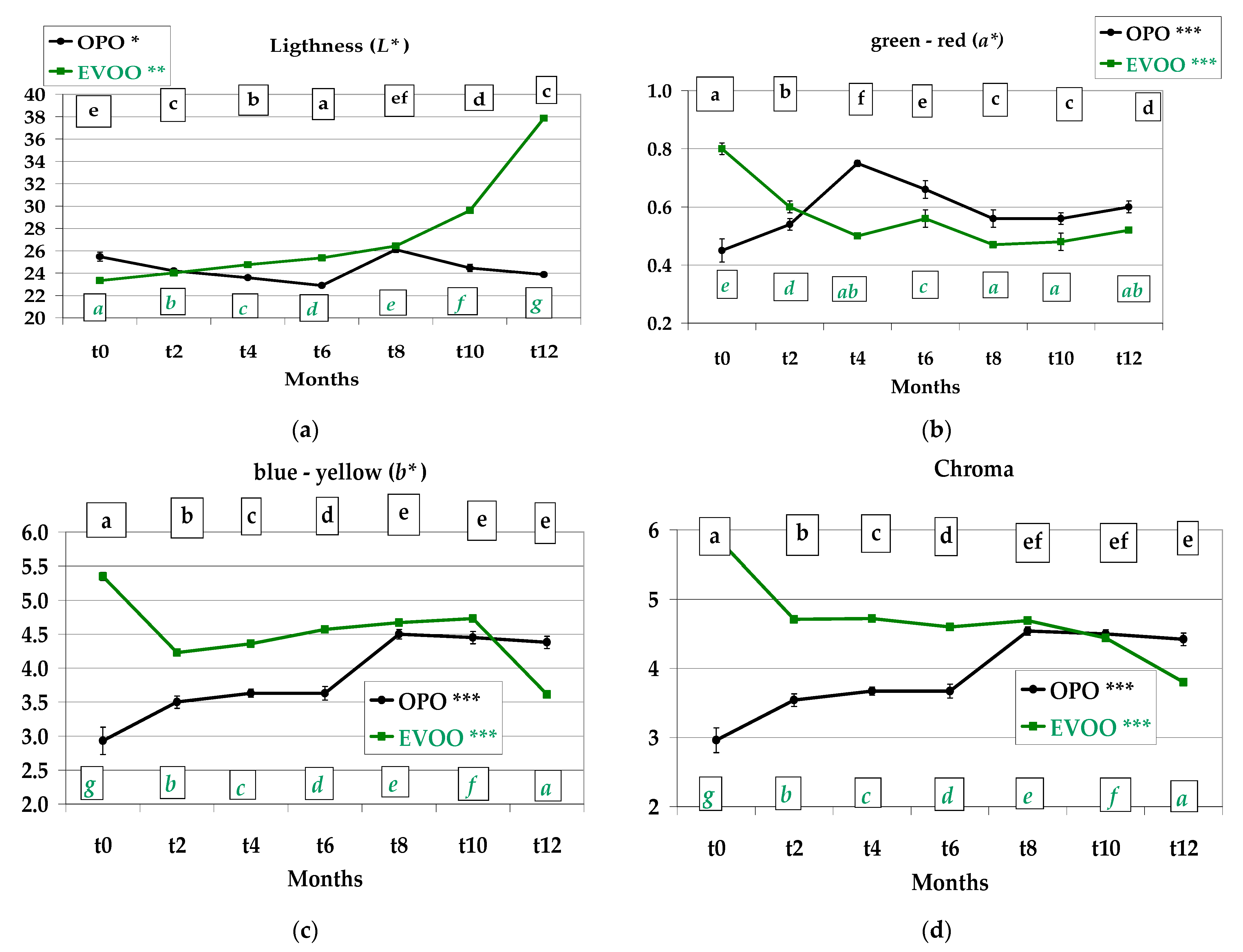
2.2.4. Colour Determination
2.2.5. Preliminary Sensory Analysis
2.3. Oil Analysis
2.3.1. Oil Extraction
2.3.2. Free Acidity Determination
2.3.3. Peroxide Value Determination
2.3.4. Spectrophotometric Investigation in Ultraviolet
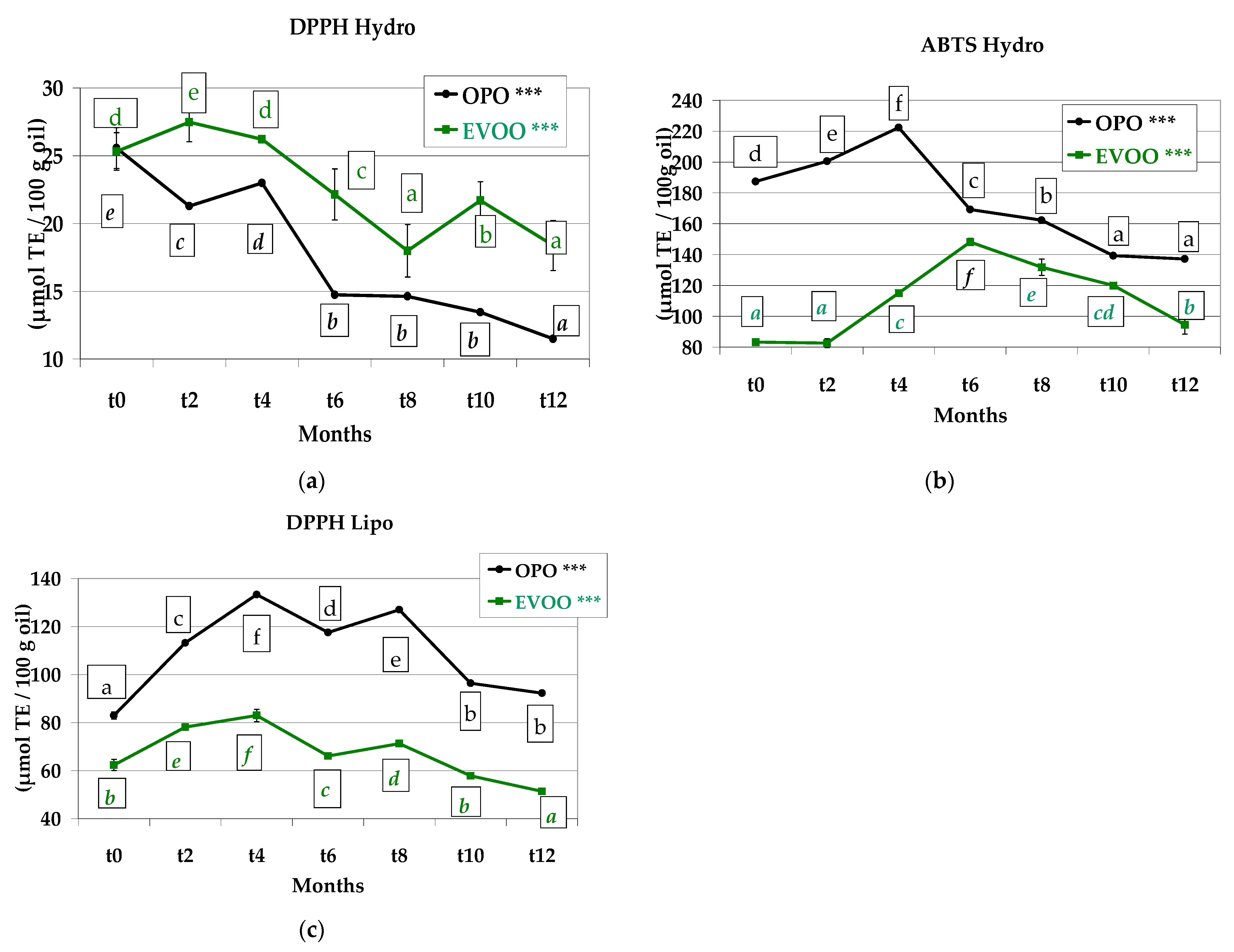
2.3.5. Antioxidant Capacity of the Extracted Oil
2.3.6. ABTS Assay on Hydrophilic Antioxidant Extract
2.3.7. DPPH Assay on Hydrophilic Antioxidant Extract
2.3.8. DPPH Assay on the Extracted Oil
2.3.9. Colour of the Extracted Oil
2.3.10. Determination of Fatty Acids by Gas Chromatography
2.3.11. Statistical Analysis
3. Results
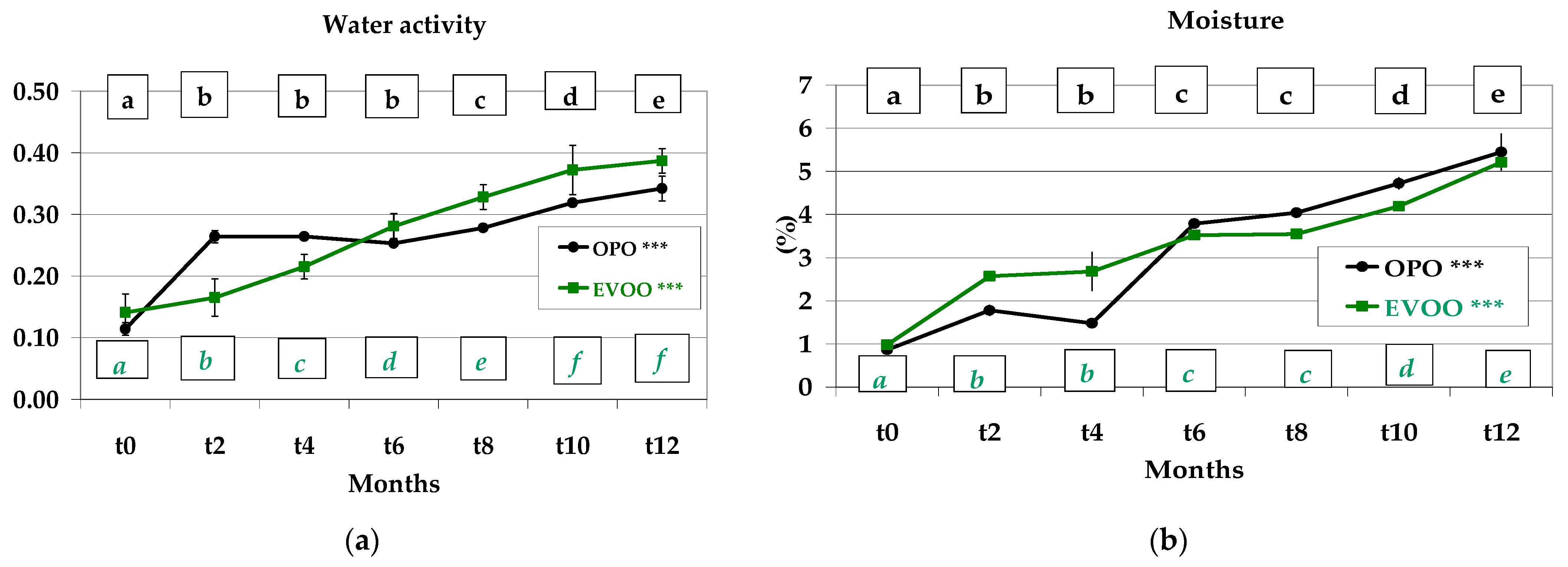
3.1. Water Activity and Moisture Content
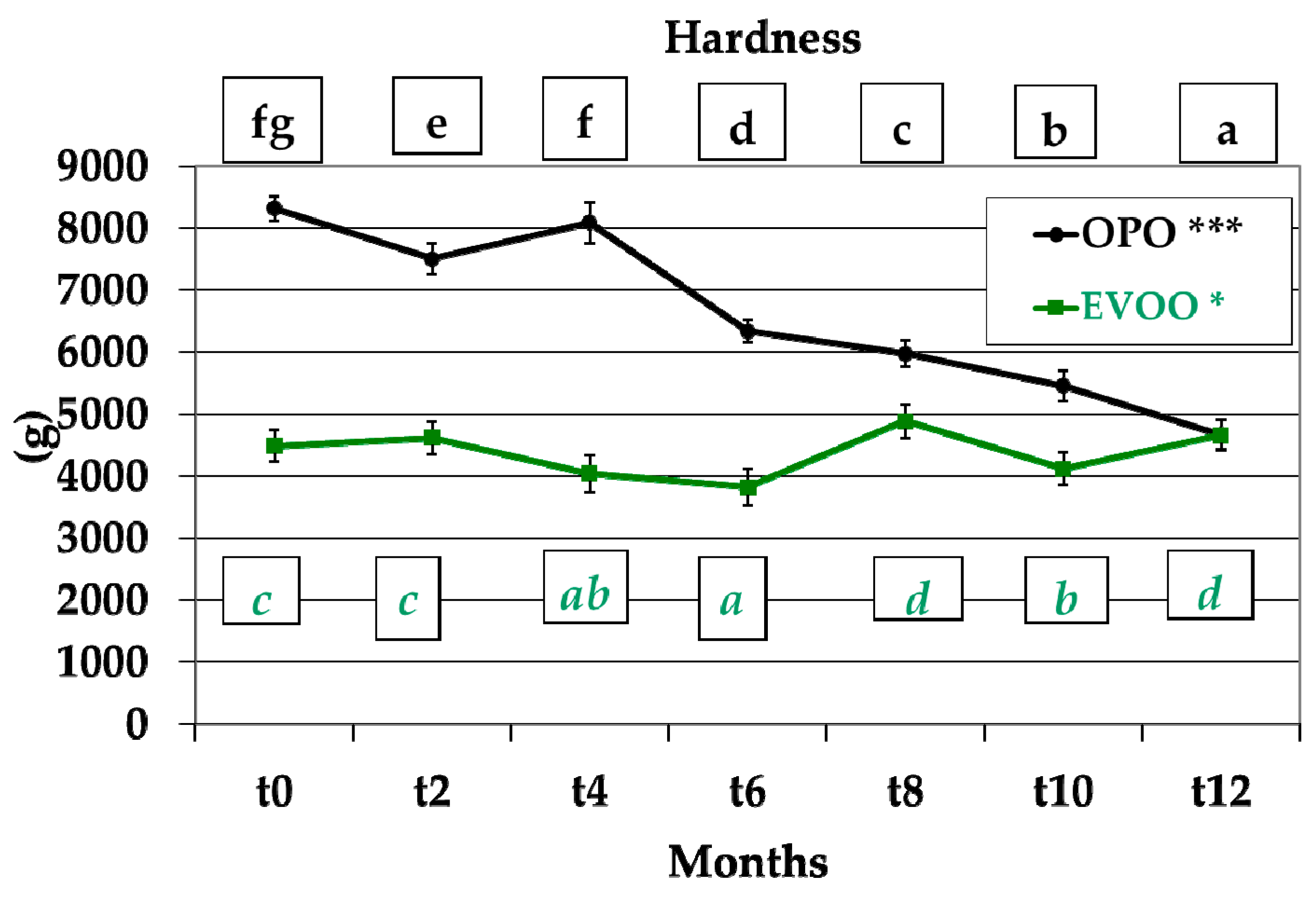
3.2. Hardness
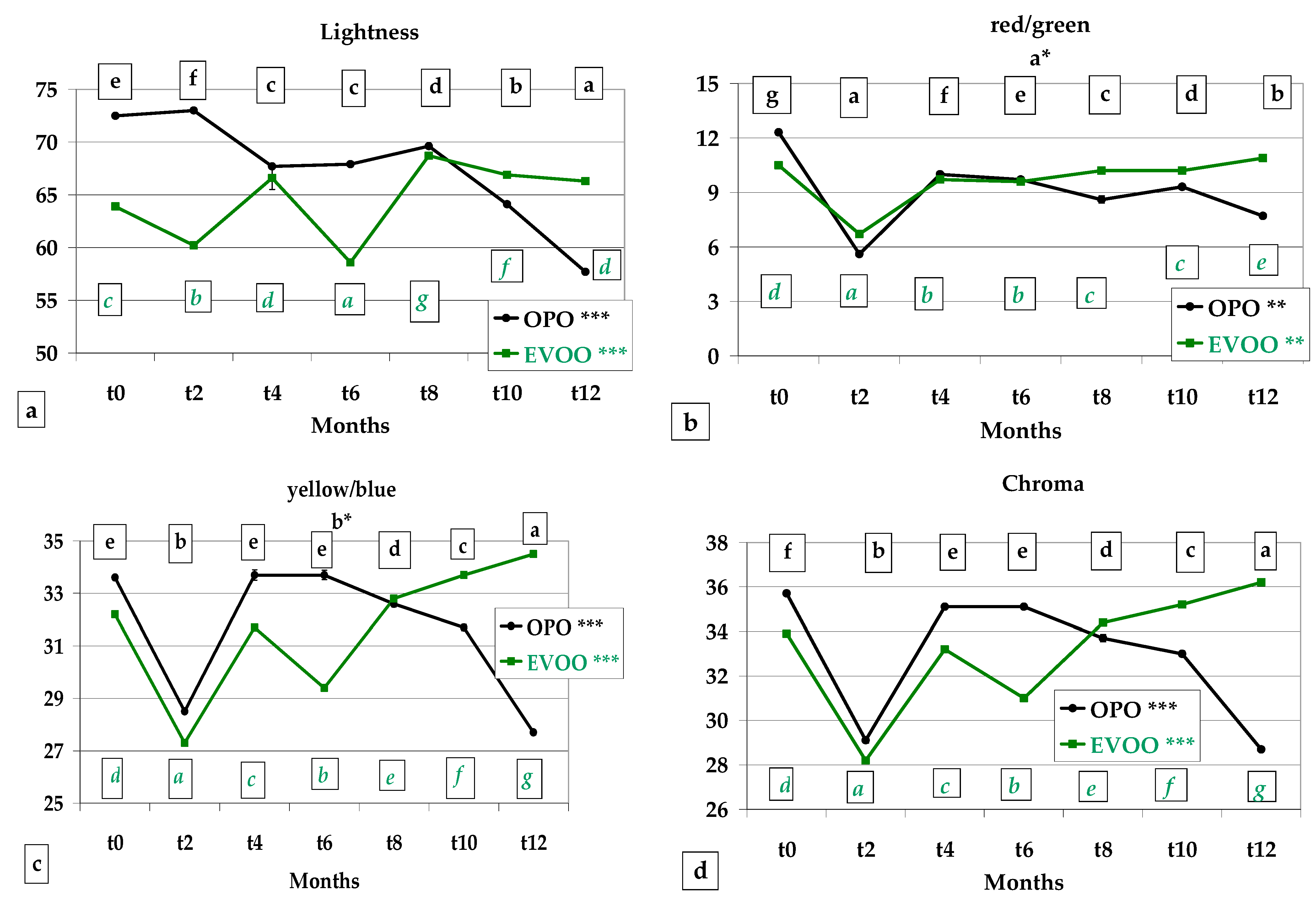
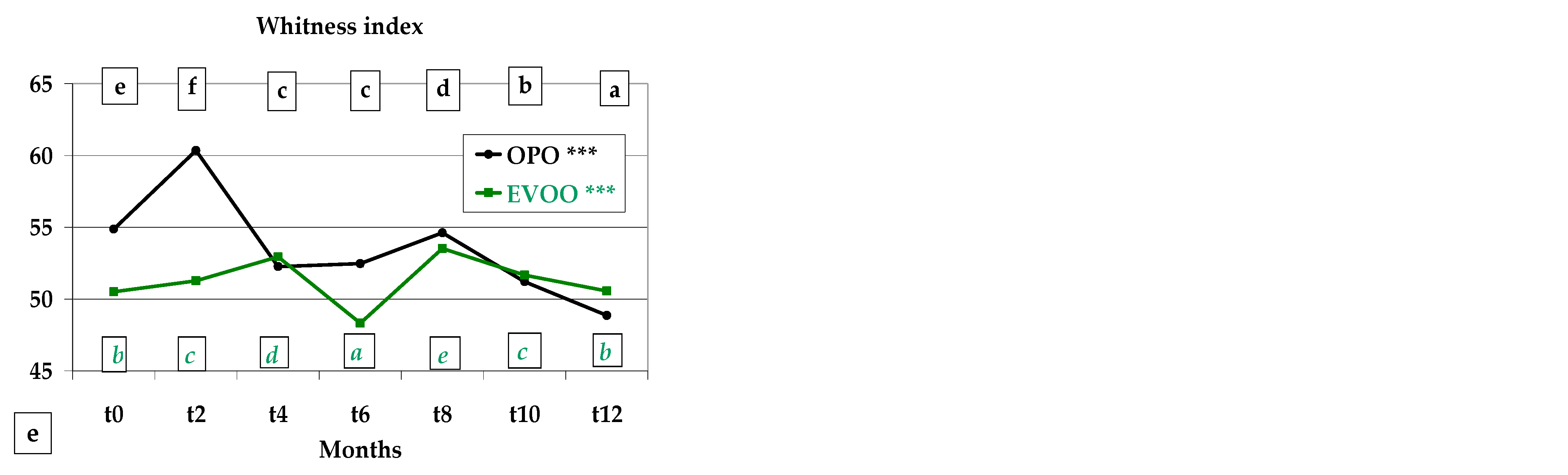
3.3. Colour, Instrumental Analysis
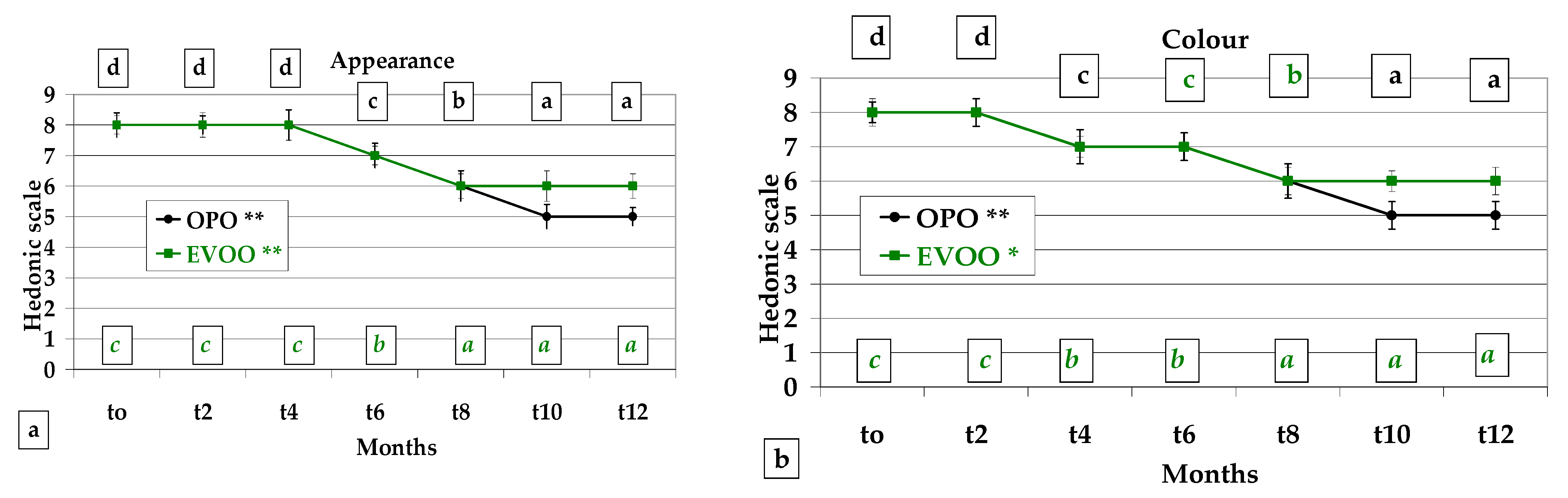
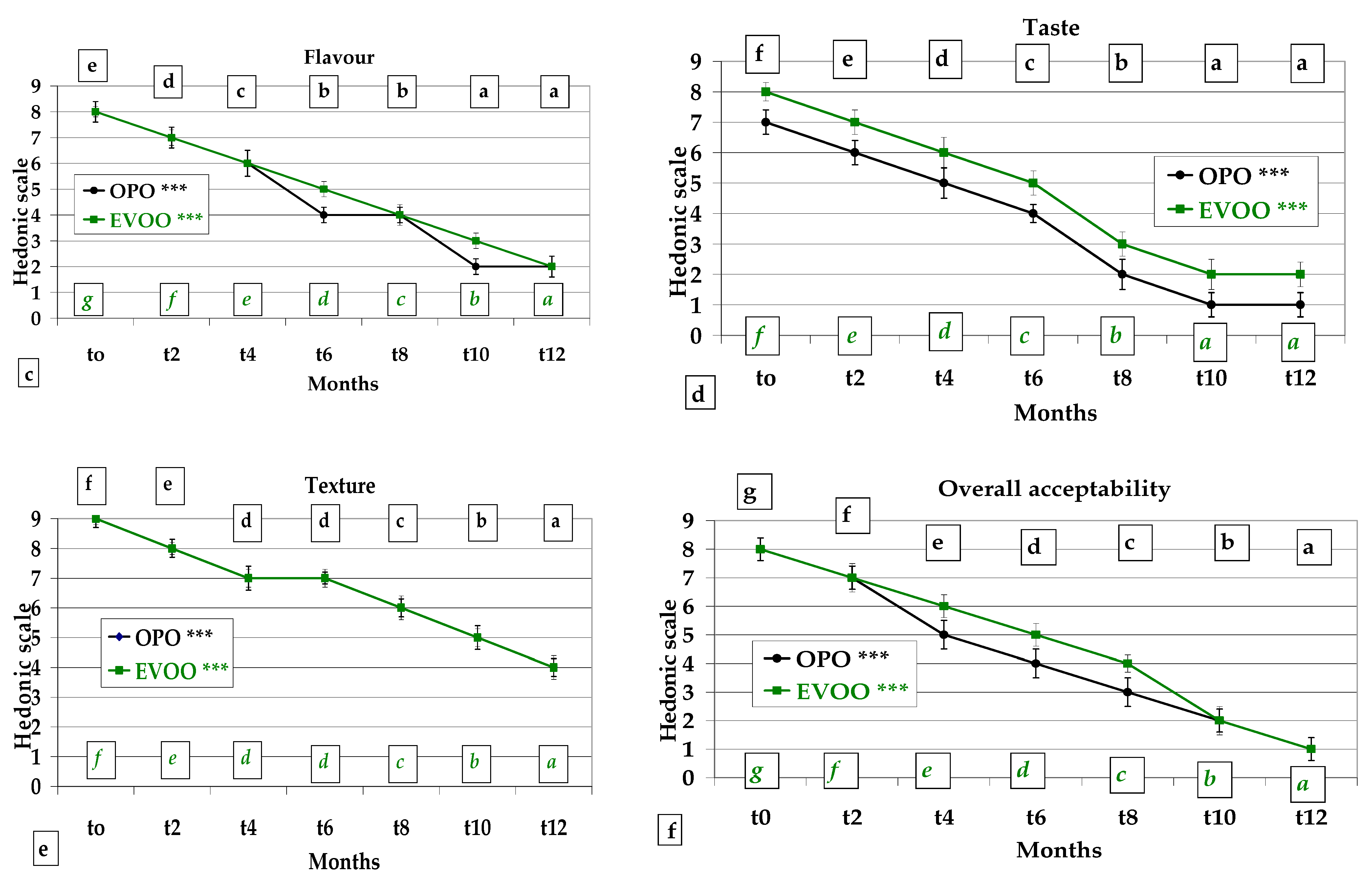
3.4. Sensory Analysis
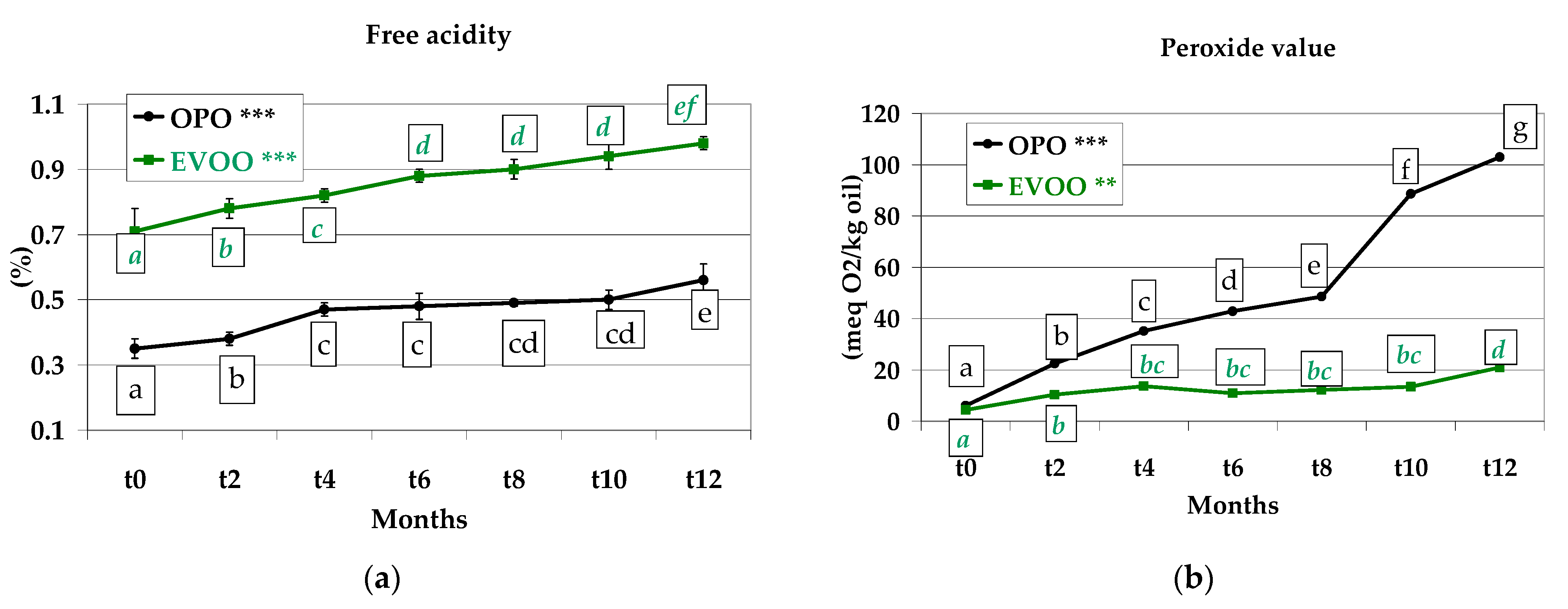
3.5. Free Acidity and Peroxide Value of the Extracted Oils
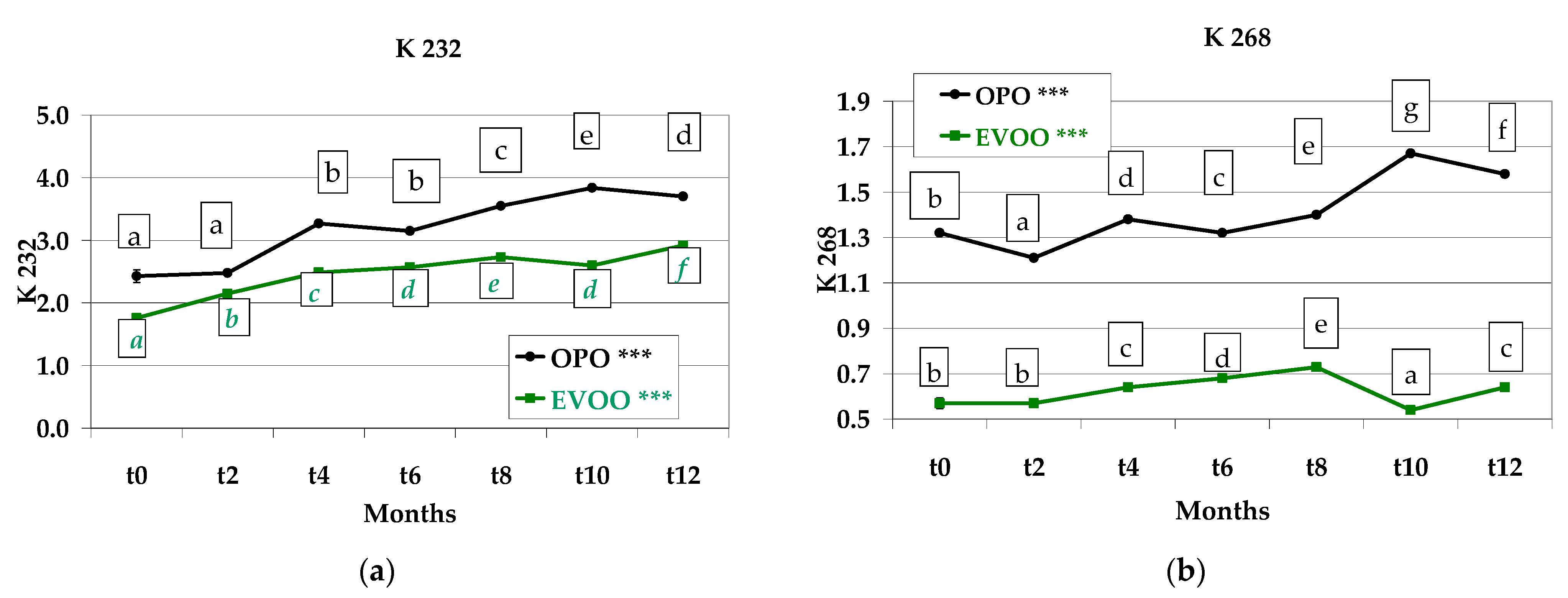
3.6. Spectrophotometric Indices of the Extracted Oils
3.7. Antioxidant Capacity
3.8. Color of the Extracted Oil
3.9. Fatty Acid Composition of the Oil Extracted from Treccine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giuffrè, A.M.; Caracciolo, M.; Capocasale, M.; Zappia, C.; Poiana, M. Effects of shortening replacement with extra virgin olive oil on the physical–chemical–sensory properties of Italian Cantuccini biscuits. Foods 2022, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Laganà, V.; Giuffrè, A.M.; De Bruno, A.; Poiana, M. Formulation of Biscuits Fortified with a Flour Obtained from Bergamot By-Products (Citrus bergamia, Risso). Foods 2022, 11, 1137. [Google Scholar] [CrossRef]
