Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers
Abstract
1. Introduction
2. Materials and Methods
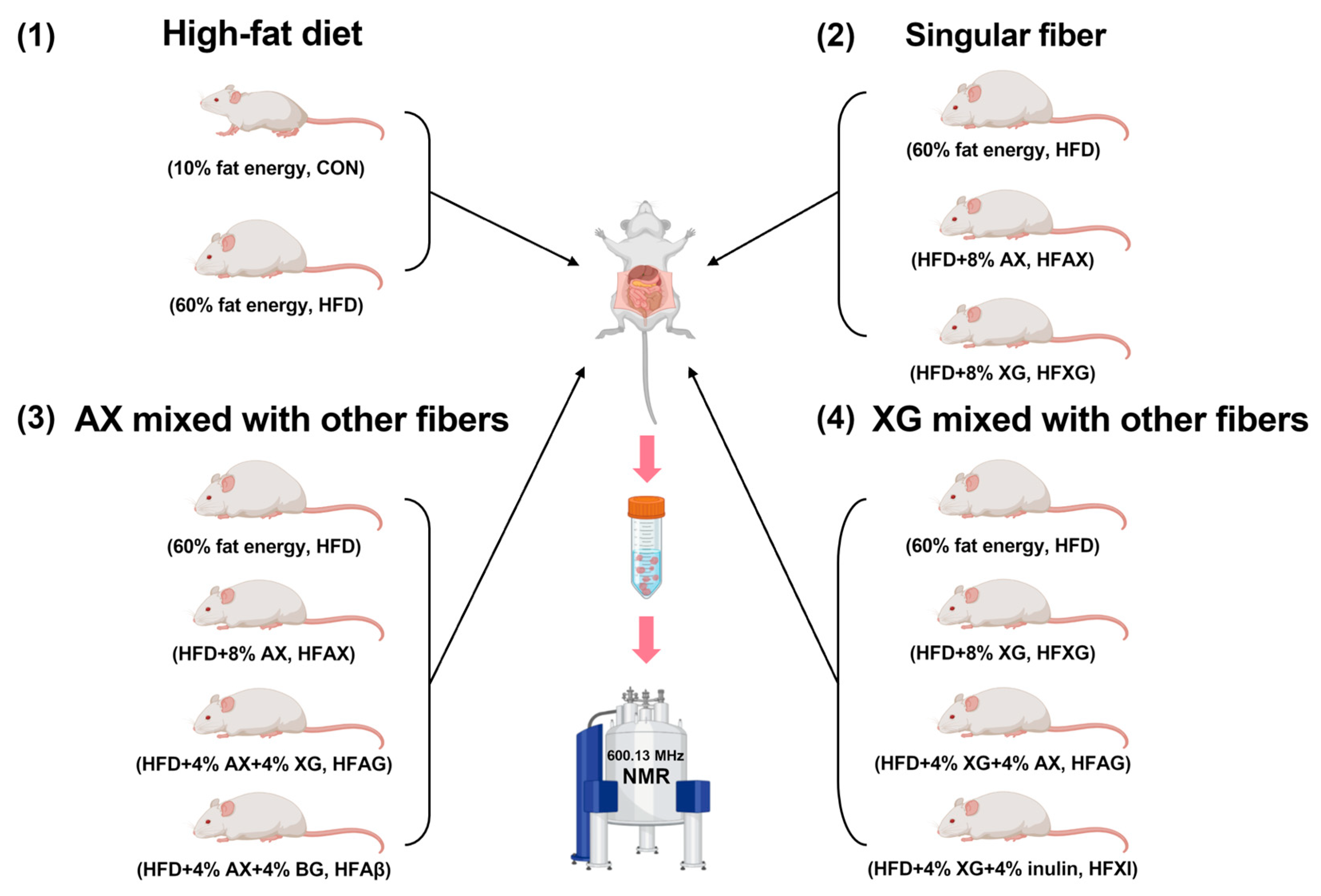
2.1. Experimental Design
2.2. Metabolomic Analysis
2.3. Statistical Analysis
3. Results
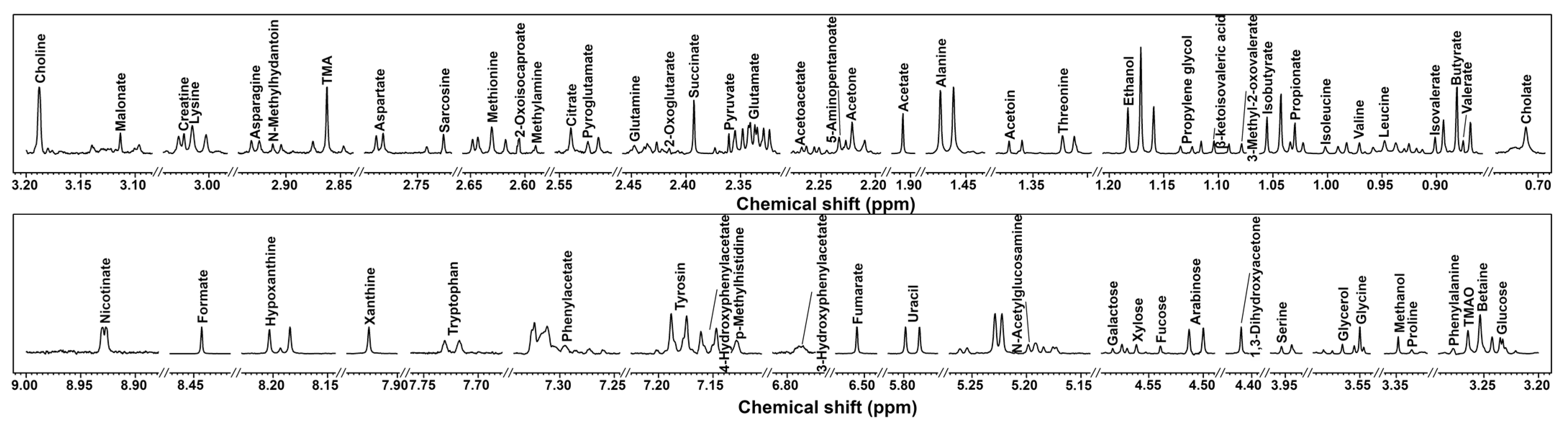
3.1. H-NMR Spectra of Cecum Contents
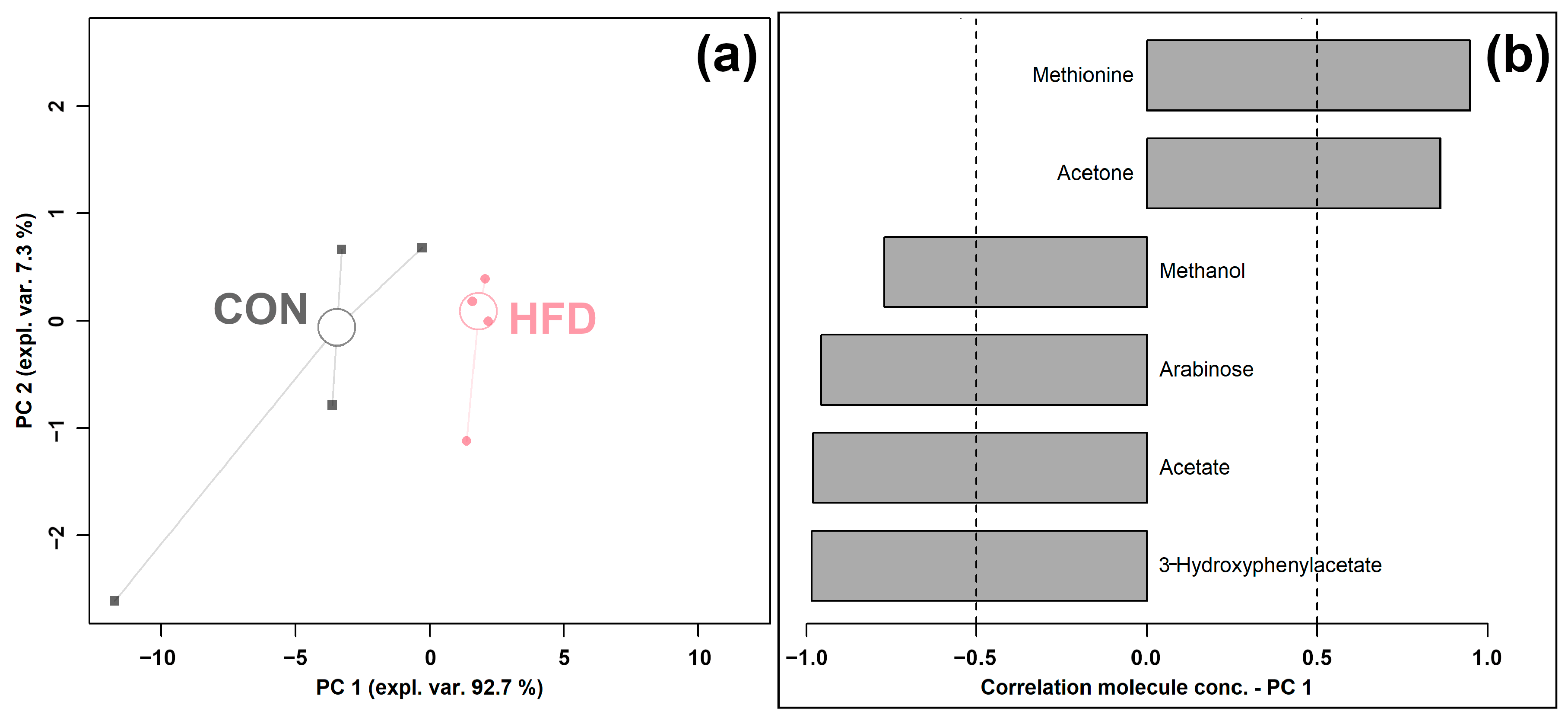
3.2. Effect of High-Fat Die on the Cecum Content Metabolome
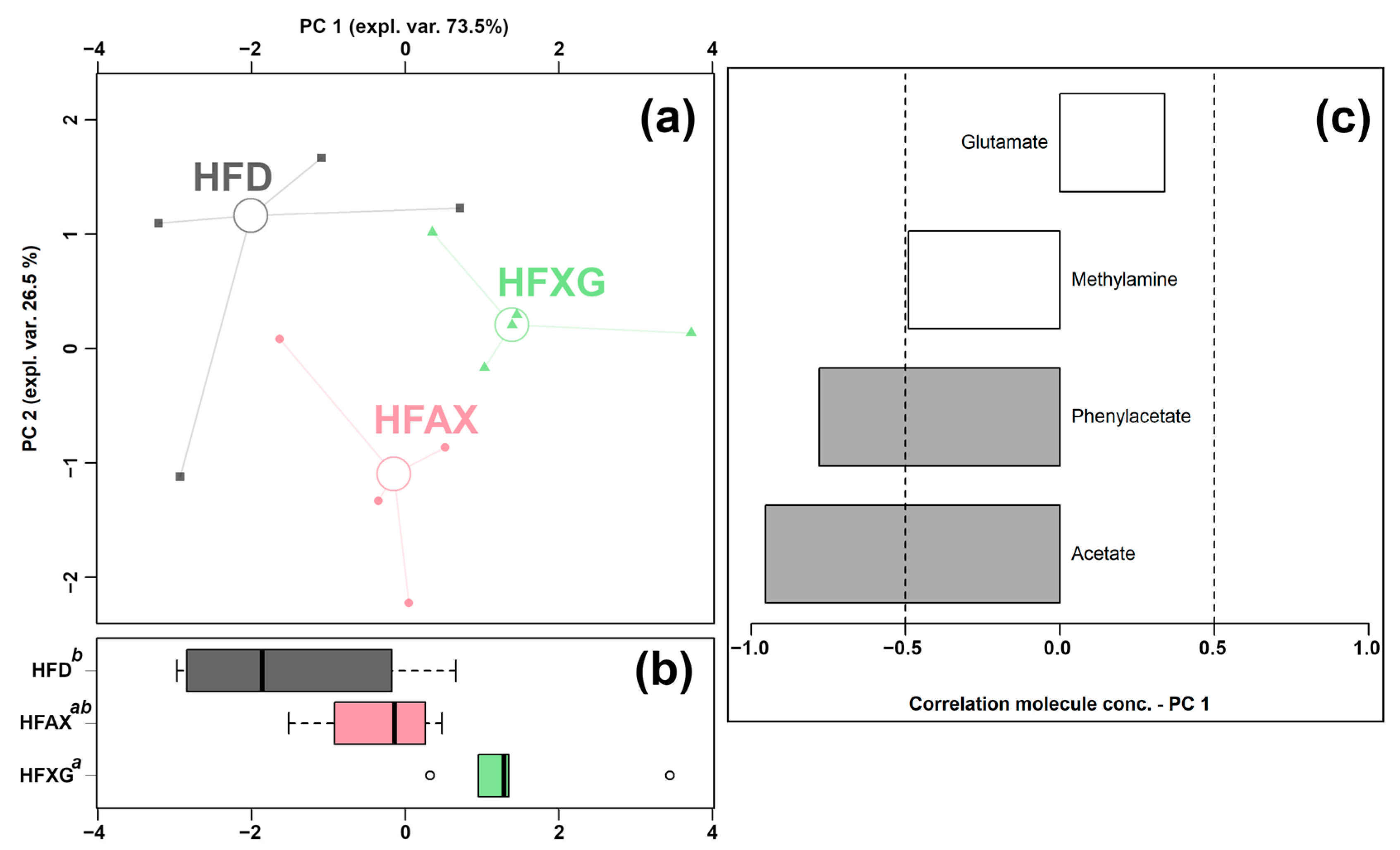
3.3. Effect of Single Fiber (AX and XG) on the Cecum Metabolome
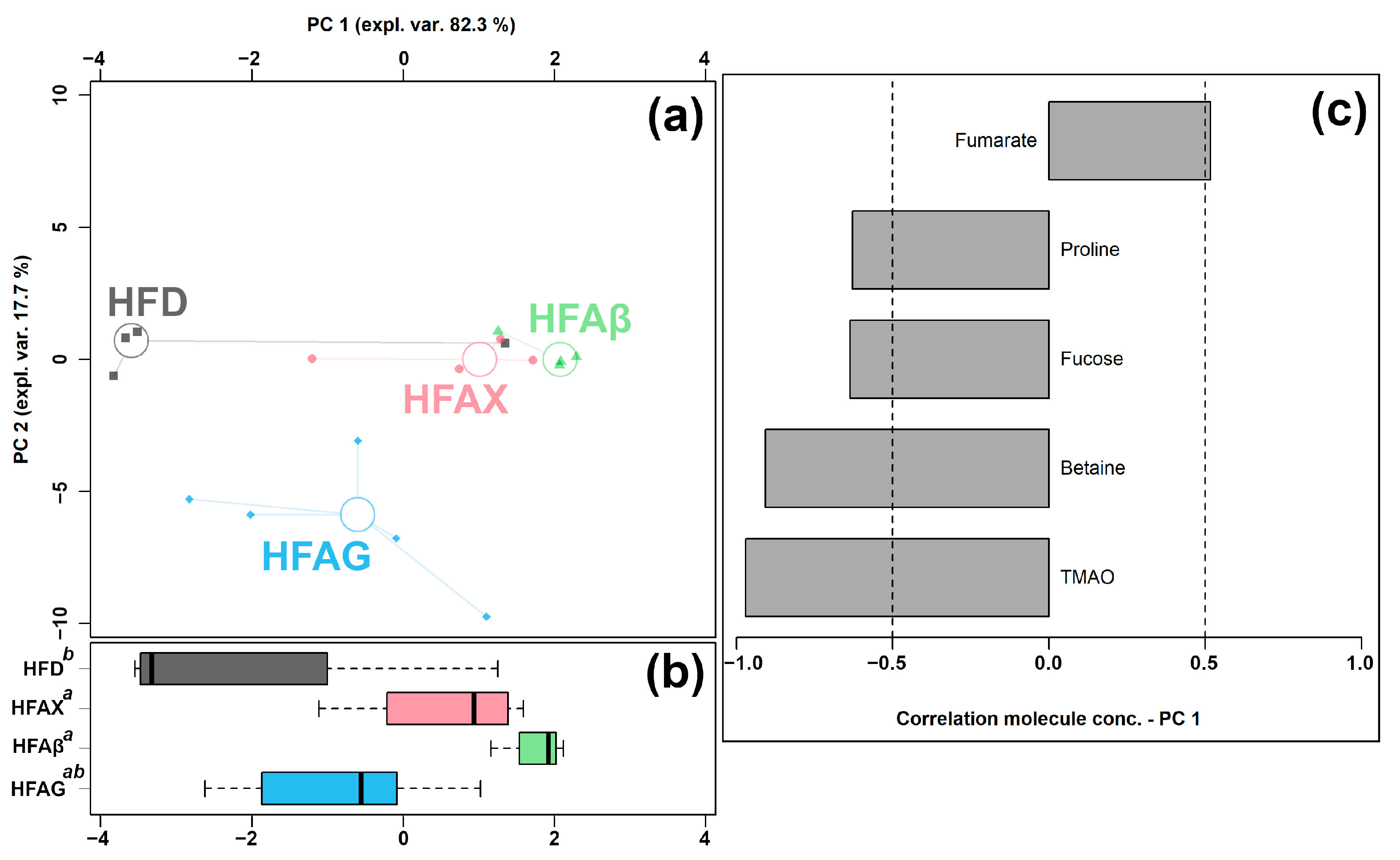
3.4. Effect of AX in Combination with Different Fibers (BG and XG) on the Cecum Metabolome
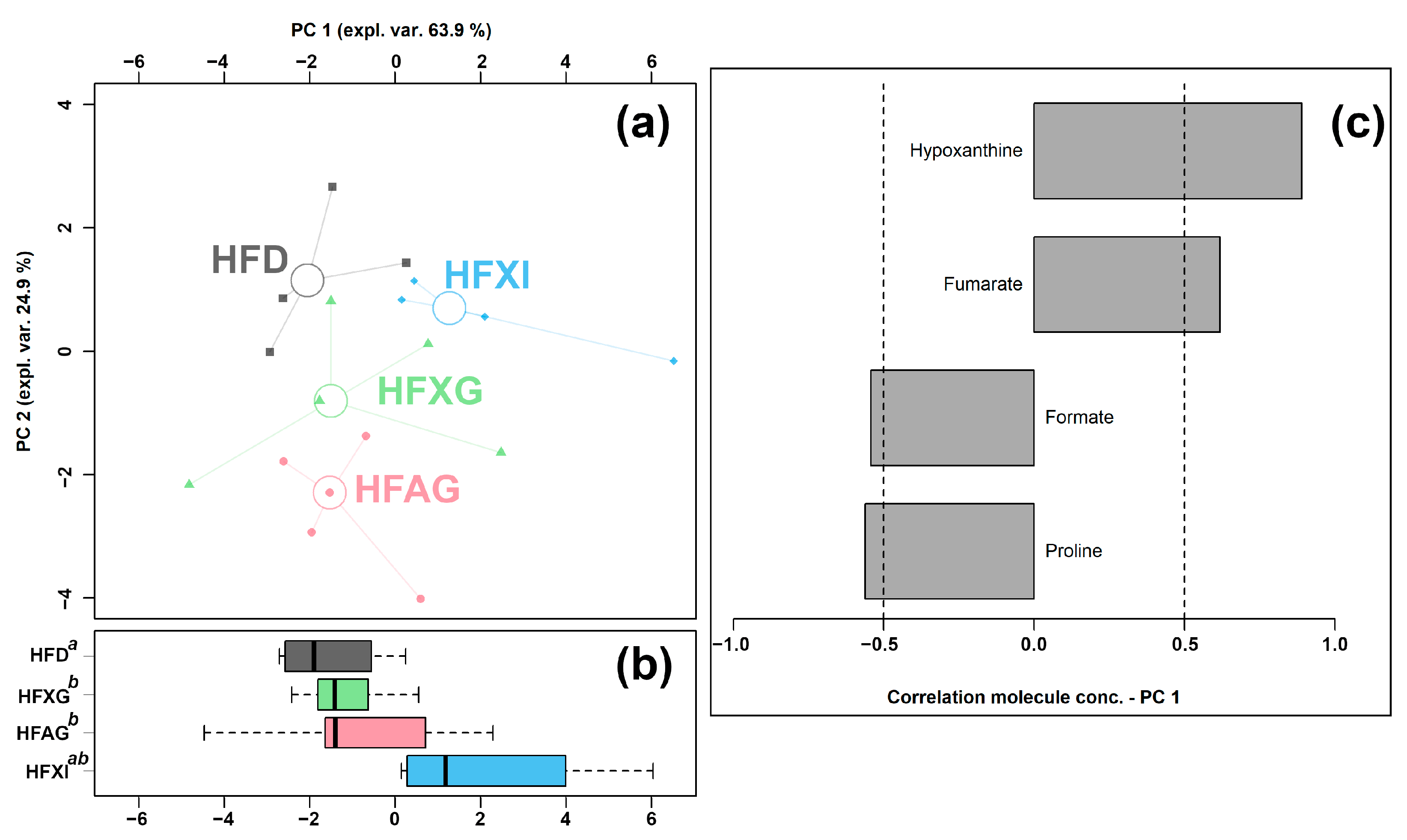
3.5. Effect of XG Combined with Different Fibers (AG and Inulin) on the Cecum Metabolome
3.6. Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nam, M.; Choi, M.S.; Jung, S.; Jung, Y.; Choi, J.Y.; Ryu, D.H.; Hwang, G.S. Lipidomic Profiling of Liver Tissue from Obesity-Prone and Obesity-Resistant Mice Fed a High Fat Diet. Sci. Rep. 2015, 5, 16984. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.N.; Ard, J.D. COVID-19, Obesity, and Structural Racism: Understanding the Past and Identifying Solutions for the Future. Cell Metab. 2021, 33, 234–241. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, L.; Li, Y.; Xia, G.; Chen, C.; Zhang, Y. Bamboo-Shaving Polysaccharide Protects against High-Diet Induced Obesity and Modulates the Gut Microbiota of Mice. J. Funct. Foods 2018, 49, 20–31. [Google Scholar] [CrossRef]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel Health and Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the Relationships among Polysaccharides, Gut Microbiota, and Human Health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Zhou, S.; Chen, D.; Qin, W.; Li, C.; Li, H.; Lin, D.; Zhang, Q.; Liu, Y.; et al. Arabinoxylan Combined with Different Glucans Improve Lipid Metabolism Disorder by Regulating Bile Acid and Gut Microbiota in Mice Fed with High-Fat Diet. Int. J. Biol. Macromol. 2021, 168, 279–288. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, J.; Chen, H.; Geng, F.; Nie, S. Arabinoxylan Ameliorates Type 2 Diabetes by Regulating the Gut Microbiota and Metabolites. Food Chem. 2022, 371, 131106. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, X.; Li, S.; Qin, W.; Huang, Z.; Luo, Y.; Li, H.; Wu, D.; Zhang, Q.; Zhao, Y.; et al. Possible Beneficial Effects of Xyloglucan from Its Degradation by Gut Microbiota. Trends Food Sci. Technol. 2020, 97, 65–75. [Google Scholar] [CrossRef]
