Unraveling the Antibacterial Mechanism of Plasma-Activated Lactic Acid against Pseudomonas ludensis by Untargeted Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Analysis
2.1.1. Preparation of Plasma-Activated Lactic Acid Solution
2.1.2. Bacterial Culture and Antibacterial Activity of PALA
2.2. Morphological Observation of Pseudomonas lundensis
2.2.1. Scanning Electron Microscopy
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Measurement of Membrane Integrity
2.2.4. Measurement of Leakage of K+
2.3. Measurement of Intracellular ATP Concentration
2.4. Measurement of Intracellular ROS and Glutathione
2.5. Detection of the Intracellular Activity of MDH and SDH
2.6. Metabolomics Analysis
2.6.1. Sample Preparation and Metabolites Extraction
2.6.2. UHPLC-MS/MS Analysis
2.6.3. Data Processing and Metabolite Identification
2.7. Statistical Analysis
3. Results and Discussion
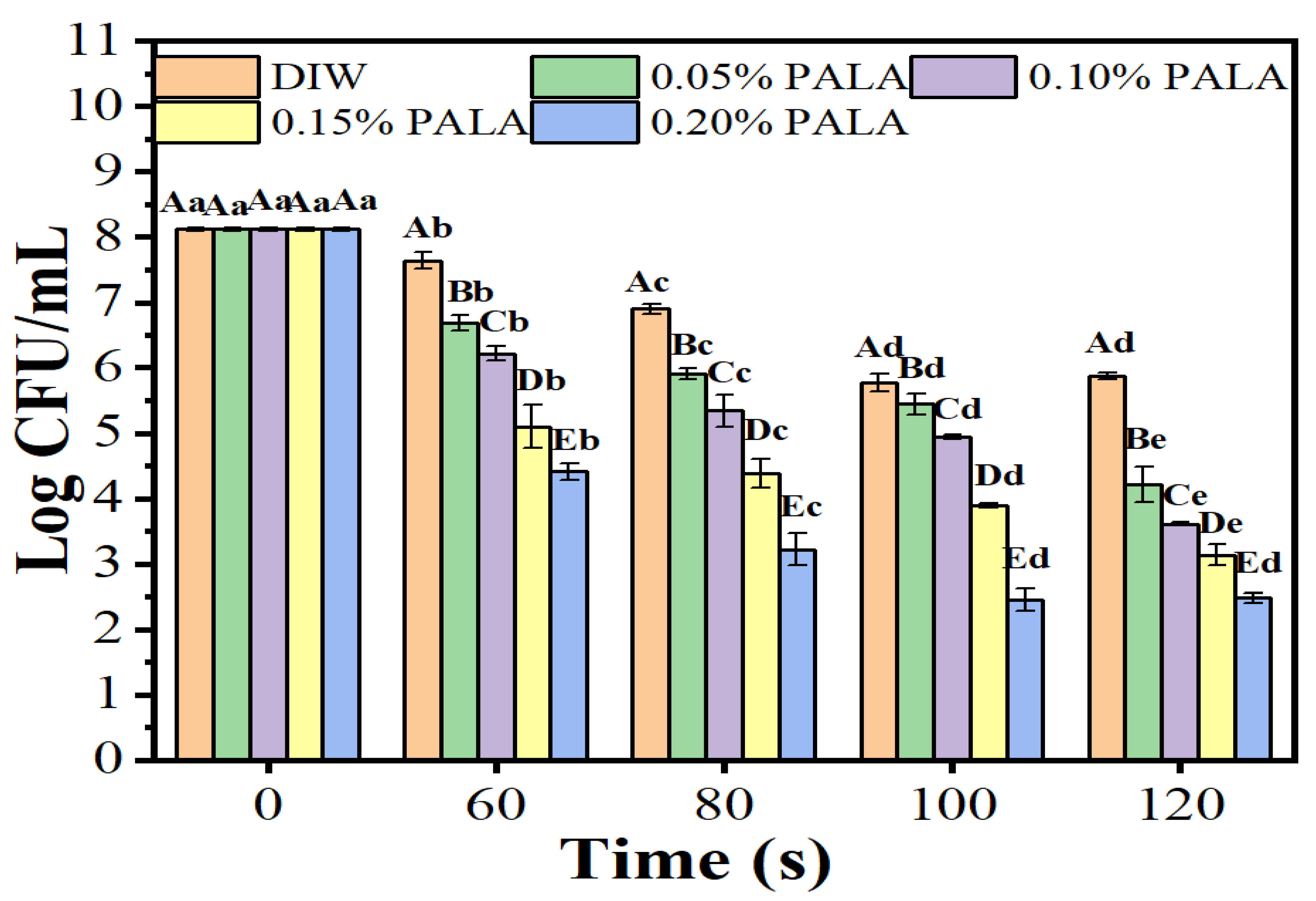
3.1. Microbial Analysis
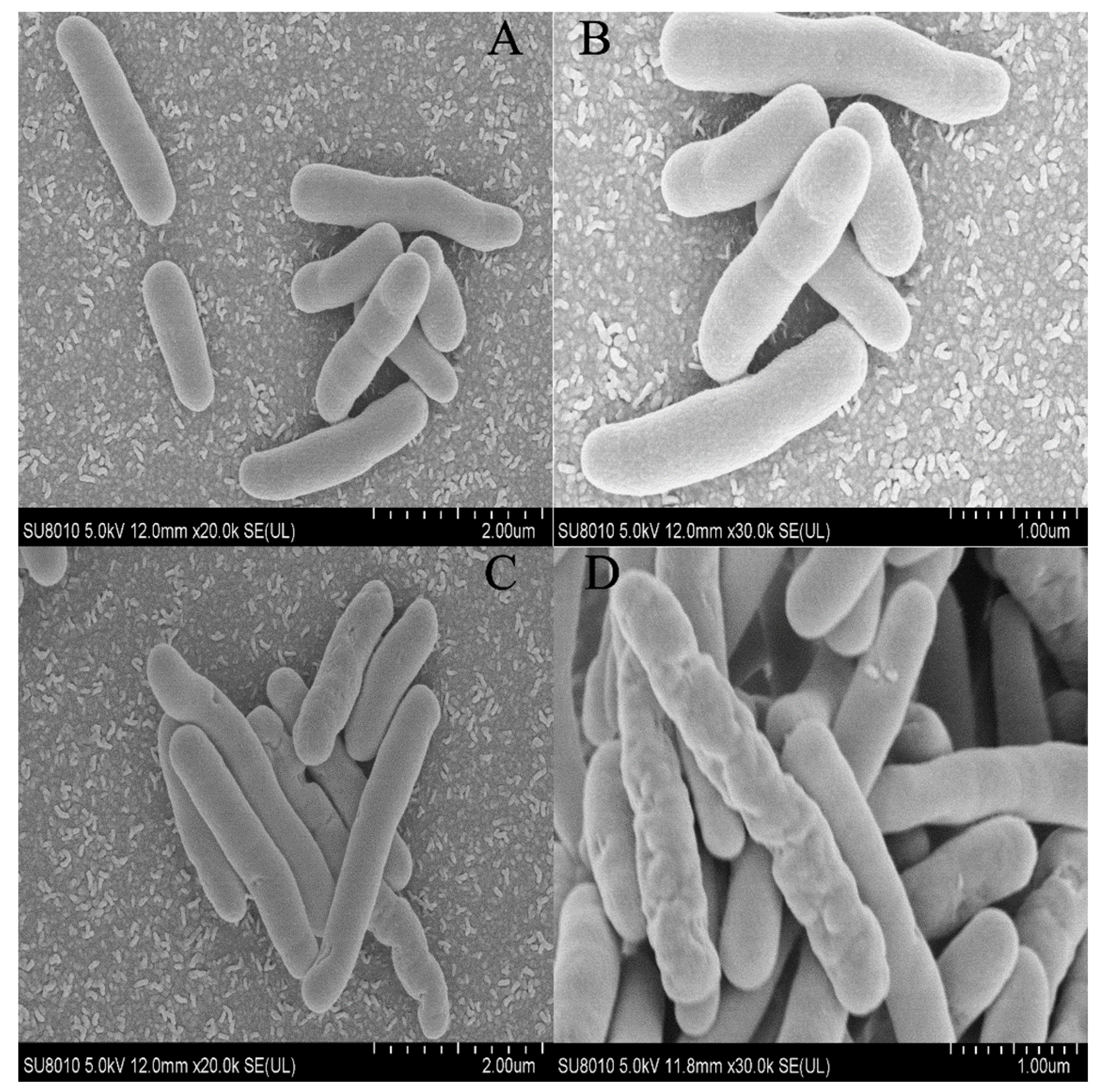
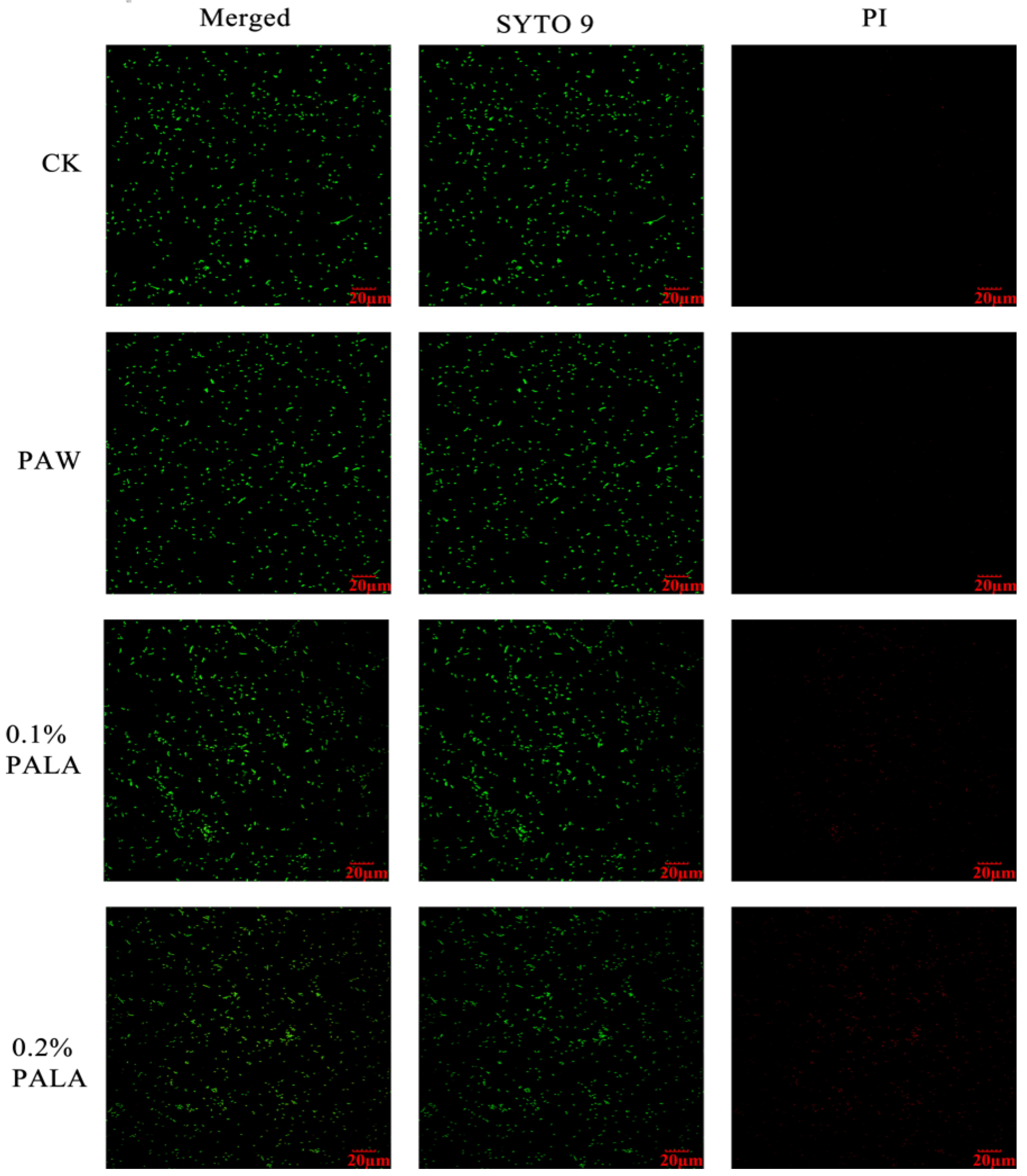
3.2. Surface Morphology and Plasma Membrane Integrity
3.3. Effect of PALA on Intracellular Organization
3.4. Intracellular ROS Level and GSH Concentration
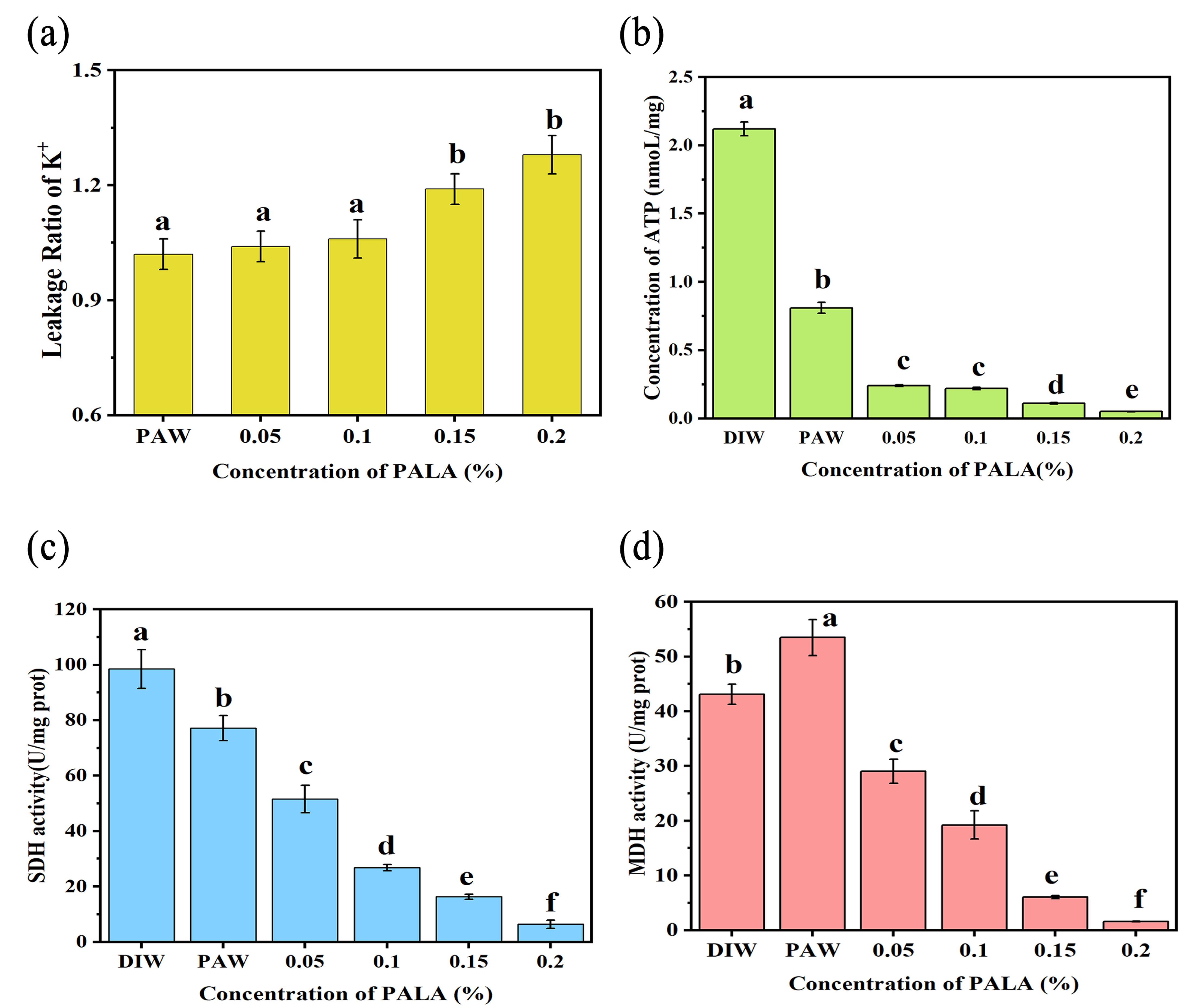
3.5. Leakage Ratio of K+, Intracellular Contents of ATP and MDH, and SDH Activity
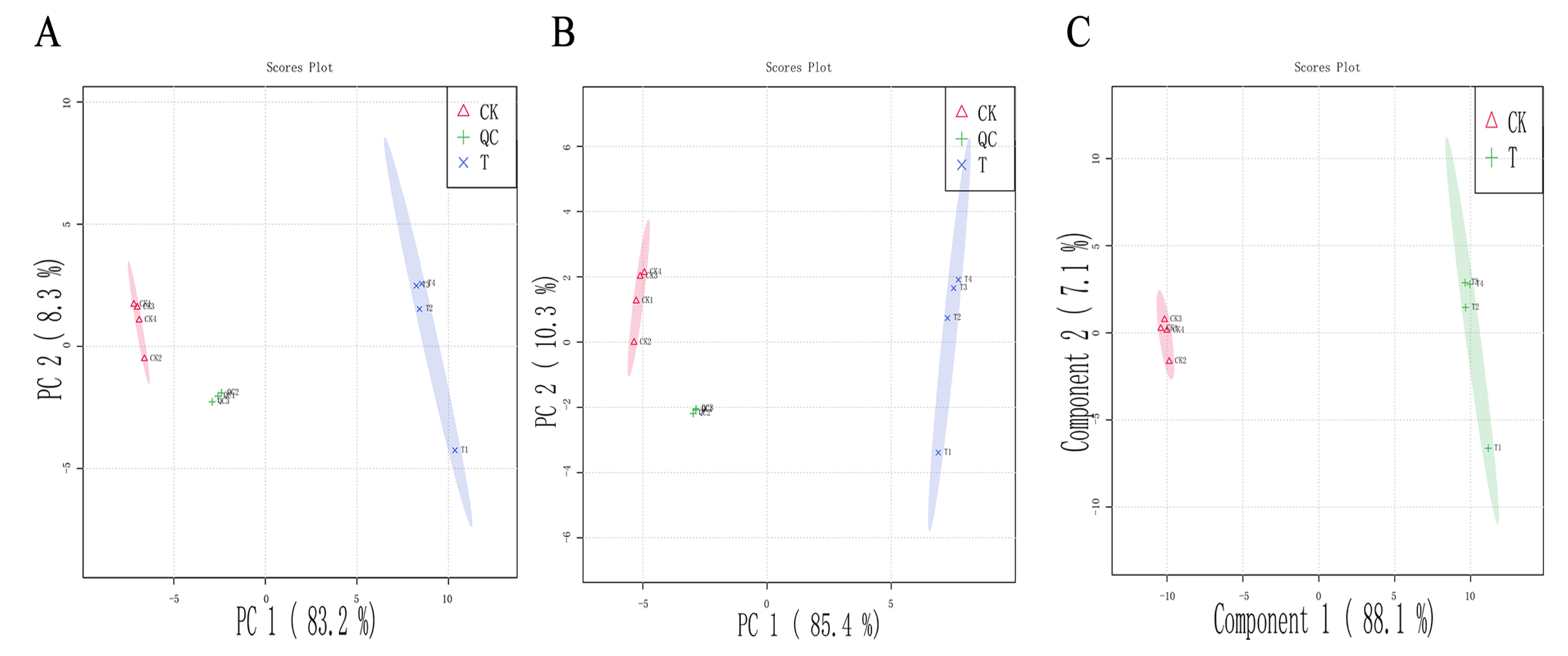
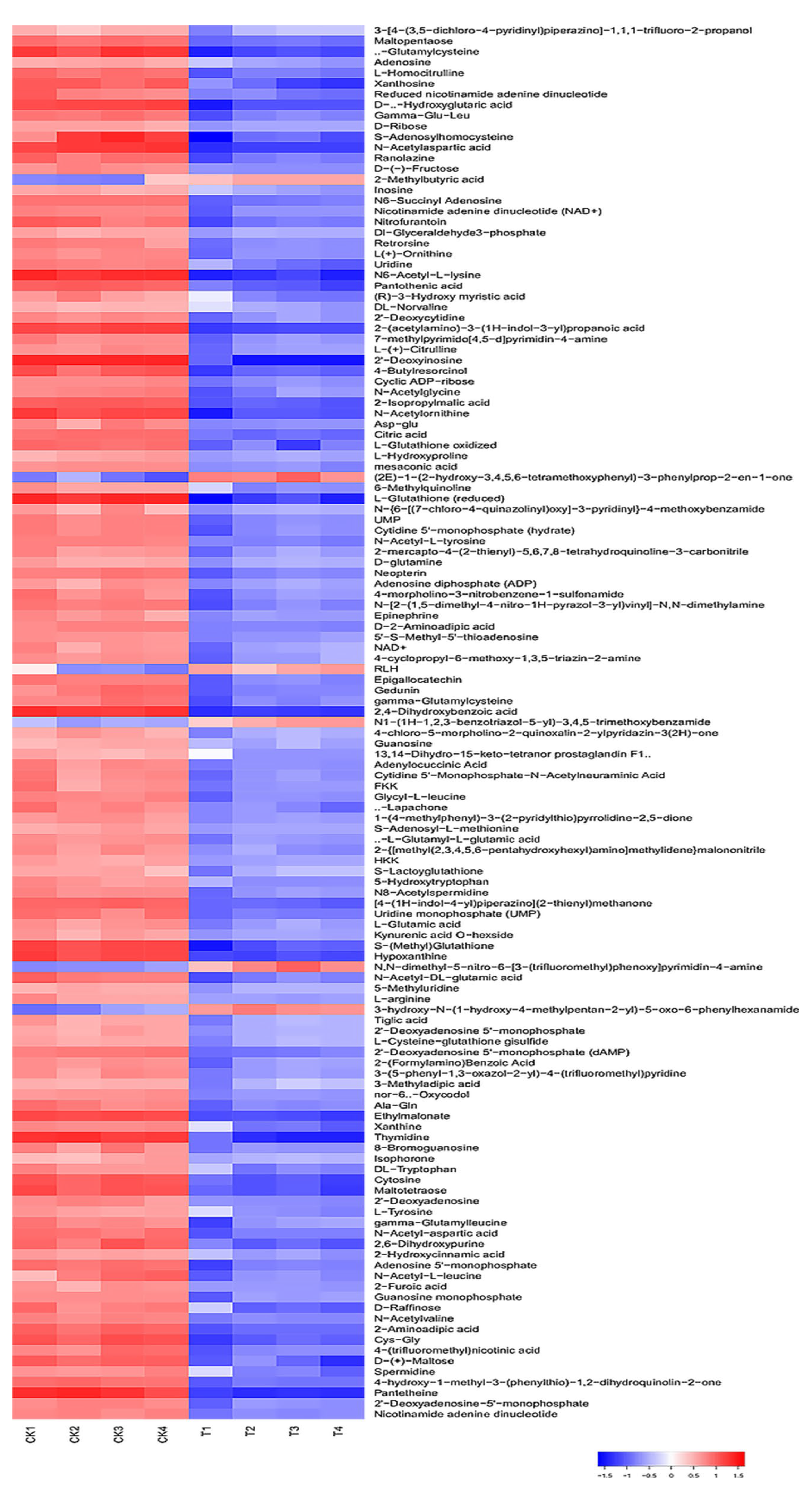
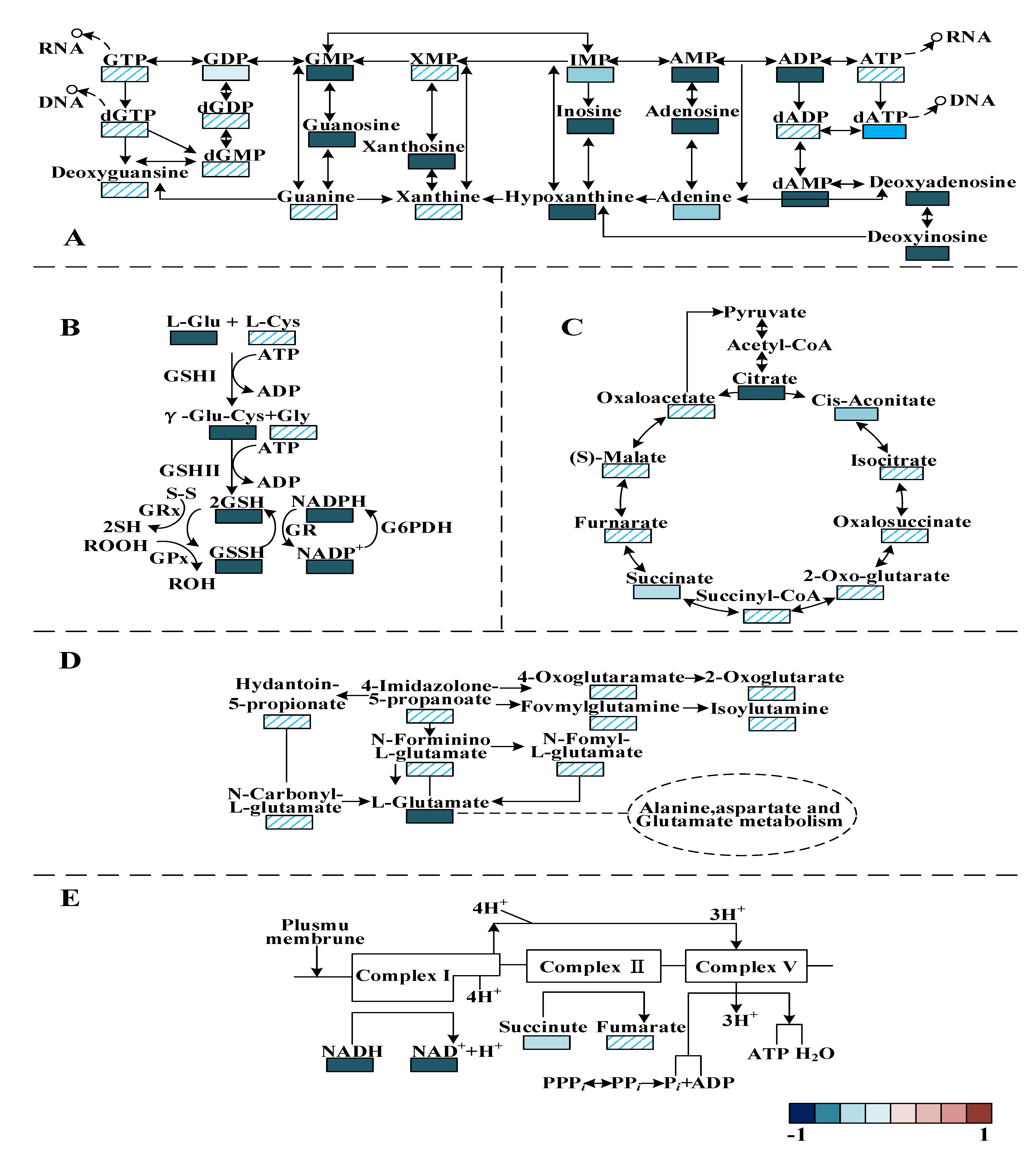
3.6. Metabolomic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, J.H.; Liu, X.M.; Wang, X.P.; Yang, H.G.; Cheng, J.R.; Lin, Y.S.; Tang, D.B. Influence of Gelatin-Chitosan-Glycerol Edible Coating Incorporated with Chlorogenic Acid, Gallic Acid, and Resveratrol on the Preservation of Fresh Beef. Foods 2022, 11, 3813. [Google Scholar] [CrossRef] [PubMed]