- Ghufran Saeed, S.M.; Ali, S.A.; Faheem, K.; Ali, R.; Giuffrè, A.M. The Impact of Innovative Plant Sources (Cordia myxa L. Fruit (Assyrian Plum) and Phoenix dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties of Gluten-Free Biscuits. Foods 2022, 11, 2346. [Google Scholar] [CrossRef] [PubMed]
- Hosseininejad, S.; Larrea, V.; Moraga, G.; Hernando, I. Evaluation of the Bioactive Compounds, and Physicochemical and Sensory Properties of Gluten-Free Muffins Enriched with Persimmon ‘Rojo Brillante’ Flour. Foods 2022, 11, 3357. [Google Scholar] [CrossRef] [PubMed]
- Ansorena, D.; Cartagena, L.; Astiasaran, I. A Cake Made with No Animal Origin Ingredients: Physical Properties and Nutritional and Sensory Quality. Foods 2023, 12, 54. [Google Scholar] [CrossRef]
- Pinto, D.; Moreira, M.M.; Vieira, E.F.; Švarc-Gajíc, J.; Vallverdú-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds. Foods 2023, 12, 640. [Google Scholar] [CrossRef]
- Dhal, S.; Anis, A.; Shaikh, H.M.; Alhamidi, A.; Pal, K. Effect of Mixing Time on Properties of Whole Wheat Flour-Based Cookie Doughs and Cookies. Foods 2023, 12, 941. [Google Scholar] [CrossRef]
- Ali, R.; Saeed, S.M.G.; Ali, S.A.; Sayed, S.A.; Ahmed, R.; Mobin, L. Effect of black gram flour as egg replacer on microstructure of biscuit dough and its impact on edible qualities. J. Food Meas. Charact. 2018, 12, 1641–1647. [Google Scholar] [CrossRef]
- Saeed, S.M.G.; Urooj, S.; Ali, S.A.; Ali, R.; Mobin, L.; Ahmed, R.; Sayeed, S.A. Impact of the incorporation of date pit flour an underutilized biowaste in dough and its functional role as a fat replacer in biscuits. J. Food Process. Preserv. 2018, 45, e15218. [Google Scholar] [CrossRef]
- Saeed, S.M.G.; Tayyaba, S.; Ali, S.A.; Tayyab, S.; Sayeed, S.A.; Ali, R.; Mobin, L.; Naz, S. Evaluation of the potential of Lotus root (Nelumbo nucifera) flour as a fat mimetic in biscuits with improved functional and nutritional properties. CYTA J. Food 2020, 18, 624–634. [Google Scholar] [CrossRef]