- Reyna-Villasmil, N.; Bermúdez-Pirela, V.; Mengual-Moreno, E.; Arias, N.; Cano-Ponce, C.; Leal-Gonzalez, E.; Souki, A.; Inglett, G.E.; Israili, Z.H.; Hernández-Hernández, R.; et al. Oat-Derived β-Glucan Significantly Improves HDLC and Diminishes LDLC and Non-HDL Cholesterol in Overweight Individuals with Mild Hypercholesterolemia. Proc. Am. J. Ther. 2007, 14, 203–212. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Singh, V.; Choi, C.; Choi, S.I.; Kim, Y.M.; Unno, T.; Cho, M. Dietary Intervention Using (1,3)/(1,6)-β-Glucan, a Fungus-Derived Soluble Prebiotic Ameliorates High-Fat Diet-Induced Metabolic Distress and Alters Beneficially the Gut Microbiota in Mice Model. Eur. J. Nutr. 2020, 59, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Watanabe, K.; Taira, S.; Kasubuchi, M.; Li, X.; Irie, J.; Itoh, H.; Kimura, I. Barley β-Glucan Improves Metabolic Condition via Short-Chain Fatty Acids Produced by Gut Microbial Fermentation in High Fat Diet Fed Mice. PLoS ONE 2018, 13, e0196579. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Titgemeier, B.; Kirkpatrick, K.; Golubic, M.; Roizen, M.F. Major Cereal Grain Fibers and Psyllium in Relation to Cardiovascular Health. Nutrients 2013, 5, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Rose, D.J.; Rumpagaporn, P.; Patterson, J.A.; Hamaker, B.R. In VitroBatch Fecal Fermentation Comparison of Gas and Short-Chain Fatty Acid Production Using “Slowly Fermentable” Dietary Fibers. J. Food Sci. 2011, 76, H137–H142. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, C.M.; Canfora, E.E.; Kip, A.M.; Gorissen, S.H.M.; Olde Damink, S.W.M.; van Eijk, H.M.; Holst, J.J.; Blaak, E.E.; Dejong, C.H.C.; Lenaerts, K. The Prebiotic Inulin Improves Substrate Metabolism and Promotes Short-Chain Fatty Acid Production in Overweight to Obese Men. Metabolism 2018, 87, P25–P35. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary Inulin Alleviates Diverse Stages of Type 2 Diabetes Mellitus: Via Anti-Inflammation and Modulating Gut Microbiota in Db/Db Mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Li, J.; Huang, X.; Cheng, J.; Jiang, X.; Qin, W.; Liu, Y.; Liu, A.; Zhang, Q.; et al. Xyloglucan Compounded Inulin or Arabinoxylan against Glycometabolism Disorder via Different Metabolic Pathways: Gut Microbiota and Bile Acid Receptor Effects. J. Funct. Foods 2020, 74, 104162. [Google Scholar] [CrossRef]
