Application of Stable Isotope Analysis for Detecting the Geographical Origin of the Greek Currants “Vostizza”: A Preliminary Study
Abstract
1. Introduction
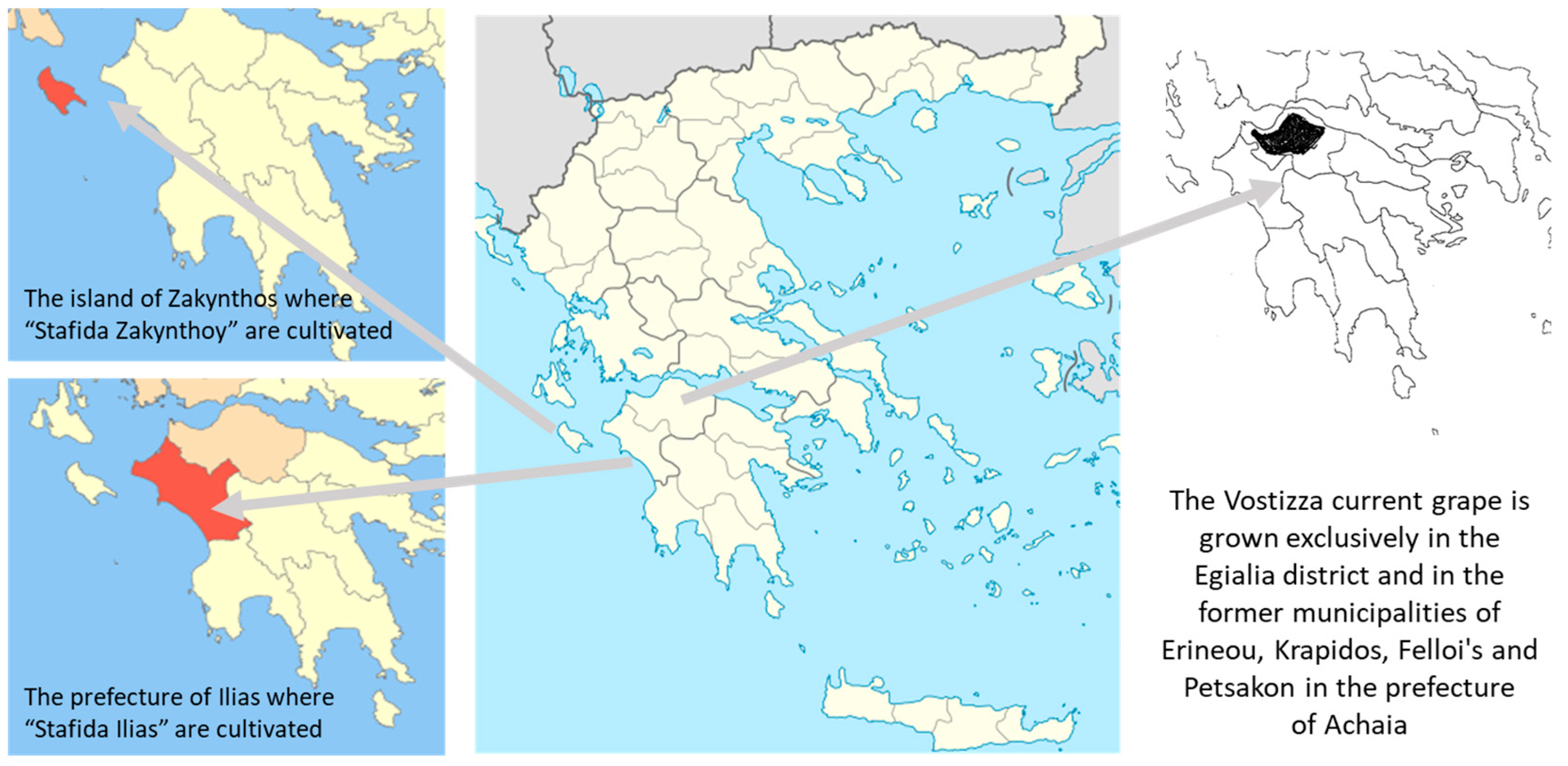
The Examined Product
2. Materials and Methods
2.1. Sampling
2.2. Sample Preparation
2.3. EA-IRMS Analysis
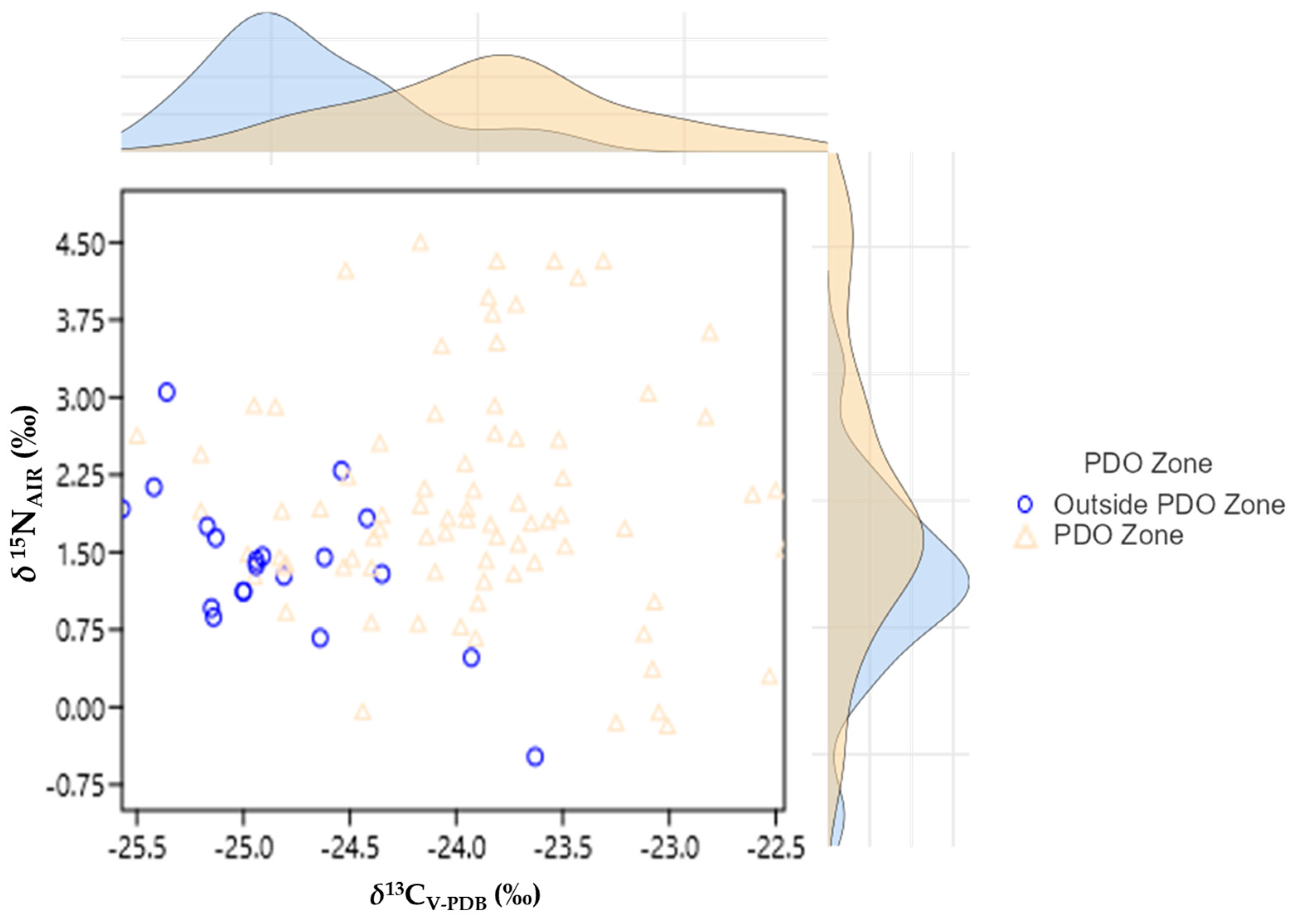
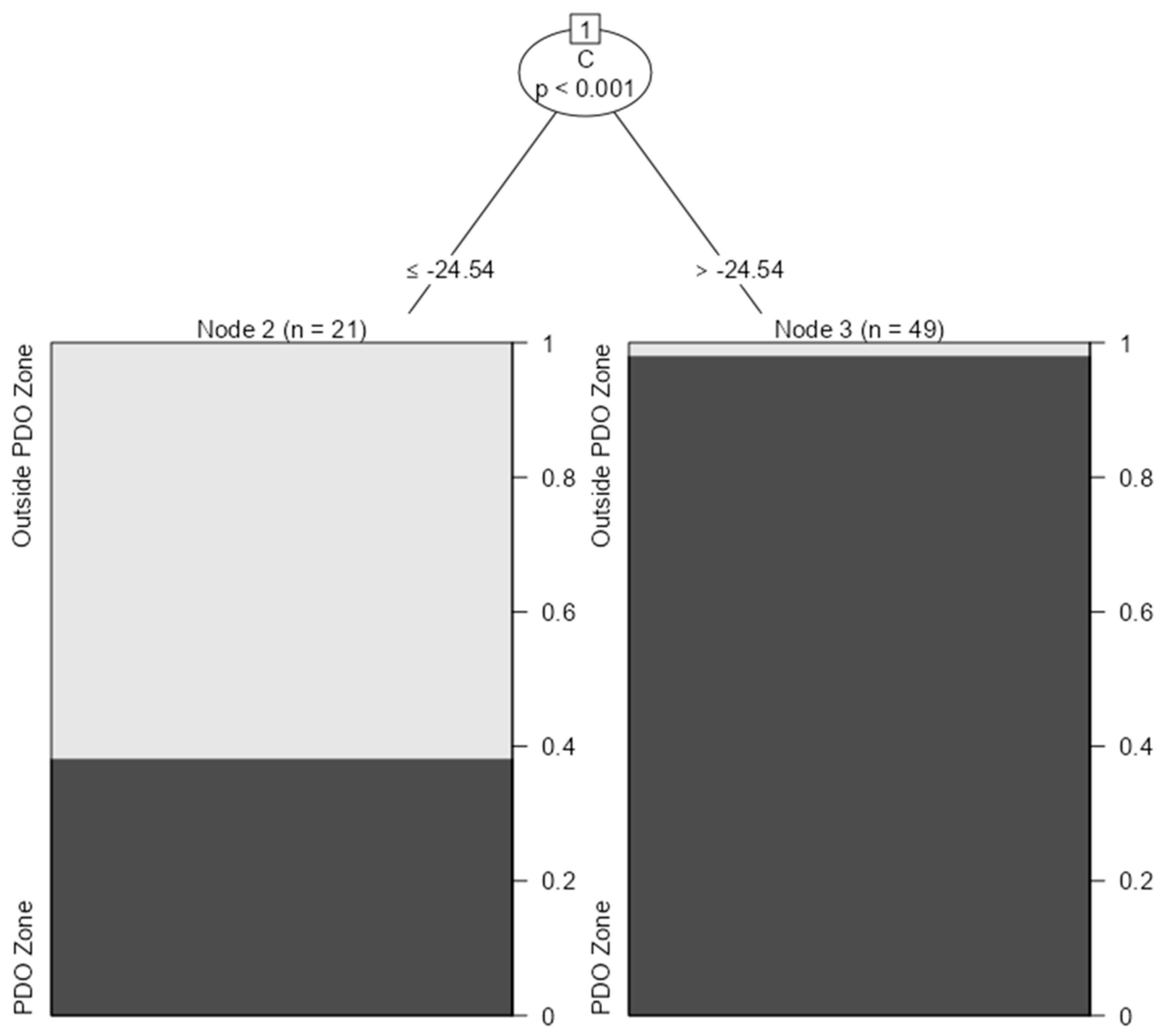
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample N. | Area | δ15NAIR (‰) | δ13CV-PDB (‰) |
|---|---|---|---|
| EP_1 | Outside PDO Zone | 1.27 | −24.81 |
| EP_2 | Outside PDO Zone | 3.05 | −25.36 |
| EP_3 | Outside PDO Zone | 0.96 | −25.15 |
| EP_4 | Outside PDO Zone | 1.12 | −25.00 |
| EP_5 | Outside PDO Zone | 2.13 | −25.42 |
| EP_6 | Outside PDO Zone | 1.92 | −25.57 |
| EP_7 | Outside PDO Zone | 1.37 | −24.94 |
| EP_8 | Outside PDO Zone | 1.45 | −24.62 |
| EP_9 | Outside PDO Zone | 0.48 | −23.93 |
| EP_10 | Outside PDO Zone | 1.29 | −24.35 |
| EP_11 | Outside PDO Zone | 0.87 | −25.14 |
| EP_12 | Outside PDO Zone | 1.46 | −24.91 |
| EP_13 | Outside PDO Zone | 1.12 | −25.03 |
| EP_14 | Outside PDO Zone | 1.83 | −24.42 |
| EP_15 | Outside PDO Zone | 1.75 | −25.17 |
| EP_16 | Outside PDO Zone | 1.42 | −24.94 |
| EP_17 | Outside PDO Zone | −0.48 | −23.63 |
| EP_18 | Outside PDO Zone | 2.29 | −24.54 |
| EP_19 | Outside PDO Zone | 0.67 | −24.64 |
| EP_20 | Outside PDO Zone | 1.64 | −25.13 |
| 21 | PDO Zone “Vostizza” | 3.97 | −23.85 |
| 22 | PDO Zone “Vostizza” | 4.23 | −24.52 |
| 23 | PDO Zone “Vostizza” | 1.77 | −23.84 |
| 24 | PDO Zone “Vostizza” | 1.29 | −23.73 |
| 25 | PDO Zone “Vostizza” | 1.48 | −24.98 |
| 26 | PDO Zone “Vostizza” | 0.82 | −24.4 |
| 27 | PDO Zone “Vostizza” | 1.02 | −23.07 |
| 28 | PDO Zone “Vostizza” | 2.1 | −22.5 |
| 29 | PDO Zone “Vostizza” | 3.63 | −22.81 |
| 30 | PDO Zone “Vostizza” | 3.04 | −23.1 |
| 31 | PDO Zone “Vostizza” | 1.8 | −23.57 |
| 32 | PDO Zone “Vostizza” | 1.65 | −24.14 |
| 33 | PDO Zone “Vostizza” | 2.63 | −25.5 |