- Saeed, S.M.G.; Ali, S.A.; Ali, R.; Sayeed, S.A.; Mobin, L. Exploring the potential of bottle gourd (Lagenaria siceraria) flour as a fat mimetic in biscuits with improved physicochemical and nutritional characteristics and anti-diabetic properties. Ital. J. Food Sci. 2022, 34, 50–66. [Google Scholar] [CrossRef]
- Pokharel, A.; Dangal, A.; Karki, S.; Lamichhane, S.; Timsina, P.; Bohara, A. Study on the effect of different treatments on soy flour, and quality and sensory evaluation of prepared biscuits incorporated with oats and soy flour. Legume Sci. 2023, 1, e181. [Google Scholar] [CrossRef]
- Haider, N.N.; Altemimi, A.B.; George, S.S.; Baioumy, A.A.; El-Maksoud, A.A.A.; Pasqualone, A.; Abedelmaksoud, T.G. Nutritional Quality and Safety Characteristics of Imported Biscuits Marketed in Basrah, Iraq. Appl. Sci. 2022, 12, 9065. [Google Scholar] [CrossRef]
- Haider, N.N.; Haider, N.N.; Altemimi, A.B.; George, S.S.; Pratap-Singh, A. The Chemical Composition and Quality Parameters of Biscuits: A Review. Basrah J. Agric. Sci. 2022, 35, 257–277. [Google Scholar] [CrossRef]
- Pacher, N.; Burtscher, J.; Johler, S.; Etter, D.; Bender, D.; Fieseler, L.; Domig, K.J. Ropiness in Bread—A Re-Emerging Spoilage Phenomenon. Foods 2022, 11, 3021. [Google Scholar] [CrossRef]
- Minutillo, S.A.; Ruano-Rosa, D.; Abdelfattah, A.; Schena, L.; Malacrinò, A. The Fungal Microbiome of Wheat Flour Includes Potential Mycotoxin Producers. Foods 2022, 11, 676. [Google Scholar] [CrossRef]
- Preiti, G.; Calvi, A.; Giuffrè, A.M.; Badagliacca, G.; Virzì, N.; Bacchi, M. A Comparative Assessment of Agronomic and Baking Qualities of Modern/Old Varieties and Landraces of Wheat Grown in Calabria (Italy). Foods 2022, 11, 2359. [Google Scholar] [CrossRef]
- Ojobi Omedi, J.; Li, N.; Chen, C.; Cheng, X.; Huang, J.; Zhang, B.; Gao, T.; Liang, L.; Zhou, Z.; Huang, W. Potential Health Benefits of Yeast-Leavened Bread Containing LAB Pediococcus pentosaceus Fermented Pitaya (Hylocereus undatus): Both In Vitro and In Vivo Aspects. Foods 2022, 11, 3416. [Google Scholar] [CrossRef]