- Cheng, J.; Jiang, X.; Li, J.; Zhou, S.; Bai, T.; Qin, W.; Li, H.; Luo, Y.; Huang, Z.; Liu, Y.; et al. Xyloglucan Affects Gut-Liver Circulating Bile Acid Metabolism to Improve Liver Damage in Mice Fed with High-Fat Diet. J. Funct. Foods 2020, 64, 103651. [Google Scholar] [CrossRef]
- Laghi, L.; Zhu, C.; Campagna, G.; Rossi, G.; Bazzano, M.; Laus, F. Probiotic Supplementation in Trained Trotter Horses: Effect on Blood Clinical Pathology Data and Urine Metabolomic Assessed in Field. J. Appl. Physiol 2018, 125, 654–660. [Google Scholar] [CrossRef]
- Bervoets, L.; Massa, G.; Guedens, W.; Reekmans, G.; Noben, J.P.; Adriaensens, P. Identification of Metabolic Phenotypes in Childhood Obesity by H NMR Metabolomics of Blood Plasma. Future Sci. OA 2018, 4, 4. [Google Scholar] [CrossRef]
- Guo, F.; Han, M.; Lin, S.; Ye, H.; Chen, J.; Zhu, H.; Lin, W. Enteromorpha Prolifera Polysaccharide Prevents High- Fat Diet-Induced Obesity in Hamsters: A NMR-Based Metabolomic Evaluation. J. Food Sci. 2021, 86, 3672–3685. [Google Scholar] [CrossRef] [PubMed]
- Ammar, N.M.; Farag, M.A.; Kholeif, T.E.; Metwally, N.S.; El-Sheikh, N.M.; el Gendy, A.N.; Abdel-Hamid, A.H.Z. Serum Metabolomics Reveals the Mechanistic Role of Functional Foods and Exercise for Obesity Management in Rats. J. Pharm. Biomed. Anal. 2017, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Cui, F.; Li, W.; Wang, Y.; Wang, Z.; Gao, Y.; Wang, N.; Xu, Q.; Hu, F.; Zhang, Y. Codonopsis Pilosula Oligosaccharides Modulate the Gut Microbiota and Change Serum Metabolomic Profiles in High-Fat Diet-Induced Obese Mice. Food Funct. 2022, 13, 8143–8157. [Google Scholar] [CrossRef]
- Almanza-Aguilera, E.; Brunius, C.; Bernal-Lopez, M.R.; Garcia-Aloy, M.; Madrid-Gambin, F.; Tinahones, F.J.; Gómez-Huelgas, R.; Landberg, R.; Andres-Lacueva, C. Impact in Plasma Metabolome as Effect of Lifestyle Intervention for Weight-Loss Reveals Metabolic Benefits in Metabolically Healthy Obese Women. J. Proteome Res. 2018, 17, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the Prebiotic Concept: Lessons from an Exploratory, Double Blind Intervention Study with Inulin-Type Fructans in Obese Women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef]
- Kretowski, A.; Ruperez, F.J.; Ciborowski, M. Genomics and Metabolomics in Obesity and Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 9415646. [Google Scholar] [CrossRef]