| 34 | PDO Zone “Vostizza” | 1.01 | −23.9 |
| 35 | PDO Zone “Vostizza” | 0.67 | −23.91 |
| 36 | PDO Zone “Vostizza” | 1.78 | −23.65 |
| 37 | PDO Zone “Vostizza” | 1.44 | −24.49 |
| 38 | PDO Zone “Vostizza” | 1.73 | −23.21 |
| 39 | PDO Zone “Vostizza” | 2.92 | −24.95 |
| 40 | PDO Zone “Vostizza” | 2.91 | −24.85 |
| 41 | PDO Zone “Vostizza” | 4.32 | −23.31 |
| 42 | PDO Zone “Vostizza” | 2.6 | −23.72 |
| 43 | PDO Zone “Vostizza” | 1.92 | −24.64 |
| 44 | PDO Zone “Vostizza” | 1.90 | −25.2 |
| 45 | PDO Zone “Vostizza” | 4.5 | −24.17 |
| 46 | PDO Zone “Vostizza” | 2.81 | −22.83 |
| 47 | PDO Zone “Vostizza” | 1.36 | −24.53 |
| 48 | PDO Zone “Vostizza” | 1.42 | −23.86 |
| 49 | PDO Zone “Vostizza” | 2.59 | −23.52 |
| 50 | PDO Zone “Vostizza” | 1.58 | −23.71 |
| 51 | PDO Zone “Vostizza” | 1.53 | −22.46 |
| 52 | PDO Zone “Vostizza” | 2.06 | −22.61 |
| 53 | PDO Zone “Vostizza” | −0.04 | −24.44 |
| 54 | PDO Zone “Vostizza” | 0.30 | −22.53 |
| 55 | PDO Zone “Vostizza” | 2.92 | −23.82 |
| 56 | PDO Zone “Vostizza” | 1.27 | −24.95 |
| 57 | PDO Zone “Vostizza” | 0.81 | −24.18 |
| 58 | PDO Zone “Vostizza” | 0.71 | −23.12 |
| 59 | PDO Zone “Vostizza” | 0.37 | −23.08 |
| 60 | PDO Zone “Vostizza” | −0.18 | −23.01 |
| 61 | PDO Zone “Vostizza” | −0.05 | −23.05 |
| 62 | PDO Zone “Vostizza” | 1.39 | −23.633 |
| 63 | PDO Zone “Vostizza” | 1.36 | −24.81 |
| 64 | PDO Zone “Vostizza” | 0.92 | −24.8 |
| 65 | PDO Zone “Vostizza” | 1.22 | −23.87 |
| 66 | PDO Zone “Vostizza” | 2.22 | −23.5 |
| 67 | PDO Zone “Vostizza” | 3.53 | −23.81 |
| 68 | PDO Zone “Vostizza” | 2.84 | −24.1 |
| 69 | PDO Zone “Vostizza” | 1.95 | −24.17 |
| 70 | PDO Zone “Vostizza” | 1.35 | −24.4 |
| 71 | PDO Zone “Vostizza” | 2.45 | −25.2 |
| 72 | PDO Zone “Vostizza” | 1.31 | −24.1 |
| 73 | PDO Zone “Vostizza” | 0.78 | −23.98 |
| 74 | PDO Zone “Vostizza” | 1.69 | −24.05 |
| 75 | PDO Zone “Vostizza” | 1.64 | −24.39 |
| 76 | PDO Zone “Vostizza” | 1.65 | −23.81 |
| 77 | PDO Zone “Vostizza” | 2.12 | −24.15 |