- You, Y.S.; Her, J.Y.; Shafel, T.; Kang, T.Y.; Jun, S. Supercooling preservation on quality of beef steak. J. Food Eng. 2020, 274, 109840. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Meyer, R.L.; Peng, M.; Hibberd, A.A.; Fischer, J.; Sigmundsson, A.; Mygind, T. Binary combination of epsilon-poly-L-lysine and isoeugenol affect progression of spoilage microbiota in fresh turkey meat, and delay onset of spoilage in Pseudomonas putida challenged meat. Int. J. Food Microbiol. 2015, 215, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Vilai, J.P.; Hughes, M.E. Profile and activity of the bacterial biota of ground beef held from freshness to spoilage at 5–7 °C. Int. J. Food Microbiol. 2003, 81, 105–111. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Mao, Y.W.; Li, K.; Dong, P.C.; Liang, R.R.; Luo, X. Models of Pseudomonas Growth Kinetics and Shelf Life in Chilled Longissimus dorsi Muscles of Beef. Asian-Australas. J. Anim. Sci. 2011, 24, 713–722. [Google Scholar] [CrossRef]
- Nowak, A.; Rygala, A.; Oltuszak-Walczak, E.; Walczak, P. The prevalence and some metabolic traits of Brochothrix thermosphacta in meat and meat products packaged in different ways. J. Sci. Food Agric. 2012, 92, 1304–1310. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Lyu, F.; Zhao, Y.L.; Shen, K.J.; Zhou, X.X.; Zhang, J.Y.; Ding, Y.T. Using Pretreatment of Carbon Monoxide Combined with Chlorine Dioxide and Lactic Acid to Maintain Quality of Vacuum-Packaged Fresh Beef. J. Food Qual. 2018, 2018, 3158086. [Google Scholar] [CrossRef]
- Samelis, J.; Sofos, J.N.; Kendall, P.A.; Smith, G.C. Fate of Escherichia coli O157: H7, Salmonella Typhimurium DT 104, and Listeria monoctyogenes in fresh meat decontamination fluids at 4 and 10 °C. J. Food Prot. 2001, 64, 950–957. [Google Scholar] [CrossRef]