- Lanng, S.K.; Zhang, Y.; Christensen, K.R.; Hansen, A.K.; Nielsen, D.S.; Kot, W.; Bertram, H.C. Partial Substitution of Meat with Insect (Alphitobius diaperinus) in a Carnivore Diet Changes the Gut Microbiome and Metabolome of Healthy Rats. Foods 2021, 10, 1814. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Y.; Dong, Y. Modulation of Gut Microbiota and Gut-Generated Metabolites by Bitter Melon Results in Improvement in the Metabolic Status in High Fat Diet-Induced Obese Rats. J. Funct. Foods 2018, 41, 127–134. [Google Scholar] [CrossRef]
- Barouei, J.; Bendiks, Z.; Martinic, A.; Mishchuk, D.; Heeney, D.; Hsieh, Y.H.; Kieffer, D.; Zaragoza, J.; Martin, R.; Slupsky, C.; et al. Microbiota, Metabolome, and Immune Alterations in Obese Mice Fed a High-Fat Diet Containing Type 2 Resistant Starch. Mol. Nutr. Food Res. 2017, 61, 1700184. [Google Scholar] [CrossRef]
- Zhu, C.; Jin, L.; Luo, B.; Zhou, Q.; Dong, L.; Li, X.; Zhang, H.; Huang, Y.; Li, C.; Zou, L.; et al. Dominant Components of the Giant Panda Seminal Plasma Metabolome, Characterized by 1H-NMR Spectroscopy. Animals 2022, 12, 1536. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Z.; He, L.; Lu, X.; Tang, J.; Laghi, L. The Longer the Storage Time, the Higher the Price, the Better the Quality? A 1H-NMR Based Metabolomic Investigation of Aged Ya’an Tibetan Tea (Camellia sinensis). Foods 2022, 11, 2986. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 2018, 26, 211–243. [Google Scholar] [CrossRef]
- Wu, W.; Xie, J.; Zhang, H. Dietary Fibers Influence the Intestinal SCFAs and Plasma Metabolites Profiling in Growing Pigs. Food Funct. 2016, 7, 4644–4654. [Google Scholar] [CrossRef]
- Liu, G.; Yang, G.; Fang, T.; Cai, Y.; Wu, C.; Wang, J.; Huang, Z.; Chen, X. NMR-Based Metabolomic Studies Reveal Changes in Biochemical Profile of Urine and Plasma from Rats Fed with Sweet Potato Fiber or Sweet Potato Residue. RSC Adv. 2014, 4, 23749–23758. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Ren, L.; Du, H.; Fei, C.; Qian, C.; Li, B.; Zhang, R.; Liu, H.; Li, Z.; et al. High-Fiber Diet Ameliorates Gut Microbiota, Serum Metabolism and Emotional Mood in Type 2 Diabetes Patients. Front. Cell Infect. Microbiol. 2023, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. Gut Microbiota-Derived Metabolites in Obesity: A Systematic Review. Biosci. Microbiota Food Health 2020, 39, 65–76. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xu, W.; Li, H.; Lei, H.; Zhang, L.; Hao, F.; Duan, Y.; Yan, X.; Zhao, Y.; Wu, J.; et al. High-Fat Diet Induces Dynamic Metabolic Alterations in Multiple Biological Matrices of Rats. J. Proteome Res. 2013, 12, 3755–3768. [Google Scholar] [CrossRef]