| 78 | PDO Zone “Vostizza” | 2.23 | −24.51 |
| 79 | PDO Zone “Vostizza” | 4.32 | −23.54 |
| 80 | PDO Zone “Vostizza” | 2.1 | −23.92 |
| 81 | PDO Zone “Vostizza” | 1.83 | −24.04 |
| 82 | PDO Zone “Vostizza” | 1.39 | −24.8 |
| 83 | PDO Zone “Vostizza” | 3.9 | −23.72 |
| 84 | PDO Zone “Vostizza” | 4.16 | −23.43 |
| 85 | PDO Zone “Vostizza” | 1.45 | −24.83 |
| 86 | PDO Zone “Vostizza” | 1.82 | −23.95 |
| 87 | PDO Zone “Vostizza” | 2.65 | −23.82 |
| 88 | PDO Zone “Vostizza” | 1.86 | −23.51 |
| 89 | PDO Zone “Vostizza” | 1.56 | −23.49 |
| 90 | PDO Zone “Vostizza” | 1.98 | −23.71 |
| 91 | PDO Zone “Vostizza” | 1.92 | −23.95 |
| 92 | PDO Zone “Vostizza” | 2.56 | −24.36 |
| 93 | PDO Zone “Vostizza” | 4.32 | −23.81 |
| 94 | PDO Zone “Vostizza” | 2.36 | −23.96 |
| 95 | PDO Zone “Vostizza” | 1.72 | −24.36 |
| 96 | PDO Zone “Vostizza” | 1.90 | −24.82 |
| 97 | PDO Zone “Vostizza” | 3.5 | −24.07 |
| 98 | PDO Zone “Vostizza” | 3.81 | −23.83 |
| 99 | PDO Zone “Vostizza” | 1.86 | −24.35 |
| 100 | PDO Zone “Vostizza” | −0.15 | −23.25 |
References
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable isotope techniques for verifying the declared geographical origin of food in legal cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.E.; Vantarakis, A. Does Traceability Lead to Food Authentication? A Systematic Review from A European Perspective. Food Rev. Int. 2021, 39, 537–559. [Google Scholar] [CrossRef]
- Thomatou, A.A.; Psarra, E.; Mazarakioti, E.C.; Katerinopoulou, K.; Tsirogiannis, G.; Zotos, A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Stable Isotope Analysis for the Discrimination of the Geographical Origin of Greek Bottarga ‘Avgotaracho Messolongiou’: A Preliminary Research. Foods 2022, 11, 2960. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable Isotope Ratio Analysis for Assessing the Authenticity of Food of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, A.; Wąsowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016, 15, 714–720. [Google Scholar] [CrossRef]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Cunsolo, V.; Muccilli, V.; Sforza, S.; Dossena, A.; Drahos, L.; Vékey, K. Applications of liquid chromatography–mass spectrometry for food analysis. J. Chromatogr. A 2012, 1259, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, M.E.; Matzarapi, K.; Chasapi, S.; Vantarakis, A.; Spyroulias, G.A. Nontargeted 1H NMR fingerprinting and multivariate statistical analysis for traceability of Greek PDO “Vostizza” currants. J. Food Sci. 2021, 86, 4417–4429. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; Kelly, S.D.; Marigheto, N.; Alonso, G.L. Geographical origin differentiation of saffron spice (Crocus sativus L. stigmas)–Preliminary investigation using chemical and multi-element (H,C,N) stable isotope analysis. Food Chem. 2011, 128, 543–548. [Google Scholar] [CrossRef]
- Teye, E.; Amuah, C.L.Y.; McGrath, T.; Elliott, C. Innovative and rapid analysis for rice authenticity using hand-held NIR spectrometry and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 147–154. [Google Scholar] [CrossRef]
- Gumus, Z.P.; Celenk, V.U.; Tekin, S.; Yurdakul, O.; Ertas, H. Determination of trace elements and stable carbon isotope ratios in virgin olive oils from Western Turkey to authenticate geographical origin with a chemometric approach. Eur. Food Res. Technol. 2017, 243, 1719–1727. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.E.; Kotsalou, C.; Koudouna, M.; Katechaki, E.; Vantarakis, A. Potential biological markers by DNA-based tools for determination of Greek PDO geographical origin and authenticity: “Avgotaracho Mesolonghiou” and ““Vostizza” currant”. JSFA Rep. 2021, 1, 26–34. [Google Scholar] [CrossRef]
- Katerinopoulou, K.; Kontogeorgos, A.; Salmas, C.E.; Patakas, A.; Ladavos, A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Gonzalvez, A.; Armenta, S.; De La Guardia, M. Trace-element composition and stable-isotope ratio for discrimination of foods with Protected Designation of Origin. Trends Anal. Chem. 2009, 28, 1295–1311. [Google Scholar] [CrossRef]
