Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
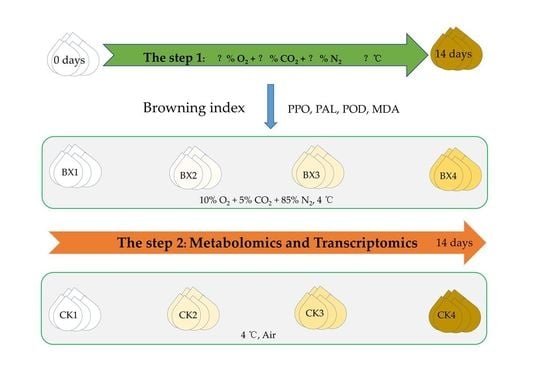
2.2. Temperatures and MAP Preservation
2.3. Determination of BD
2.4. Extraction and Assay of PPO, POD and PAL
2.5. Determination of Malondialdehyde (MDA)
2.6. Transcriptomic Profiling and Data Analysis
2.7. Metabolite Profiling and Data Analysis
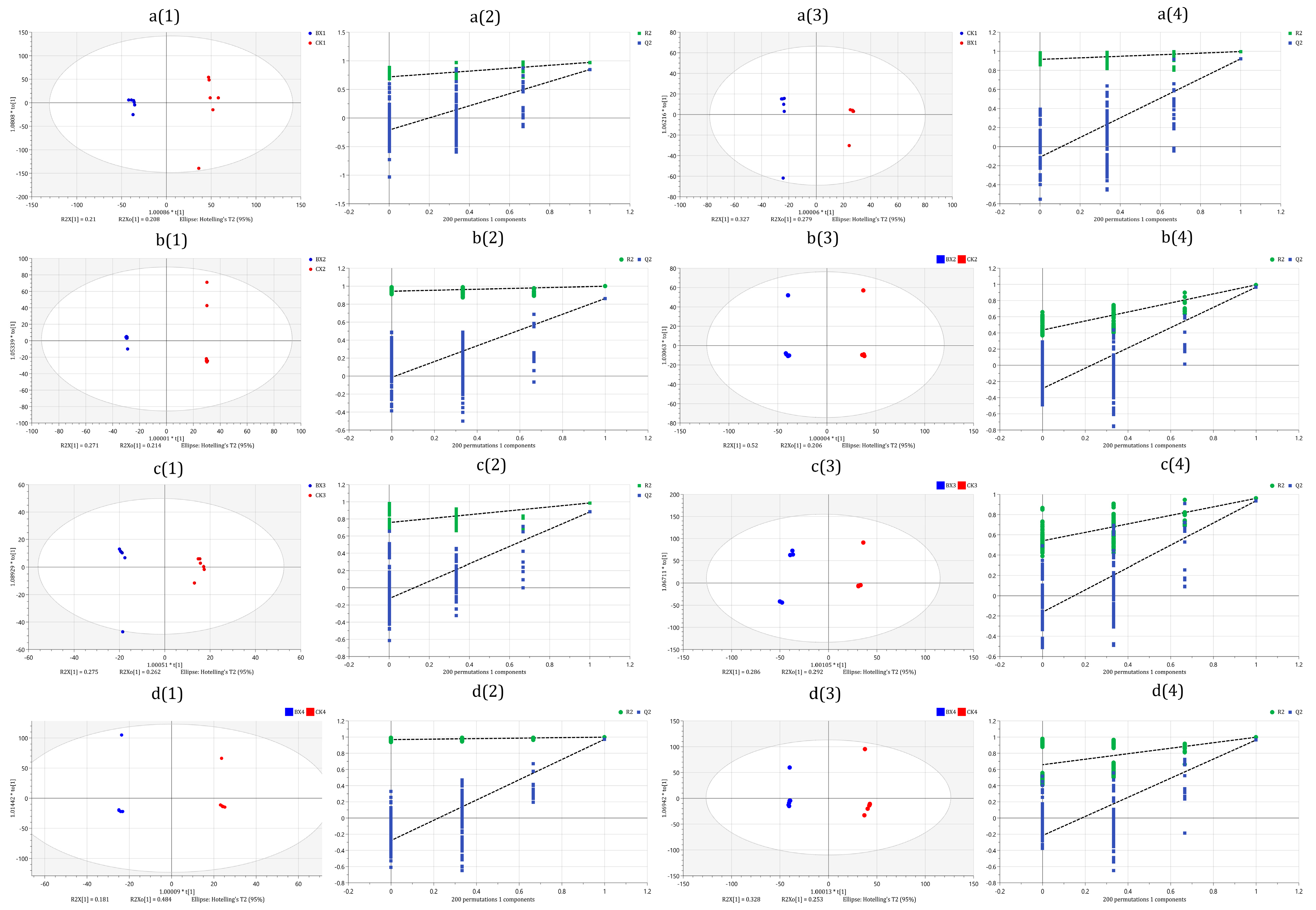
2.8. Statistical Analysis
3. Results
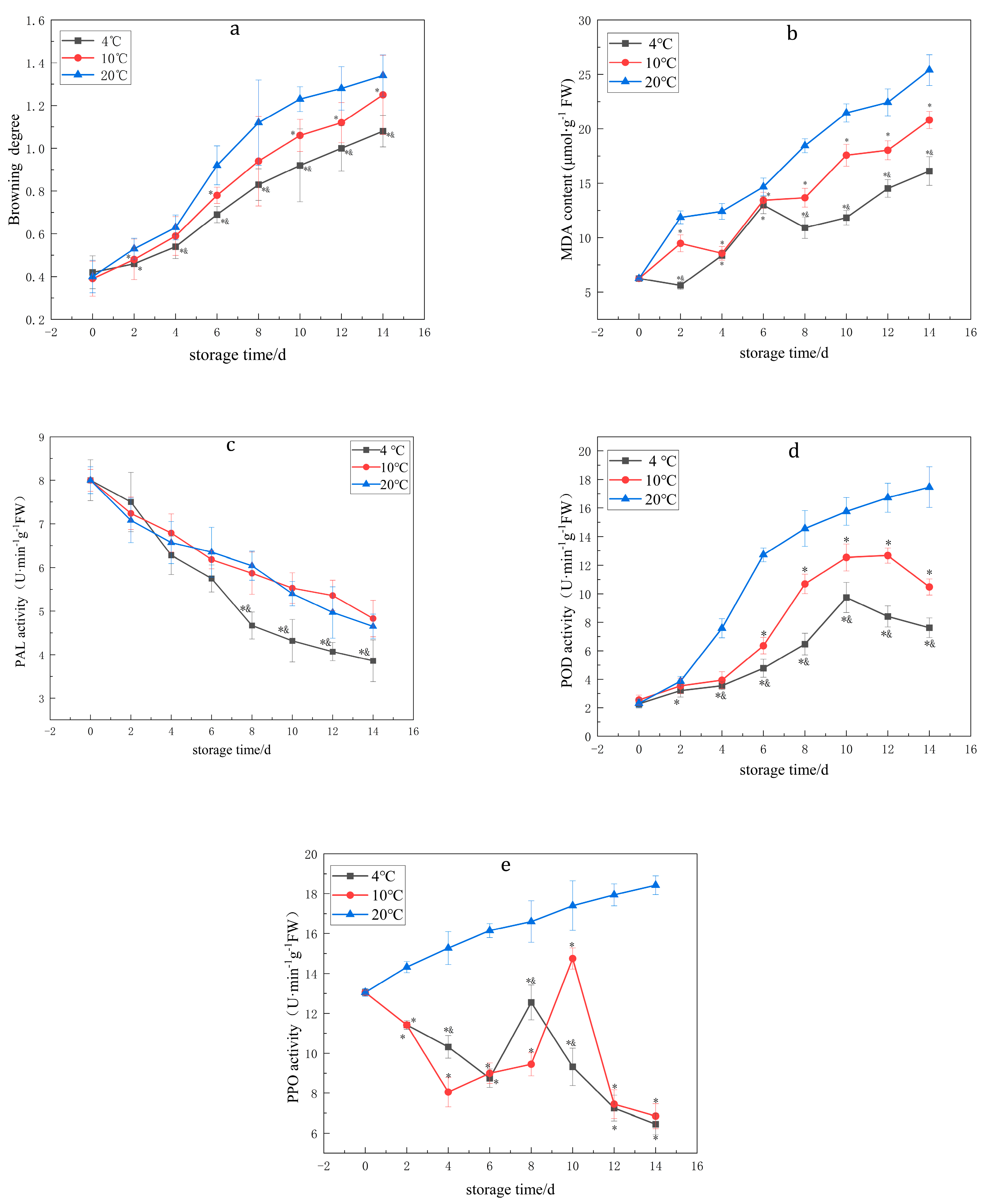