- Newsholme, P.; Lima, M.M.R.; Procopio, J.; Pithon-Curi, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and Glutamate as Vital Metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef]
- Zampiga, M.; Laghi, L.; Petracci, M.; Zhu, C.; Meluzzi, A.; Dridi, S.; Sirri, F. Effect of Dietary Arginine to Lysine Ratios on Productive Performance, Meat Quality, Plasma and Muscle Metabolomics Profile in Fast-Growing Broiler Chickens. J. Anim. Sci. Biotechnol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, C.; Cai, S.; Dong, J.; Li, X.; Feng, J.; Chen, Z. Metabolomic Profilings of Urine and Serum from High Fat-Fed Rats via 1H NMR Spectroscopy and Pattern Recognition. Appl. Biochem. Biotechnol. 2013, 169, 1250–1261. [Google Scholar] [CrossRef]
- Nokhoijav, E.; Guba, A.; Kumar, A.; Kunkli, B.; Kalló, G.; Káplár, M.; Somodi, S.; Garai, I.; Csutak, A.; Tóth, N.; et al. Metabolomic Analysis of Serum and Tear Samples from Patients with Obesity and Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 4534. [Google Scholar] [CrossRef]
- Zhu, C.; Tang, K.; Lu, X.; Tang, J.; Laghi, L. An Untargeted Metabolomics Investigation of Milk from Dairy Cows with Clinical Mastitis by 1H-NMR. Foods 2021, 10, 1707. [Google Scholar] [CrossRef]
- Maulidiani; Abas, F.; Khatib, A.; Perumal, V.; Suppaiah, V.; Ismail, A.; Hamid, M.; Shaari, K.; Lajis, N.H. Metabolic Alteration in Obese Diabetes Rats upon Treatment with Centella Asiatica Extract. J. Ethnopharmacol. 2016, 180, 60–69. [Google Scholar] [CrossRef]
- Fu, M.; Bao, T.; Yu, H.; LiSha, A.; Li, H.F.; Ba, G.; Cho, S. Metabolomics Investigation on Antiobesity Effects of Corydalis Bungeana on High-Fat High-Sugar Diet-Induced Obese Rats. Chin. Herb. Med. 2022, 14, 414–421. [Google Scholar] [CrossRef]
- Bone, D.B.J.; Antic, M.; Quinonez, D.; Hammond, J.R. Hypoxanthine Uptake by Skeletal Muscle Microvascular Endothelial Cells from Equilibrative Nucleoside Transporter 1 (ENT1)-Null Mice: Effect of Oxidative Stress. Microvasc. Res. 2015, 98, 16–22. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Kim, M.S.; Yang, H.J.; Lee, M.; Kwon, D.Y. Metabolomics Associated with Genome-Wide Association Study Related to the Basal Metabolic Rate in Overweight/Obese Korean Women. J. Med. Food 2019, 22, 499–507. [Google Scholar] [CrossRef]