- Deumier, F. Decontamination of deboned chicken legs by vacuum-tumbling in lactic acid solution. Int. J. Food Sci. Technol. 2006, 41, 23–32. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Qiu, Y.C.; Nie, A.L.; Wang, X.D. Experimental research on inactivation of bacteria by using dielectric barrier discharge. IEEE Trans. Plasma Sci. 2007, 35, 1496–1500. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhuang, H.; Zhang, J.H. Inactivation of Spoilage Bacteria in Package by Dielectric Barrier Discharge Atmospheric Cold Plasma-Treatment Time Effects. Food Bioprocess Technol. 2016, 9, 1648–1652. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.N.; Zhang, Q.; Feng, H.Q.; Liang, Y.D.; Zhang, J.; Fang, J. Assessment of the Physicochemical Properties and Biological Effects of Water Activated by Non-Thermal Plasma Above and Beneath the Water Surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A.; Khort, D.; Filippov, R.; Lukanin, V.; Lukina, N.; Astashev, M.; Konchekov, E. Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple. Plants 2023, 12, 385. [Google Scholar] [CrossRef]
- Lotfy, K.; Khalil, S. Effect of plasma-activated water on microbial quality and physicochemical properties of fresh beef. Open Phys. 2022, 20, 573–586. [Google Scholar] [CrossRef]
- Lee, J.; Jo, K.; Lim, Y.; Jeon, H.J.; Choe, J.H.; Jo, C.; Jung, S. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chem. 2018, 240, 430–436. [Google Scholar] [CrossRef]