3.1. Effects of Temperature on the BD, PPO, POD and PAL Enzyme Activities and MDA Content of Lanzhou Lily Bulbs
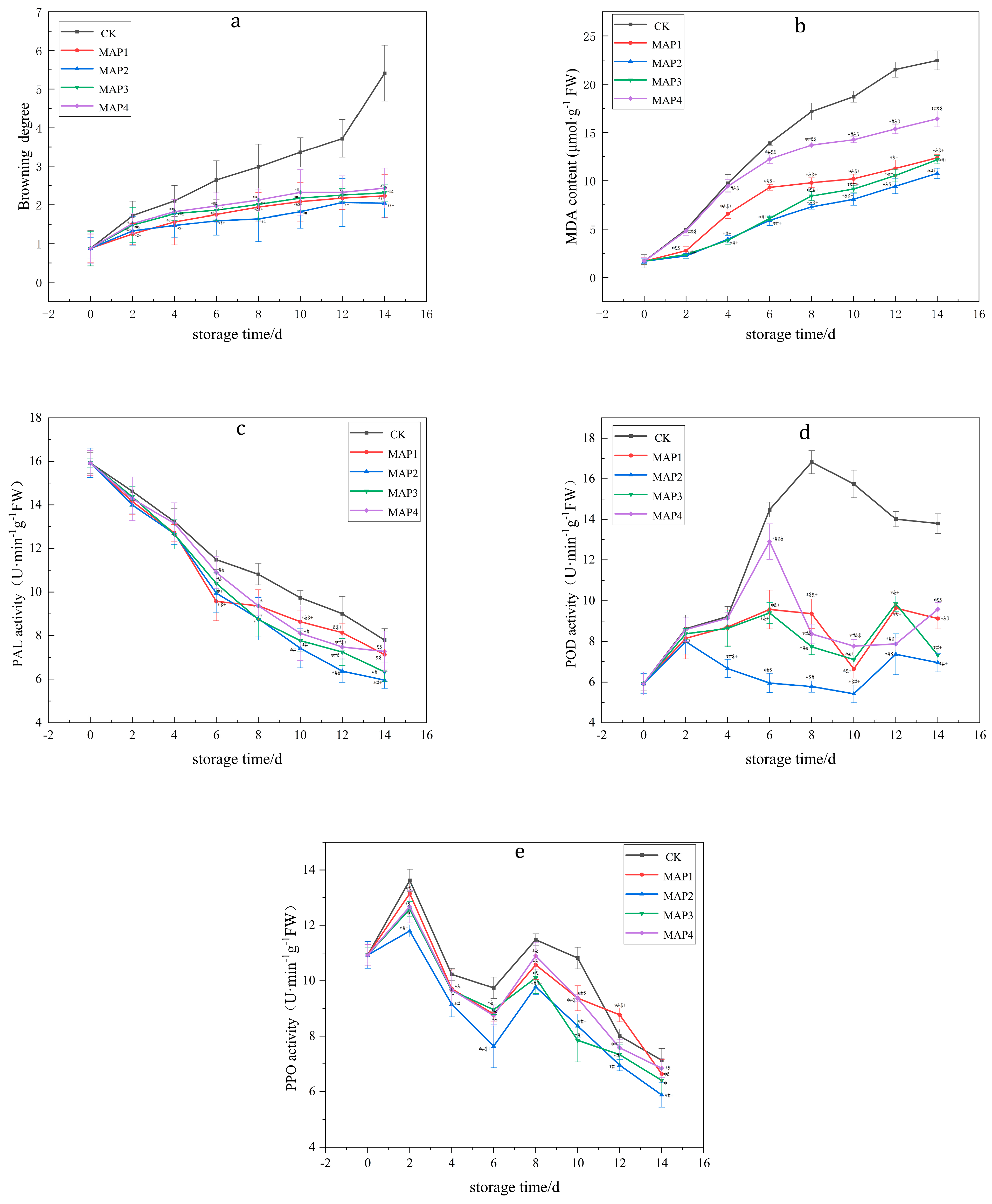
3.2. Effects of MAP on the