- Wu, W.; Hu, Y.; Zhang, S.; Liu, D.; Li, Q.; Lin, Y.; Liu, Z. Untargeted Metabolomic and Lipid Metabolism-Related Gene Expression Analyses of the Effects and Mechanism of Aged Liupao Tea Treatment in HFD-Induced Obese Mice. RSC Adv. 2021, 11, 23791–23800. [Google Scholar] [CrossRef]
- Lavallee, C.M.; Bruno, A.; Ma, C.; Raman, M. The Role of Intermittent Fasting in the Management of Nonalcoholic Fatty Liver Disease: A Narrative Review. Nutrients 2022, 14, 4655. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, C.; Han, J.; Ming, T.; Zhou, J.; Lu, C.; Li, Y.; Wang, Z.; Su, X. Dose Effect of High-Docosahexaenoic Acid Tuna Oil on Dysbiosis in High-Fat Diet Mice. J. Sci. Food Agric. 2022, 102, 5531–5543. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, S.; Tang, L.; Zhang, M.; Yuan, M.; Wang, P.; Gao, X. Integrative Analysis of Fecal Metabolome and Gut Microbiota in High-Fat Diet-Induced Hyperlipidemic Rats Treated with Rosa Roxburghii Tratt Juice. J. Funct. Foods 2022, 90. [Google Scholar] [CrossRef]
- Gual-Grau, A.; Guirro, M.; Mayneris-Perxachs, J.; Arola, L.; Boqué, N. Impact of Different Hypercaloric Diets on Obesity Features in Rats: A Metagenomics and Metabolomics Integrative Approach. J. Nutr. Biochem. 2019, 71, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, L.; Wang, J.; Zheng, Q.; Jin, B.; Sun, L. Differentiation of Gestational Diabetes Mellitus by Nuclear Magnetic Resonance-Based Metabolic Plasma Analysis. J. Biomed. Res 2021, 35, 351. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Vargas-Morales, J.M.; Méndez-García, A.L.; López-Barradas, A.M.; Granados-Portillo, O.; Ordaz-Nava, G.; Rocha-Viggiano, A.K.; Gutierrez-Leyte, C.A.; Medina-Cerda, E.; Rosado, J.L.; et al. Amino Acid Profiles of Young Adults Differ by Sex, Body Mass Index and Insulin Resistance. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 393–401. [Google Scholar] [CrossRef]
- Bagheri, M.; Djazayery, A.; Farzadfar, F.; Qi, L.; Yekaninejad, M.S.; Aslibekyan, S.; Chamari, M.; Hassani, H.; Koletzko, B.; Uhl, O. Plasma Metabolomic Profiling of Amino Acids and Polar Lipids in Iranian Obese Adults. Lipids Health Dis. 2019, 18, 94. [Google Scholar] [CrossRef]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut Microbiota-Derived Metabolite Trimethylamine N-Oxide (TMAO) Potentially Increases the Risk of Obesity in Adults: An Exploratory Systematic Review and Dose-Response Meta- Analysis. Obes. Rev. 2020, 21, e12993. [Google Scholar] [CrossRef]