- Mahne Opatić, A.; Nečemer, M.; Lojen, S.; Masten, J.; Zlatić, E.; Šircelj, H.; Stopar, D.; Vidrih, R. Determination of geographical origin of commercial tomato through analysis of stable isotopes, elemental composition and chemical markers. Food Control 2018, 89, 133–141. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.-H.; Bong, Y.-S. Geographical origin authentication of onions using stable isotope ratio and compositions of C, H, O, N, and S. Food Control 2019, 101, 121–125. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, D.; Dong, H.; Wan, J.; Luo, H.; Xian, Y.; Guo, X.; Qin, F.; Han, W.; Wang, L.; et al. Geographical origin of cereal grains based on element analyser-stable isotope ratio mass spectrometry (EA-SIRMS). Food Chem. 2015, 174, 553–557. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Karathanos, V.T. Determination of water content of dried fruits by drying kinetics. J. Food Eng. 1999, 39, 337–344. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kountouri, A.M.; Karathanos, V.T. Antioxidant Properties of Raisins (Vitis vinifera L.). J. Med. Food 2009, 12, 1302–1309. [Google Scholar] [CrossRef]
- Breksa III, A.P.; Takeoka, G.R.; Hidalgo, M.B.; Vilches, A.; Vasse, J.; Ramming, D.W. Antioxidant activity and phenolic content of 16 raisin grape (Vitis vinifera L.) cultivars and selections. Food Chem. 2010, 121, 740–745. [Google Scholar] [CrossRef]
- Chiou, A.; Panagopoulou, E.A.; Gatzali, F.; De Marchi, S.; Karathanos, V.T. Anthocyanins content and antioxidant capacity of Corinthian currants (Vitis vinifera L., var. Apyrena). Food Chem. 2014, 146, 157–165. [Google Scholar] [CrossRef]
- Vasilopoulou, E.; Trichopoulou, A. Greek raisins: A traditional nutritious delicacy. J. Berry Res. 2014, 4, 117–125. [Google Scholar] [CrossRef]