- Chen, T.P.; Liang, J.F.; Su, T.L. Plasma-activated water: Antibacterial activity and artifacts? Environ. Sci. Pollut. Res. 2018, 25, 26699–26706. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Tian, Y.; Ma, R.N.; Liu, Q.H.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Pavlovich, M.J.; Chang, H.W.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J. Phys. D Appl. Phys. 2013, 46, 145202. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hahnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.D.; von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Jung, S.; Lee, C.W.; Lee, J.; Yong, H.I.; Yum, S.J.; Jeong, H.G.; Jo, C. Increase in nitrite content and functionality of ethanolic extracts of Perilla frutescens following treatment with atmospheric pressure plasma. Food Chem. 2017, 237, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Boselli, M.; Colombo, V.; Gherardi, M.; Laurita, R.; Liguori, A.; Sanibondi, P. Characterization of a Cold Atmospheric Pressure Plasma Jet Driven by Nanosecond High-Voltage Pulses. In Proceedings of the 2015 IEEE International Conference on Plasma Sciences (ICOPS), Antalya, Turkey, 24–28 May 2015. [Google Scholar]
- Qian, J.; Zhuang, H.; Nasiru, M.M.; Muhammad, U.; Zhang, J.H.; Yan, W.J. Action of plasma-activated lactic acid on the inactivation of inoculated Salmonella Enteritidis and quality of beef. Innov. Food Sci. Emerg. Technol. 2019, 57, 102196. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Zhuang, H.; Zhang, J.H.; Yan, W.J. Oxidative stress responses of pathogen bacteria in poultry to plasma-activated lactic acid solutions. Food Control 2020, 118, 107355. [Google Scholar] [CrossRef]
- Wang, H.H.; Ding, S.J.; Wang, G.Y.; Xu, X.L.; Zhou, G.H. In situ characterization and analysis of Salmonella biofilm formation under meat processing environments using a combined microscopic and spectroscopic approach. Int. J. Food Microbiol. 2013, 167, 293–302. [Google Scholar] [CrossRef]