- Bianchi, A.; Taglieri, I.; Zinnai, A.; Macaluso, M.; Sanmartin, C.; Venturi, F. Effect of Argon as Filling Gas of the Storage Atmosphere on the Shelf-Life of Sourdough Bread—Case Study on PDO Tuscan Bread. Foods 2022, 11, 3470. [Google Scholar] [CrossRef]
- Piscopo, A.; Zappia, A.; Mincione, A.; Silletti, R.; Summo, C.; Pasqualone, A. Effect of Oil Type Used in Neapolitan Pizza TSG Topping on Its Physical, Chemical, and Sensory Properties. Foods 2023, 12, 41. [Google Scholar] [CrossRef]
- Coţovanu, I.; Stroe, S.G.; Ursachi, F.; Mironeasa, S. Addition of Amaranth Flour of Different Particle Sizes at Established Doses in Wheat Flour to Achieve a Nutritional Improved Wheat Bread. Foods 2023, 12, 133. [Google Scholar] [CrossRef]
- Huang, C.; Huang, J.; Zhang, B.; Ojobi Omedi, J.; Chen, C.; Zhou, L.; Liang, L.; Zou, Q.; Zheng, J.; Zeng, Y.; et al. Rheo-Fermentation Dough Properties, Bread-Making Quality and Aroma Characteristics of Red Bean (Vigna angularis) Sourdough Induced by LAB Weissella confusa QS813 Strain Fermentation. Foods 2023, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.M.G.; Ayesha, R.; Ali, S.A.; Ali, R.; Ahmed, R. Lotus root (Nelumbo nucifera Gaertn) flour a novel ingredient for the formulation of traditional unleavened flatbread: Rheological, physical and nutritional characteristics, and sensory attributes. J. Food Process. Preserv. 2021, 45, e16078. [Google Scholar] [CrossRef]
- Nicolosi, A.; Laganà, V.R.; Di Gregorio, D. Habits, Health and Environment in the Purchase of Bakery Products: Consumption Preferences and Sustainable Inclinations before and during COVID-19. Foods 2023, 12, 1661. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Caracciolo, M.; Zappia, C.; Capocasale, M.; Poiana, M. Effect of heating on chemical parameters of extra virgin olive oil, pomace olive oil, soybean oil and palm oil. Ital. J. Food Sci. 2018, 30, 715–739. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Zappia, C.; Capocasale, M. Effects of high temperatures and duration of heating on olive oil properties for food use and biodiesel production. J. Am. Oil Chem. Soc. 2017, 94, 819–830. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C.; Poiana, M. Influence of high temperature and duration of heating on the sunflower seed oil properties for food use and bio-diesel production. J. Oleo Sci. 2017, 66, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.L.; Arnal, C.; Navarro, M.A.; Osada, J. Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Mol. Nutr. Food Res. 2012, 56, 1043–1057. [Google Scholar] [CrossRef]
- Mele, M.A.; Islam, M.Z.; Kang, H.M.; Giuffrè, A.M. Pre-and post-harvest factors and their impact on oil composition and quality of olive fruit. Emir. J. Food Agric. 2018, 30, 592–603. [Google Scholar] [CrossRef]
- Giuffrè, A.M. Biometric evaluation of twelve olive cultivars under rainfed conditions in the region of Calabria, South Italy. Emir. J. Food Agric. 2017, 29, 696–709. [Google Scholar] [CrossRef]
- Giuffrè, A.M. The evolution of free acidity and oxidation related parameters in olive oil during olive ripening from cultivars grown in the region of Calabria, South Italy. Emir. J. Food Agric. 2018, 30, 539–548. [Google Scholar] [CrossRef]
- Esti, M.; Cinquanta, L.; La Notte, E. Phenolic Compounds in Different Olive Varieties. J. Agric. Food Chem. 1998, 46, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Squeo, G.; Difonzo, G.; Caponio, F.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Montanari, L.; Montinaro, A.; Piga, A. Change in quality during ripening of olive fruits and related oils extracted from three minor autochthonous Sardinian cultivars. Emir. J. Food Agric. 2019, 31, 196–205. [Google Scholar] [CrossRef]