BD, PPO, POD and PAL Enzyme Activities and MDA Content of Lanzhou Lily Bulbs
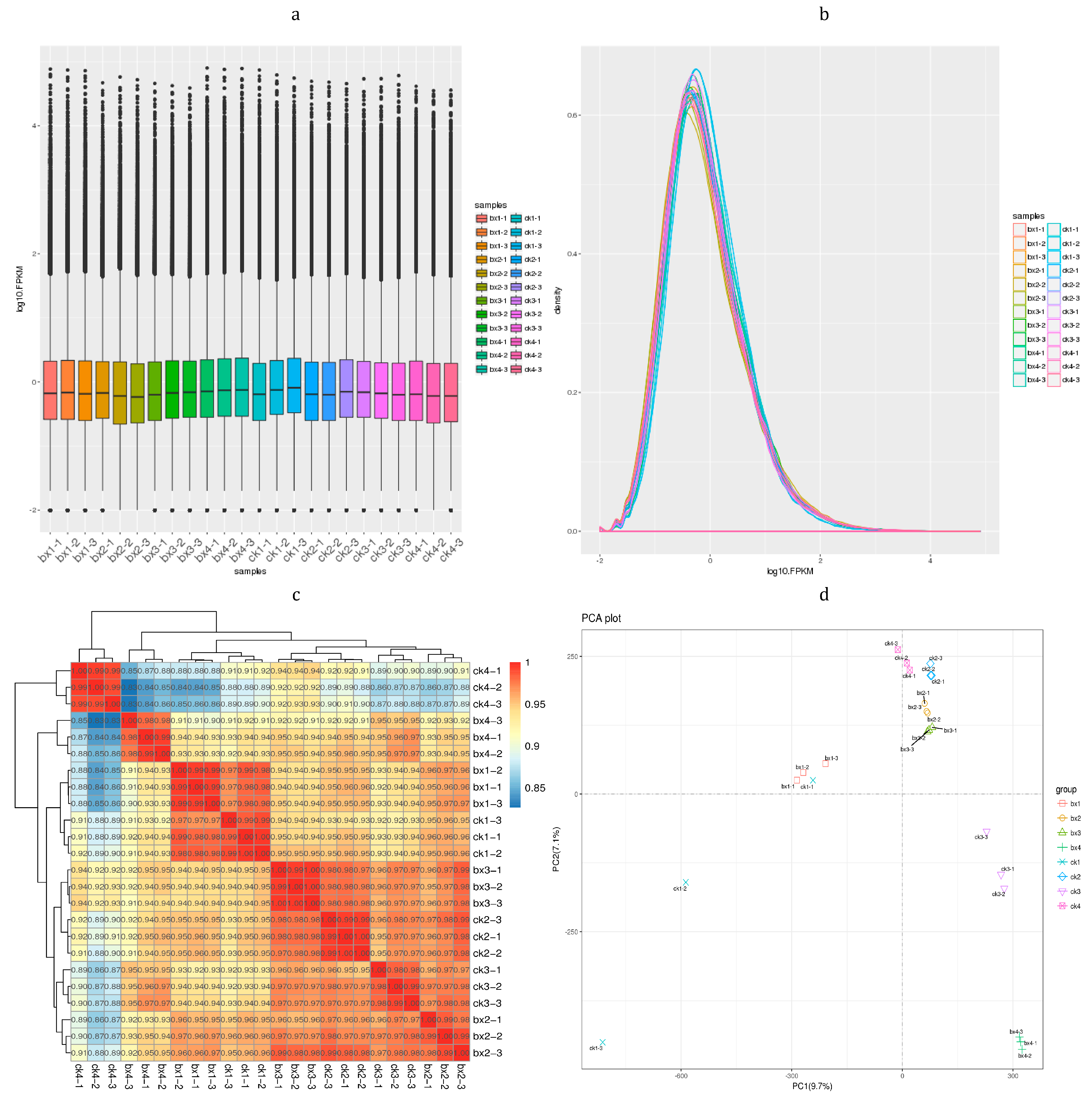
3.3. Sequencing Data Quality Assessment