- Yanni, A.E.; Efthymiou, V.; Lelovas, P.; Agrogiannis, G.; Kostomitsopoulos, N.; Karathanos, V.T. Effects of dietary Corinthian currants (Vitis vinifera L., var. Apyrena) on atherosclerosis and plasma phenolic compounds during prolonged hypercholesterolemia in New Zealand White rabbits. Food Funct. 2015, 6, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Nikolidaki, E.K.; Chiou, A.; Christe, M.; Gkegka, A.P.; Karvelas, M.; Karathanos, V.T. Sun dried Corinthian currant (Vitis vinifera L., var. Apyrena) simple sugar profile and macronutrient characterization. Food Chem. 2017, 221, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, E.A.; Chiou, A.; Karathanos, V.T. Watersoluble vitamin content of sun-dried Corinthian raisins (Vitis vinifera L., var. Apyrena). J. Sci. Food Agric. 2019, 99, 5327–5333. [Google Scholar] [CrossRef]
- Langová, R.; Jůzl, M.; Cwiková, O.; Kos, I. Effect of different method of drying of five varieties grapes (Vitis vinifera L.) on the bunch stem on physicochemical, microbiological, and sensory quality. Foods 2020, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L. Raisin Grape Varieties. Raisin Production Manual; Agricultural and Natural Resources Publication: Oakland, CA, USA, 2000; pp. 38–47. [Google Scholar]
- Dutra, S.V.; Adami, L.; Marcon, A.R.; Carnieli, G.J.; Roani, C.A.; Spinelli, F.R.; Leonardelli, S.; Vanderlinde, R. Characterization of wines according the geographical origin by analysis of isotopes and minerals and the influence of harvest on the isotope values. Food Chem. 2013, 141, 2148–2153. [Google Scholar] [CrossRef]
- Jiang, W.; Xue, J.; Liu, X.; Wang, D.; Guo, Y.; Wang, L. The application of SNIF-NMR and IRMS combined with C, H and O isotopes for detecting the geographical origin of Chinese wines. Int. J. Food Sci. Technol. 2015, 50, 774–781. [Google Scholar] [CrossRef]
- Durante, C.; Bertacchini, L.; Bontempo, L.; Camin, F.; Manzini, D.; Lambertini, P.; Marchetti, A.; Paolini, M. From soil to grape and wine: Variation of light and heavy elements isotope ratios. Food Chem. 2016, 210, 648–659. [Google Scholar] [CrossRef]