- Hui, D.Y. Intestinal Phospholipid and Lysophospholipid Metabolism in Cardiometabolic Disease. Curr. Opin. Lipidol. 2016, 27, 507–512. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Ocampo-Medina, E.; Gutierrez-Aguilar, R.; MacIás-Kauffer, L.; Villamil-Ramírez, H.; López-Contreras, B.E.; León-Mimila, P.; Vega-Badillo, J.; Gutierrez-Vidal, R.; Villarruel-Vazquez, R.; et al. An Amino Acid Signature Associated with Obesity Predicts 2-Year Risk of Hypertriglyceridemia in School-Age Children. Sci. Rep. 2017, 7, 5607. [Google Scholar] [CrossRef]

| CON | HFD | p Value | Trend | |
|---|---|---|---|---|
| 3-Hydroxyphenylacetate | 1.87 × 10−4 ± 8.15 × 10−5 | 4.69 × 10−5 ± 1.28 × 10−5 | 0.0017 | ↓ |
| Acetate | 3.94 × 10−2 ± 1.10 × 10−2 | 2.00 × 10−2 ± 3.04 × 10−3 | 0.0077 | ↓ |
| Acetone | 2.68 × 10−5 ± 2.08 × 10−5 | 6.52 × 10−5 ± 9.25 × 10−6 | 0.0266 | ↑ |
| Arabinose | 1.09 × 10−3 ± 1.27 × 10−3 | 1.16 × 10−4 ± 4.41 × 10−5 | 0.0147 | ↓ |
| Methanol | 7.83 × 10−4 ± 2.06 × 10−4 | 1.92 × 10−4 ± 2.06 × 10−4 | 0.0067 | ↓ |
| Methionine | 3.81 × 10−4 ± 5.55 × 10−5 | 4.87 × 10−4 ± 1.89 × 10−5 | 0.0260 | ↑ |
| N-Methylhydantoin | 5.30 × 10−5 ± 9.35 × 10−6 | 1.81 × 10−5 ± 6.64 × 10−6 | 0.0013 | ↓ |
| Sarcosine | 2.60 × 10−4 ± 1.27 × 10−4 | 9.84 × 10−5 ± 7.13 × 10−5 | 0.0418 | ↓ |
| HFD | HFAX | HFXG | |
|---|---|---|---|
| Acetate | 2.04 × 10−2 ± 3.58 × 10−3 a,* | 1.62 × 10−2 ± 3.02 × 10−3 ab | 1.25 × 10−2 ± 4.23 × 10−3 b |
| Glutamate | 2.41 × 10−3 ± 4.96 × 10−4 a | 1.61 × 10−3 ± 3.42 × 10−4 b | 2.37 × 10−3 ± 3.10 × 10−4 a |
| Methylamine | 2.31 × 10−5 ± 1.37 × 10−5 ab | 2.89 × 10−5 ± 9.76 × 10−6 b | 1.42 × 10−5 ± 4.76 × 10−6 a |
| Phenylacetate | 2.27 × 10−4 ± 1.09 × 10−4 a | 1.43 × 10−4 ± 4.02 × 10−5 ab | 9.79 × 10−5 ± 3.26 × 10−5 b |
| HFD | HFAX | HFAβ | HFAG | |
|---|---|---|---|---|
| Acetate | 2.04 × 10−2 ± 3.58 × 10−3 a,* | 1.62 × 10−2 ± 3.02 × 10−3 ab | 9.62 × 10−3 ± 1.25 × 10−3 b | 9.13 × 10−3 ± 5.64 × 10−3 b |
| Betaine | 2.59 × 10−4 ± 1.49 × 10−4 a | 1.47 × 10−4 ± 6.54 × 10−5 ab | 5.84 × 10−5 ± 2.10 × 10−5 b | 1.52 × 10−4 ± 7.50 × 10−5 ab |
| Fucose | 1.01 × 10−4 ± 4.49 × 10−5 ab | 9.26 × 10−5 ± 4.33 × 10−5 ab | 4.43 × 10−5 ± 4.90 × 10−6 a | 1.04 × 10−4 ± 4.18 × 10−5 b |