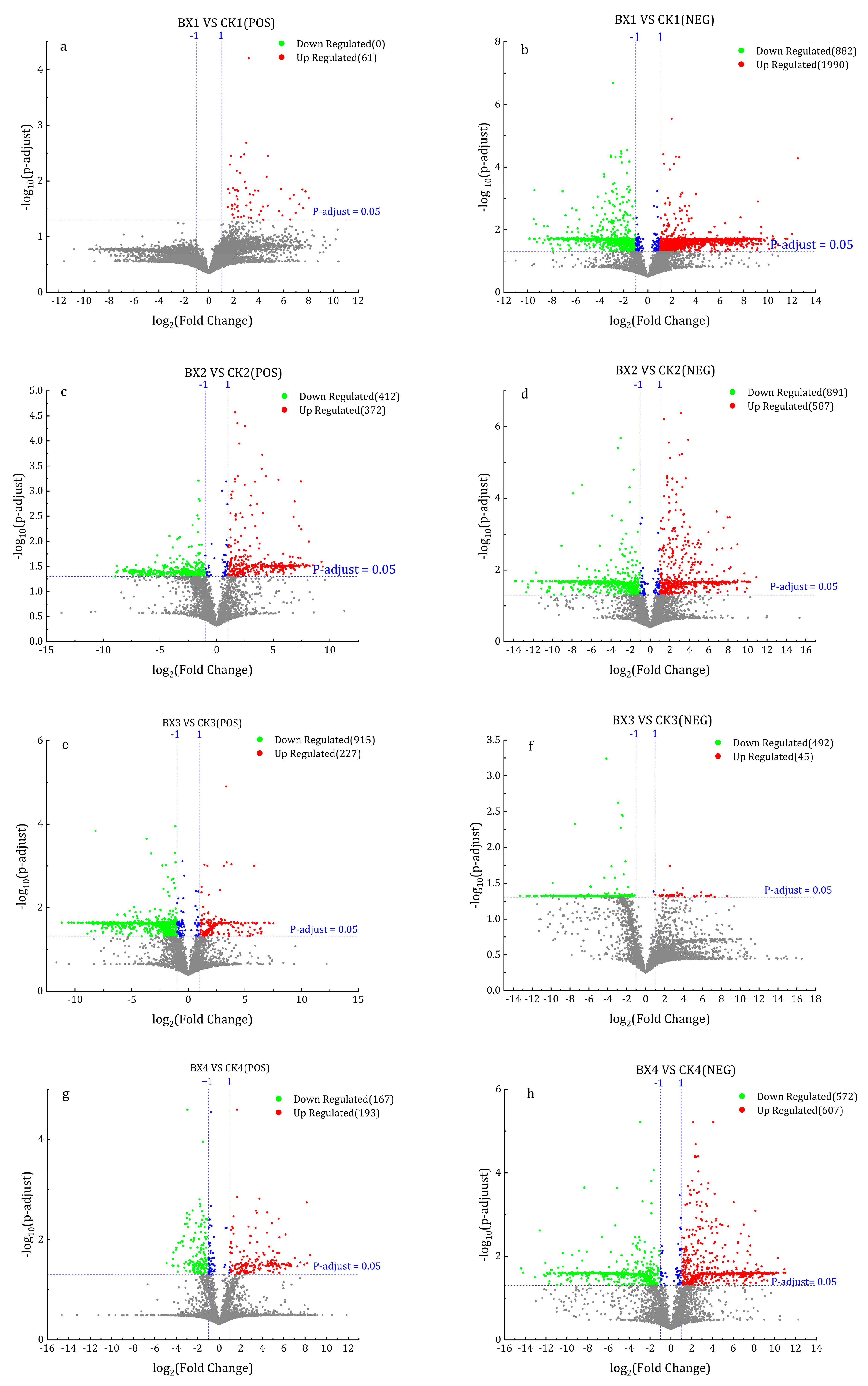
3.4. Differentially Expressed Genes (DEGs) Analysis
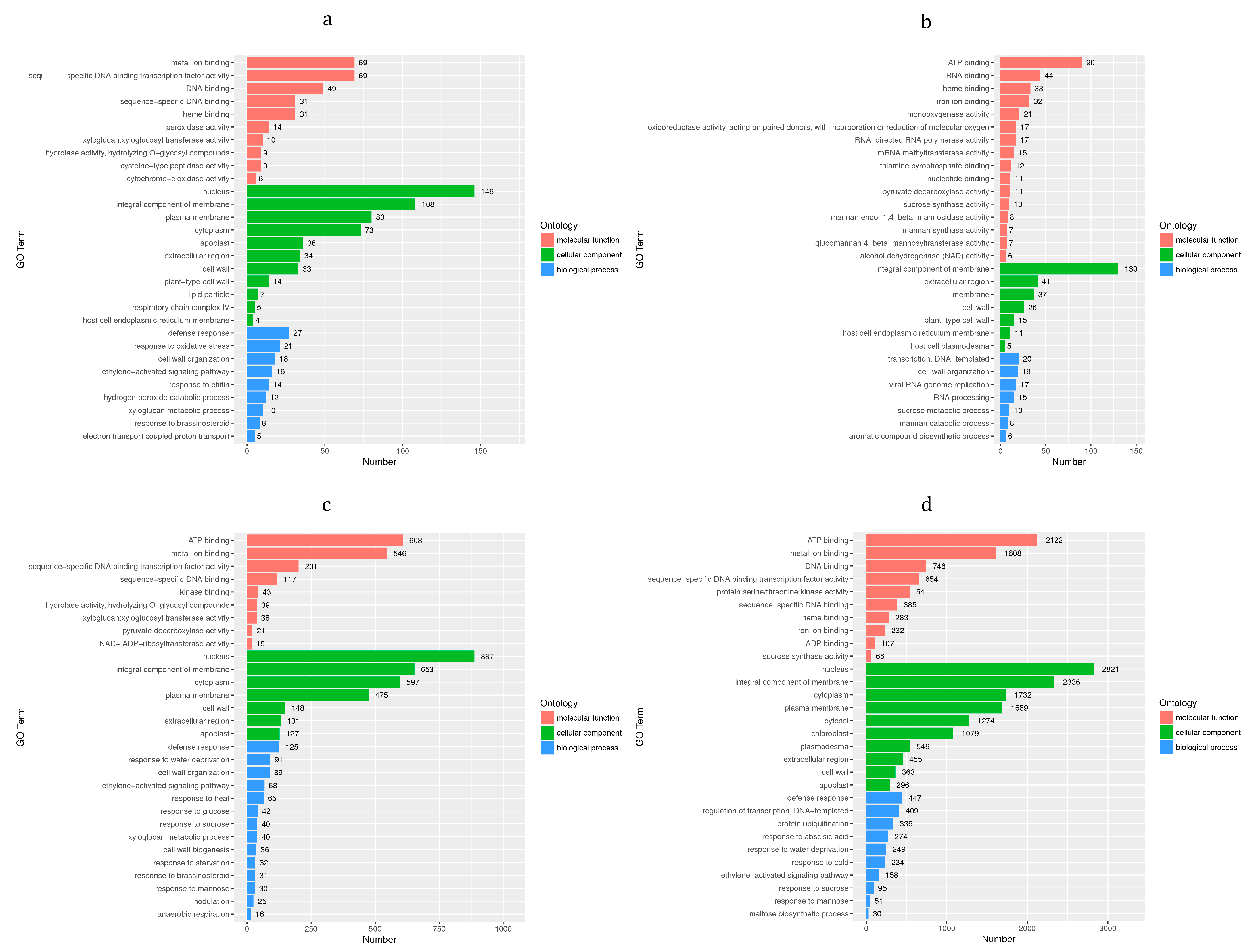
3.5. GO Enrichment Analysis of DEGs