- Longobardi, F.; Casiello, G.; Centonze, V.; Catucci, L.; Agostiano, A. Isotope ratio mass spectrometry in combination with chemometrics for characterization of geographical origin and agronomic practices of table grape. J. Sci. Food Agric. 2017, 97, 3173–3180. [Google Scholar] [CrossRef]
- Su, Y.Y.; Gao, J.; Zhao, Y.F.; Wen, H.S.; Zhang, J.J.; Zhang, A.; Yuan, C.L. Geographical Origin Classification of Chinese Wines Based on Carbon and Oxygen Stable Isotopes and Elemental Profiles. J. Food Prot. 2020, 83, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, F.; Shimizu, H.; Hayashi, S.; Kamada, A.; Igi, Y.; Koyama, K.; Yamada, O.; Goto-Yamamoto, N. Chemometric approaches for determining the geographical origin of Japanese Chardonnay wines using oxygen stable isotope and multi-element analyses. Food Chem. 2022, 371, 131113. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Ding, Q.; Lin, G. The stable isotope signatures of blackcurrant (Ribes nigrum L.) in main cultivation regions of China: Implications for tracing geographic origin. Eur. Food Res. Technol. 2013, 237, 109–116. [Google Scholar] [CrossRef]
- Perini, M.; Giongo, L.; Grisenti, M.; Bontempo, L.; Camin, F. Stable isotope ratio analysis of different European raspberries, blackberries, blueberries, currants and strawberries. Food Chem. 2018, 239, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using SPSS; SAGE Publication: London, UK, 2005. [Google Scholar]
- Carelli, M.L.C.; Queiroz-Voltan, R.B.; Fahl, J.I. Trivelin PCO. Braz. J. Plant Physiol. 2003, 15, 19–24. [Google Scholar] [CrossRef]
- Li, G.; Wu, Z.; Wang, Y.; Dong, X.; Li, B.; He, W.; Wang, S.; Cui, J. Identification of geographical origins of Schisandra fruits in China based on stable carbon isotope ratio analysis. Eur. Food Res. Technol. 2011, 232, 797–802. [Google Scholar] [CrossRef]
| δ (‰) | Area | N | Mean δ (‰) | Std. Error | ANOVA (F-Value) | Sig. |
|---|---|---|---|---|---|---|