- El Chami, A.; Conte, P.; Hassoun, G.; Piga, A. Effect of region of cultivation, tree age, and harvest time on the quality of Lebanese virgin olive oil. Ital. J. Food Sci. 2023, 35, 57–71. [Google Scholar] [CrossRef]
- Mansouri, F.; Moumen, A.B.; Belhaj, K.; Richard, G.; Fauconnier, M.L.; Sindic, M.; Serghini Caid, H.; Elamrani, A. Effect of crop season on the quality and composition of extra virgin olive oils from Greek and Spanish varieties grown in the Oriental region of Morocco. Emir. J. Food Agric. 2018, 30, 549–562. [Google Scholar] [CrossRef]
- Ben Hmida, R.; Gargouri, B.; Chtourou, F.; Abichou, M.; Sevim, D.; Bouaziz, M. Study on the Effect of Climate Changes on the Composition and Quality Parameters of Virgin Olive Oil “Zalmati” Harvested at Three Consecutive Crop Seasons: Chemometric Discrimination. ACS Omega 2022, 7, 40078–400908. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Esti, M.; La Notte, E. Evolution of Phenolic Compounds in Virgin Olive Oil During Storage. J. Am. Oil Chem. Soc. 1997, 74, 1259–1264. [Google Scholar] [CrossRef]
- Sakar, E.H.; Khtira, A.; Aalam, Z.; Zeroual, A.; Gagour, J.; Gharby, S. Variations in Physicochemical Characteristics of Olive Oil (cv ‘Moroccan Picholine’) According to Extraction Technology as Revealed by Multivariate Analysis. AgriEngineering 2022, 4, 922–938. [Google Scholar] [CrossRef]
- Saffar Taluri, S.; Jafari, S.M.; Bahrami, A. Evaluation of changes in the quality of extracted oil from olive fruits stored under different temperatures and time intervals. Sci. Rep. 2019, 9, 19688. [Google Scholar] [CrossRef]
- Mousavi, S.; hùMariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G.M. Evolution of Extra Virgin Olive Oil Quality under Different Storage Conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. (OJ L 248, 5.9.1991, p. 1). Consolidated Text. 01991R2568—EN—04.12.2016—031.005—1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01991R2568-20161204 (accessed on 20 April 2023).