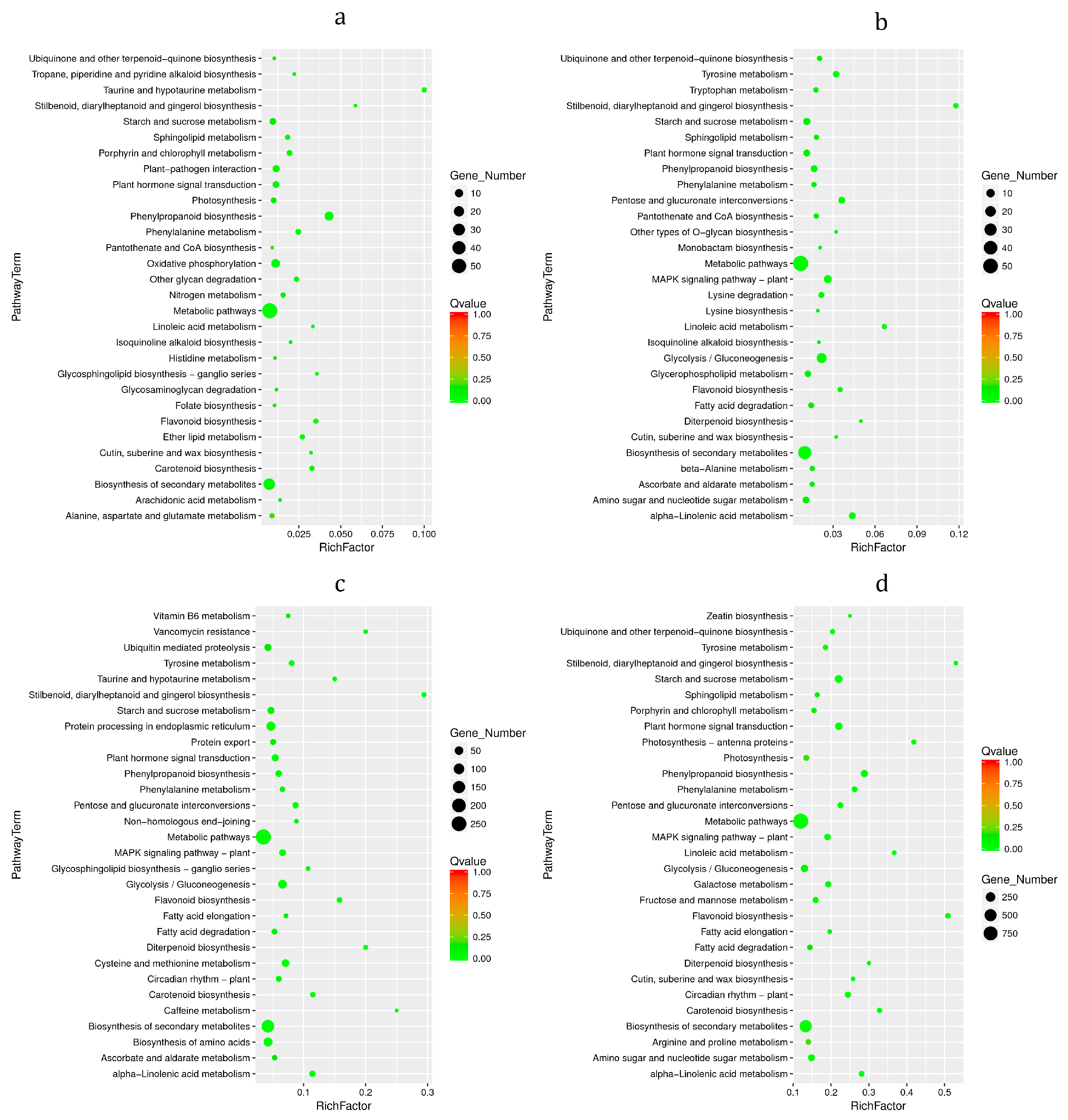
3.6. KEGG Enrichment Analysis of DEGs
3.7. Differential Metabolites (DMs) Analysis
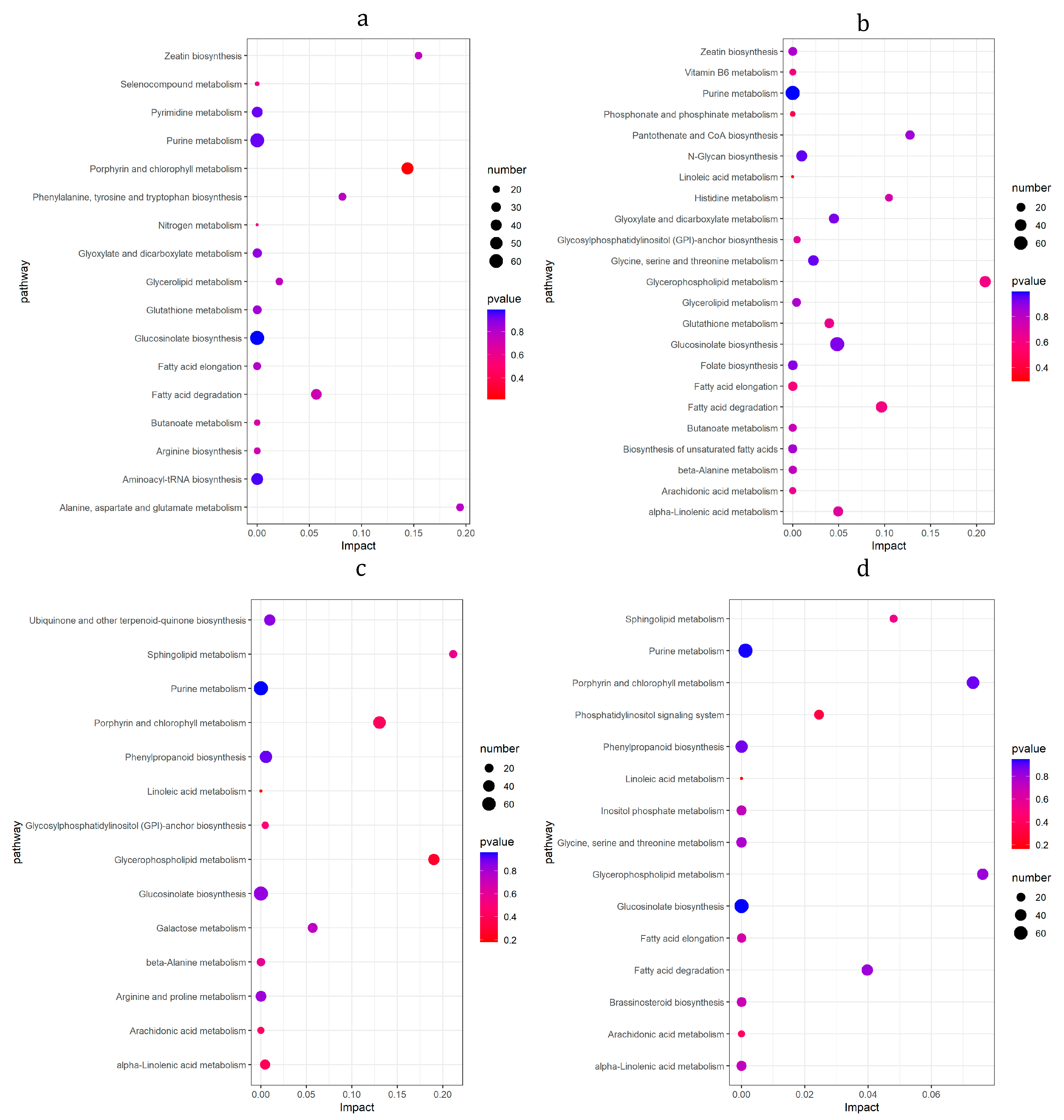