- Park, J.H.; Kumar, N.; Park, D.H.; Yusupov, M.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A.; Kang, M.H.; Uhm, H.S.; Choi, E.H.; et al. A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nano-second pulsed plasma. Sci. Rep. 2015, 5, 13849. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhang, Q.; Ma, R.N.; Yu, S.; Wang, K.L.; Zhang, J.; Fang, J. Reactive radical-driven bacterial inactivation by hydrogen-peroxide-enhanced plasma-activated-water. Eur. Phys. J. Spec. Top. 2017, 226, 2887–2899. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Y.D.; Feng, H.Q.; Ma, R.N.; Tian, Y.; Zhang, J.; Fang, J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 2013, 102, 203701. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljevic, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microb. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Qian, J.; Luo, J.; Huang, M.M.; Yan, W.J.; Zhang, J.H. Morphophysiological Changes in Staphylococcus aureus Biofilms Treated with Plasma-Activated Hydrogen Peroxide Solution. Appl. Sci. 2021, 11, 11597. [Google Scholar] [CrossRef]
- Moussa, M.; Perrier-Cornet, J.M.; Gervais, P. Damage in Escherichia coli cells treated with a combination of high hydrostatic pressure and subzero temperature. Appl. Environ. Microb. 2007, 73, 6508–6518. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.H.; Shi, C.X.; Guo, H.; Ni, R.Y.; Qu, J.L.; Tang, J.N.; Liu, S.D. Effect of oxidative stress from nanoscale TiO2 particles on a Physarum polycephalum macroplasmodium under dark conditions. Environ. Sci. Pollut. Res. 2017, 24, 17241–17249. [Google Scholar] [CrossRef]