- COI/T.15/NC No 3/Rev; Trade Standard Applying to Olive Oils and Olive Pomace Oils. International Olive Council: Madrid, Spain, 2018; English Version; Original: French Version.
- ISO 8589:1988; Sensory Analysis—General Guidance for the Design of Test Rooms. International Standard ISO: Geneva, Switzerland, 1988.
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.D.; Stathopoulos, C.E.; Golding, J.B.; Roach, P.D. Fate of the phenolic compounds during olive oil production with the traditional press method. Int. Food Res. 2014, 21, 101–109. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kalantzakis, G.; Blekas, G.; Pegklidou, K.; Boskou, D. Stability and radical-scavenging activity of heated olive oil and other vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 329–335. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Physicochemical composition of tomato seed oil for an edible use: The effect of cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Myers, A.W.; Stannett, V.; Szwarc, M. The permeability of polypropylene to gases and vapors. J. Polym. Sci. Part A Gen. Pap. 1959, 35, 285–286. [Google Scholar] [CrossRef]
- Blanco, I.; Romani, S.; Tylewicz, U.; Dalla Rosa, M. Gas Permeability and Thermal Behavior of Polypropylene Films Used for Packaging Minimally Processed Fresh-Cut Potatoes: A Case Study. J. Food Sci. 2012, 77, 10. [Google Scholar] [CrossRef]
- Conte, P.; Pulina, S.; Del Caro, A.; Fadda, C.; Urgeghe, P.P.; De Bruno, A.; Difonzo, G.; Caponio, F.; Romeo, R.; Piga, A. Gluten-Free Breadsticks Fortified with Phenolic-Rich Extracts from Olive Leaves and Olive Mill Wastewater. Foods 2021, 10, 923. [Google Scholar] [CrossRef]
- Alamprese, C.; Cappa, C.; Ratti, S.; Limbo, S.; Signorelli, M.; Fessas, D.; Lucisano, M. Shelf life extension of whole-wheat breadsticks: Formulation and packaging strategies. Food Chem. 2017, 230, 532–539. [Google Scholar] [CrossRef]
- Jemziya, M.B.F.; Mahendran, T. Storage evaluation of cookies produced from composite blends of wheat and sweet potato flour. JSc EUSL 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of starch–water interactions and their effects on two key functional properties: Starch gelatinization and retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Scott, G.; Awika, J.M. Effect of protein–starch interactions onstarch retrogradation and implications for foodproduct quality. Compr. Rev. Food Sci. Food Saf. 2023, 1–31. [Google Scholar] [CrossRef]
- Surojanametakusul, V.; Karnasuta, S.; Satmalee, P. Effect of oil type and batter ingredients on the properties of deep-frying flakes. Food Sci. Technol. (Camp.) 2020, 40, 592–596. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Patricia, A.; Hampana, N.N.; Mahalakshmi, D. Effect of Fats and Oils on Different Properties of Flours Used in Bakery Products: A Review. J. Nutr. Food Sci. 2022, 12, 353. [Google Scholar]
- Cox, S.; Abu-Ghannam, N. Incorporation of Himanthalia elongata seaweed to enhance the phytochemical content of breadsticks using Response Surface Methodology (RSM). Int. Food Res. J. 2013, 20, 1537–1545. [Google Scholar]
- De Gennaro, G.; Difonzo, G.; Summo, C.; Pasqualone, A.; Caponio, F. Olive cake powder as functional ingredient to improve the quality of gluten-free breadsticks. Foods 2022, 11, 552. [Google Scholar] [CrossRef]
- Rakshit, M.; Srivastav, P.P. Sensory evaluation and storage stability of fat reduced shortdough biscuit using hydrolysable tannin encapsulated double emulsion as fat replacer. LWT-Food Sci. Technol. 2022, 154, 112816. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Chaurin, V.; Reis, S.F.; Gallagher, E. Brewer’s spent grain as a functional ingredient for breadsticks. Int. J. Food Sci. Technol. 2012, 47, 1765–1771. [Google Scholar] [CrossRef]
- Hammad, K.S.M.; Morsy, N.F.S.; Abd El-Salam, E.A. Improving the oxidative stability of breadsticks with ginkgo (Ginkgo biloba) and ginseng (Panax ginseng) dried extracts. Grasas Aceites 2021, 3, e424. [Google Scholar] [CrossRef]
- Caponio, F.; Giarnetti, M.; Paradiso, V.M.; Summo, C.; Gomes, T. Potential use of extra virgin olive oil in bakery products rich in fats: A comparative study with refined oils. Int. J. Food Sci. Technol. 2013, 48, 82–88. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.P.; Ramo, T.; Macià, A.; Motilva, M.J. Methods for preparing phenolic extracts from olive cake for potential application as food antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphellyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.M.G.; Ali, S.A.; Ali, R.; Naz, S.; Sayeed, S.A.; Mobin, L.; Ahmed, R. Utilization of Vigna mungo flour as fat mimetic in biscuits: Its impact on antioxidant profile, polyphenolic content, storage stability, and quality attributes. Legume Sci. 2020, 2, e58. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Cookie making behavior of wheat–barley flour blends and effects on antioxidant properties. LWT-Food Sci. Technol. 2014, 55, 301–307. [Google Scholar] [CrossRef]
- Barison, A.; Da Silva, C.W.; Campos, F.R.; Simonelli, F.; Lenz, C.A.; Ferreira, A.G. A simple methodology for the determination of fatty acid composition in edible oils through 1H NMR spectroscopy. Magn. Reson. Chem. 2010, 48, 642–650. [Google Scholar] [CrossRef]