| Fumarate | 8.70 × 10−5 ± 3.96 × 10−5 b | 1.52 × 10−4 ± 7.42 × 10−5 ab | 4.52 × 10−4 ± 3.23 × 10−4 a | 2.21 × 10−4 ± 6.58 × 10−5 a |
| Glutamate | 2.41 × 10−3 ± 4.96 × 10−4 ab | 1.61 × 10−3 ± 3.42 × 10−4 b | 2.46 × 10−3 ± 5.24 × 10−4 a | 2.25 × 10−3 ± 1.58 × 10−4 ab |
| N-Acetylglucosamine | 3.42 × 10−4 ± 1.01 × 10−4 b | 3.68 × 10−4 ± 6.99 × 10−5 b | 2.44 × 10−4 ± 8.41 × 10−5 b | 1.21 × 10−3 ± 3.35 × 10−4 a |
| Proline | 1.05 × 10−3 ± 4.36 × 10−4 ab | 7.03 × 10−4 ± 2.05 × 10−4 ab | 5.85 × 10−4 ± 9.86 × 10−5 b | 1.25 × 10−3 ± 2.99 × 10−4 a |
| TMAO | 1.40 × 10−4 ± 7.57 × 10−5 b | 6.37 × 10−5 ± 3.73 × 10−5 ab | 3.03 × 10−5 ± 1.27 × 10−5 a | 8.82 × 10−5 ± 4.95 × 10−5 ab |
| HFD | HFAG | HFXG | HFXI | |
|---|---|---|---|---|
| Acetate | 2.04 × 10−2 ± 3.58 × 10−3 a,* | 9.13 × 10−3 ± 5.64 × 10−3 b | 1.25 × 10−2 ± 4.23 × 10−3 ab | 1.15 × 10−2 ± 4.88 × 10−3 ab |
| Formate | 5.55 × 10−4 ± 4.41 × 10−4 ab | 6.71 × 10−4 ± 3.20 × 10−4 b | 2.33 × 10−3 ± 2.88 × 10−3 b | 1.86 × 10−4 ± 6.06 × 10−5 a |
| Fumarate | 8.70 × 10−5 ± 3.96 × 10−5 b | 2.21 × 10−4 ± 6.58 × 10−5 ab | 2.17 × 10−4 ± 1.40 × 10−4 ab | 4.21 × 10−4 ± 3.76 × 10−4 a |
| Hypoxanthine | 2.23 × 10−4 ± 1.30 × 10−4 b | 3.52 × 10−4 ± 1.53 × 10−4 ab | 4.24 × 10−4 ± 1.88 × 10−4 ab | 5.89 × 10−4 ± 3.62 × 10−4 a |
| N-Acetylglucosamine | 3.42 × 10−4 ± 1.01 × 10−4 b | 1.21 × 10−3 ± 3.35 × 10−4 a | 3.71 × 10−4 ± 1.78 × 10−4 b | 2.84 × 10−4 ± 7.60 × 10−5 b |
| Proline | 1.05 × 10−3 ± 4.36 × 10−4 ab | 1.25 × 10−3 ± 2.99 × 10−4 a | 1.07 × 10−3 ± 8.74 × 10−5 ab | 6.85 × 10−4 ± 1.54 × 10−4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Cheng, J.; Jiang, X.; Tang, J.; Zhu, C.; Chen, H.; Laghi, L. Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers. Foods 2023, 12, 1403. https://doi.org/10.3390/foods12071403
Zhang Q, Cheng J, Jiang X, Tang J, Zhu C, Chen H, Laghi L. Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers. Foods. 2023; 12(7):1403. https://doi.org/10.3390/foods12071403
Chicago/Turabian StyleZhang, Qian, Jinhua Cheng, Xiaole Jiang, Junni Tang, Chenglin Zhu, Hong Chen, and Luca Laghi. 2023. "Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers" Foods 12, no. 7: 1403. https://doi.org/10.3390/foods12071403
APA StyleZhang, Q., Cheng, J., Jiang, X., Tang, J., Zhu, C., Chen, H., & Laghi, L. (2023). Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers. Foods, 12(7), 1403. https://doi.org/10.3390/foods12071403