- Chen, H.X.; Bai, F.W.; Xiu, Z.L. Oxidative Stress Induced in Saccharomyces Cerevisiae Exposed to Dielectric Barrier Discharge Plasma in Air at Atmospheric Pressure. IEEE Trans. Plasma Sci. 2010, 38, 1885–1891. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, F.Q.; Wang, Q.; Rensing, C.; Yu, P.; Gong, J.; Wang, G.J. Disrupting ROS-protection mechanism allows hydrogen peroxide to accumulate and oxidize Sb(III) to Sb(V) in Pseudomonas stutzeri TS44. BMC Microbiol. 2016, 16, 279. [Google Scholar] [CrossRef]
- Lee, J.W.; Romeo, A.; Kosman, D.J. Transcriptional remodeling and G(1) arrest in dioxygen stress in Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 24885–24893. [Google Scholar] [CrossRef]
- Liu, G.R.; Ren, G.M.; Zhao, L.; Cheng, L.; Wang, C.T.; Sun, B.G. Antibacterial activity and mechanism of bifidocin A against Listeria monocytogenes. Food Control 2017, 73, 854–861. [Google Scholar] [CrossRef]
- Liao, X.Y.; Liu, D.H.; Ding, T. Nonthermal Plasma Induces the Viable-but-Nonculturable State in Staphylococcus aureus via Metabolic Suppression and the Oxidative Stress Response. Appl. Environ. Microb. 2020, 86, e02216-19. [Google Scholar] [CrossRef]
- Yao, Y.X.; Li, M.; Zhai, H.; You, C.X.; Hao, Y.J. Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis. J. Plant Physiol. 2011, 168, 474–480. [Google Scholar] [CrossRef]
- Fattoretti, P.; Bertoni-Freddari, C.; Caselli, U.; Paoloni, R.; Meier-Ruge, W. Impaired succinic dehydrogenase activity of rat Purkinje cell mitochondria during aging. Mech. Ageing Dev. 1998, 101, 175–182. [Google Scholar] [CrossRef]
- Li, G.H.; Xu, Y.F.; Wang, X.; Zhang, B.G.; Shi, C.; Zhang, W.S.; Xia, X.D. Tannin-Rich Fraction from Pomegranate Rind Damages Membrane of Listeria monocytogenes. Foodborne Pathog. Dis. 2014, 11, 313–319. [Google Scholar] [CrossRef]
- Cai, L.L.; Hu, H.J.; Lu, Q.; Wang, H.H.; Xu, X.L.; Zhou, G.H.; Kang, Z.L.; Ma, H.J. Morphophysiological responses of detached and adhered biofilms of Pseudomonas fluorescens to acidic electrolyzed water. Food Microbiol. 2019, 82, 89–98. [Google Scholar] [CrossRef]