- Toker, O.S.; Ozturk, I.; Karaman, S.; Yalcin, H.; Kayacier, A.; Dogan, M.; Sagdic, O. Change in major fatty acid composition of vegetable oil depending on phenolic incorporation and storage period. Qual. Assur. Saf. Crops Foods 2016, 8, 179–188. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Beltrán, G.; Ortega, D.; Uceda, M. Characterisation of virgin olive oil of Italian olive cultivars: “Frantoio” and “Leccino”, grown in Andalusia. Food Chem. 2005, 8, 387–391. [Google Scholar] [CrossRef]
- Ranalli, A.; Contento, S.; Di Simone, G. Full characterization of virgin olive oils from new olive germplasm. VI Int. Symp. Olive Grow. 2008, 949, 77–83. [Google Scholar] [CrossRef]
- Khaleghi, E.; Arzani, K.; Moallemi, N.; Barzegar, M. The efficacy of kaolin particle film on oil quality indices of olive trees (Olea europaea L.) cv “Zard” grown under warm and semi-arid region of Iran. Food Chem. 2015, 166, 35–41. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Lercker, G.; Fernández-Gutiérrez, A. Evaluation of the influence of thermal oxidation on the phenolic composition and on the antioxidant activity of extra-virgin olive oils. J. Agric. Food Chem. 2007, 55, 4771–4780. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Pasqualone, A.; Gomes, T. Changes in the fatty acid composition of vegetable oils in model doughs submitted to conventional or microwave heating. Int. J. Food Sci. Technol. 2003, 38, 481–486. [Google Scholar] [CrossRef]
- Escudero, A.-; Ramos, N.; La Rubia, M.D.; Pacheco, R. Influence of extreme storage conditions on extra virgin olive oil parameters: Traceability study. J. Anal. Methods Chem. 2016, 2016, 7506807. [Google Scholar] [CrossRef] [PubMed]

| 14:0 | 16:0 | 16:1 | 17:0 | 17:1 | 18:0 | 18:1 | 18:2 | 18:3 | 20:0 | 20:1 | 22:0 | 24:0 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t0 | 0.02 | 12.03 | 0.77 | 0.09 | 0.14 | 2.56 d | 71.08 a | 12.00 c | 0.05 | 0.49 | 0.43 | 0.22 | 0.12 |
| t2 | 0.02 | 12.06 | 0.76 | 0.09 | 0.12 | 2.23 c | 71.45 b | 11.99 c | 0.05 | 0.49 | 0.41 | 0.22 | 0.11 |
| t4 | 0.02 | 12.35 | 0.76 | 0.09 | 0.12 | 2.15 b | 71.68 bc | 11.61 b | 0.05 | 0.48 | 0.40 | 0.20 | 0.09 |
| t6 | 0.02 | 12.51 | 0.78 | 0.09 | 0.13 | 2.24 c | 71.48 b | 11.52 b | 0.05 | 0.47 | 0.41 | 0.21 | 0.09 |
| t8 | 0.01 | 12.33 | 0.76 | 0.07 | 0.12 | 1.47 a | 72.92 d | 11.13 b | 0.04 | 0.47 | 0.40 | 0.20 | 0.08 |
| t10 | 0.02 | 12.26 | 0.77 | 0.09 | 0.13 | 1.45 a | 73.28 e | 10.76 a | 0.04 | 0.50 | 0.40 | 0.20 | 0.09 |
| t12 | 0.02 | 12.32 | 0.90 | 0.11 | 0.18 | 1.41 a | 72.88 d | 10.97 a | 0.04 | 0.48 | 0.39 | 0.21 | 0.09 |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | * | * | * | n.s. | n.s. | n.s. | n.s. | n.s. |
| Σ Saturated | Σ Unsaturated | Σ Mono -Unsaturated | Σ Poly -Unsaturated | Unsaturated/ Saturated | |
|---|---|---|---|---|---|
| t0 | 15.51 | 84.49 | 72.44 a | 12.05 c | 5.45 |
| t2 | 15.21 | 84.79 | 72.75 a | 12.04 c | 5.57 |
| t4 | 15.37 | 84.63 | 72.97 b | 11.66 b | 5.51 |
| t6 | 15.61 | 84.39 | 72.82 ab | 11.57 b | 5.41 |
| t8 | 14.62 | 85.38 | 74.21 c | 11.17 ab | 5.84 |
| t10 | 14.60 | 85.40 | 74.60 d | 10.80 a | 5.85 |
| t12 | 14.62 | 85.38 | 74.37 c | 11.01 a | 5.84 |
| Sign. | n.s. | n.s. | * | * | n.s. |
| 14:0 | 16:0 | 16:1 | 17:0 | 17:1 | 18:0 | 18:1 | 18:2 | 18:3 | 20:0 | 20:1 | 22:0 | 24:0 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t0 | 0.01 | 13.09 | 1.22 | 0.15 | 0.32 | 2.38 | 72.60 | 8.61 | 0.58 | 0.48 | 0.32 | 0.16 | 0.08 |
| t2 | 0.01 | 13.27 | 1.27 | 0.15 | 0.34 | 2.00 | 72.45 | 8.91 | 0.60 | 0.48 | 0.29 | 0.15 | 0.08 |
| t4 | 0.01 | 13.04 | 1.23 | 0.15 | 0.33 | 1.76 | 73.43 | 8.47 | 0.59 | 0.46 | 0.30 | 0.15 | 0.08 |
| t6 | 0.01 | 13.36 | 1.18 | 0.18 | 0.31 | 1.70 | 73.65 | 8.07 | 0.57 | 0.45 | 0.29 | 0.16 | 0.08 |
| t8 | 0.01 | 12.76 | 1.22 | 0.16 | 0.34 | 2.24 | 73.07 | 8.56 | 0.60 | 0.48 | 0.33 | 0.15 | 0.08 |
| t10 | 0.01 | 12.93 | 1.21 | 0.15 | 0.32 | 2.02 | 73.44 | 8.33 | 0.57 | 0.47 | 0.31 | 0.16 | 0.08 |
| t12 | 0.01 | 13.17 | 1.26 | 0.17 | 0.34 | 2.39 | 72.64 | 8.41 | 0.58 | 0.48 | 0.31 | 0.17 | 0.08 |
| n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Σ Saturated | Σ Unsaturated | Σ Mono -Unsaturated | Σ Poly -Unsaturated | Unsaturated/ Saturated | |
|---|---|---|---|---|---|
| t0 | 16.33 | 83.67 | 74.48 | 9.19 | 5.12 |
| t2 | 16.13 | 83.87 | 74.36 | 9.51 | 5.20 |
| t4 | 15.64 | 84.36 | 75.30 | 9.06 | 5.39 |
| t6 | 15.92 | 84.09 | 75.45 | 8.64 | 5.28 |
| t8 | 15.87 | 84.13 | 74.97 | 9.16 | 5.30 |
| t10 | 15.80 | 84.20 | 75.30 | 8.90 | 5.33 |
| t12 | 16.45 | 83.56 | 74.57 | 8.99 | 5.08 |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuffrè, A.M.; Caracciolo, M.; Zappia, C.; Capocasale, M.; Poiana, M. Breadsticks Flavoured with Olives and Onions: One-Year Shelf Life. Foods 2023, 12, 1798. https://doi.org/10.3390/foods12091798
Giuffrè AM, Caracciolo M, Zappia C, Capocasale M, Poiana M. Breadsticks Flavoured with Olives and Onions: One-Year Shelf Life. Foods. 2023; 12(9):1798. https://doi.org/10.3390/foods12091798
Chicago/Turabian StyleGiuffrè, Angelo Maria, Manuela Caracciolo, Clotilde Zappia, Marco Capocasale, and Marco Poiana. 2023. "Breadsticks Flavoured with Olives and Onions: One-Year Shelf Life" Foods 12, no. 9: 1798. https://doi.org/10.3390/foods12091798
APA StyleGiuffrè, A. M., Caracciolo, M., Zappia, C., Capocasale, M., & Poiana, M. (2023). Breadsticks Flavoured with Olives and Onions: One-Year Shelf Life. Foods, 12(9), 1798. https://doi.org/10.3390/foods12091798