| δ15NAIR (‰) | PDO Zone “Vostizza” | 80 | 1.38 | 1.115 | 9.05 | 0.004 |
| Outside PDO Zone | 20 | 2.01 | 0.735 | |||
| δ13CV-PDB (‰) | PDO Zone “Vostizza” | 80 | −23.93 | 0.668 | 46.85 | <0.001 |
| Outside PDO Zone | 20 | −24.83 | 0.486 |
| Observed | Predicted | |
|---|---|---|
| Outside PDO Zone | PDO Zone | |
| Outside PDO Zone | 3 | 5 |
| PDO Zone | 3 | 19 |
| Predictor | Estimate/Coefficient | SE | Z | p |
|---|---|---|---|---|
| Intercept | 77.37 | 18.508 | 4.18 | <0.001 |
| δ 15NAIR (‰) | 1.57 | 0.561 | 2.81 | 0.005 |
| δ13CV-PDB (‰) | 3.21 | 0.768 | 4.18 | <0.001 |
| Observed | Predicted | ||
|---|---|---|---|
| Outside PDO Zone | PDO Zone | % Correct | |
| Outside PDO Zone | 17 | 3 | 85% |
| PDO Zone | 16 | 64 | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomatou, A.-A.; Mazarakioti, E.C.; Zotos, A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Application of Stable Isotope Analysis for Detecting the Geographical Origin of the Greek Currants “Vostizza”: A Preliminary Study. Foods 2023, 12, 1672. https://doi.org/10.3390/foods12081672
Thomatou A-A, Mazarakioti EC, Zotos A, Kontogeorgos A, Patakas A, Ladavos A. Application of Stable Isotope Analysis for Detecting the Geographical Origin of the Greek Currants “Vostizza”: A Preliminary Study. Foods. 2023; 12(8):1672. https://doi.org/10.3390/foods12081672
Chicago/Turabian StyleThomatou, Anna-Akrivi, Eleni C. Mazarakioti, Anastasios Zotos, Achilleas Kontogeorgos, Angelos Patakas, and Athanasios Ladavos. 2023. "Application of Stable Isotope Analysis for Detecting the Geographical Origin of the Greek Currants “Vostizza”: A Preliminary Study" Foods 12, no. 8: 1672. https://doi.org/10.3390/foods12081672
APA StyleThomatou, A.-A., Mazarakioti, E. C., Zotos, A., Kontogeorgos, A., Patakas, A., & Ladavos, A. (2023). Application of Stable Isotope Analysis for Detecting the Geographical Origin of the Greek Currants “Vostizza”: A Preliminary Study. Foods, 12(8), 1672. https://doi.org/10.3390/foods12081672