- Szeto, S.S.W.; Reinke, S.N.; Sykes, B.D.; Lemire, B.D. Mutations in the Saccharomyces cerevisiae Succinate Dehydrogenase Result in Distinct Metabolic Phenotypes Revealed Through H-1 NMR-Based Metabolic Footprinting. J. Proteome Res. 2010, 9, 6729–6739. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, F.; Bojko, B.; Bessonneau, V.; Pawliszyn, J. Cinnamaldehyde Characterization as an Antibacterial Agent toward E. coli Metabolic Profile Using 96-Blade Solid-Phase Microextraction Coupled to Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2016, 15, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Diomande, S.E.; Nguyen-The, C.; Guinebretiere, M.H.; Broussolle, V.; Brillard, J. Role of fatty acids in Bacillus environmental adaptation. Front. Microbiol. 2015, 6, 813. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.; Liebisch, G. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog. Lipid Res. 2014, 54, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Cen, D.; Ge, Q.W.; Xie, C.K.; Zheng, Q.; Guo, J.S.; Zhang, Y.Q.; Wang, Y.F.; Li, X.; Gu, Z.; Cai, X.J. ZnS@BSA Nanoclusters Potentiate Efficacy of Cancer Immunotherapy. Adv. Mater. 2021, 33, 2104037. [Google Scholar] [CrossRef]
- Ramond, E.; Gesbert, G.; Rigard, M.; Dairou, J.; Dupuis, M.; Dubail, I.; Meibom, K.; Henry, T.; Barel, M.; Charbit, A. Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Francisella Phagosomal Escape. PLoS Pathog. 2014, 10, e1003893. [Google Scholar] [CrossRef]
- Rice, A.J.; Park, A.; Pinkett, H.W. Diversity in ABC transporters: Type I, II and III importers. Crit. Rev. Biochem. Mol. 2014, 49, 426–437. [Google Scholar] [CrossRef]
- Allen, R.H.; Stabler, S.P.; Savage, D.G.; Lindenbaum, J. Elevation of 2-Methylcitric Acid-I and Acid-Ii Levels in Serum, Urine, and Cerebrospinal-Fluid of Patients with Cobalamin Deficiency. Metabolism 1993, 42, 978–988. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Hung, C.H.; Hwang, T.S.; Hsu, C.H. Cloning, overexpression, purification and crystallization of malate dehydrogenase from Thermus thermophilus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 1249–1251. [Google Scholar] [CrossRef]
- Jensen, P.R.; Rohwer, J.M.; Michelsen, O.; Westerhoff, H.V. Modelling of Oxidative Phosphorylation in E. coli. Mod. Trends Biothermokinetics 1994, 3, 218–220. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, X.; Sheng, X.; Zhao, L.; Qian, J.; Zhang, J.; Wang, J. Unraveling the Antibacterial Mechanism of Plasma-Activated Lactic Acid against Pseudomonas ludensis by Untargeted Metabolomics. Foods 2023, 12, 1605. https://doi.org/10.3390/foods12081605
Wang Z, Wang X, Sheng X, Zhao L, Qian J, Zhang J, Wang J. Unraveling the Antibacterial Mechanism of Plasma-Activated Lactic Acid against Pseudomonas ludensis by Untargeted Metabolomics. Foods. 2023; 12(8):1605. https://doi.org/10.3390/foods12081605
Chicago/Turabian StyleWang, Zhaobin, Xiaoting Wang, Xiaowei Sheng, Luling Zhao, Jing Qian, Jianhao Zhang, and Jin Wang. 2023. "Unraveling the Antibacterial Mechanism of Plasma-Activated Lactic Acid against Pseudomonas ludensis by Untargeted Metabolomics" Foods 12, no. 8: 1605. https://doi.org/10.3390/foods12081605
APA StyleWang, Z., Wang, X., Sheng, X., Zhao, L., Qian, J., Zhang, J., & Wang, J. (2023). Unraveling the Antibacterial Mechanism of Plasma-Activated Lactic Acid against Pseudomonas ludensis by Untargeted Metabolomics. Foods, 12(8), 1605. https://doi.org/10.3390/foods12081605