3.8. KEGG Enrichment Analysis of DMs
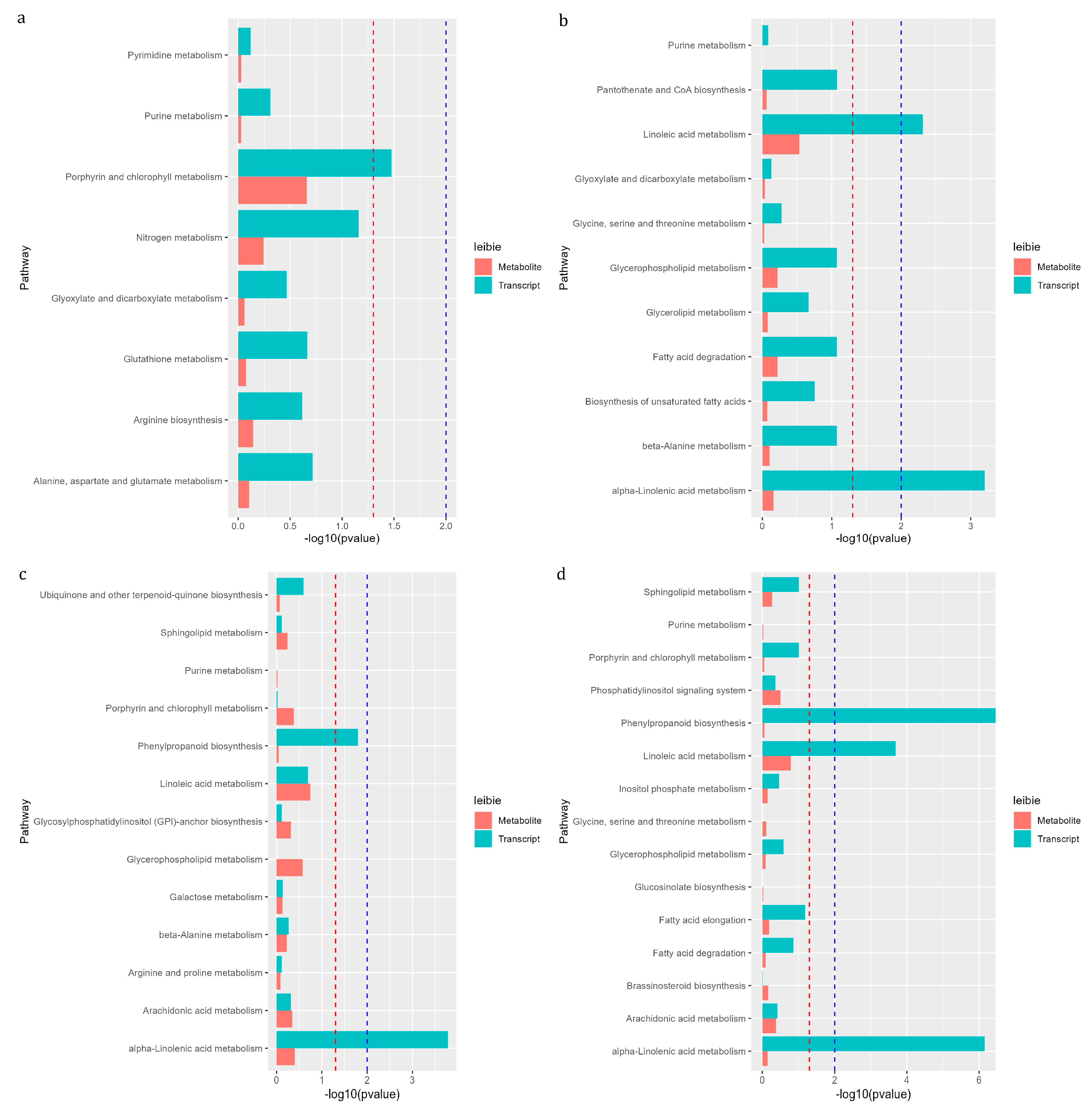
3.9. KEGG Pathway of Differential Gene and Metabolite Co-Enrichment Analysis
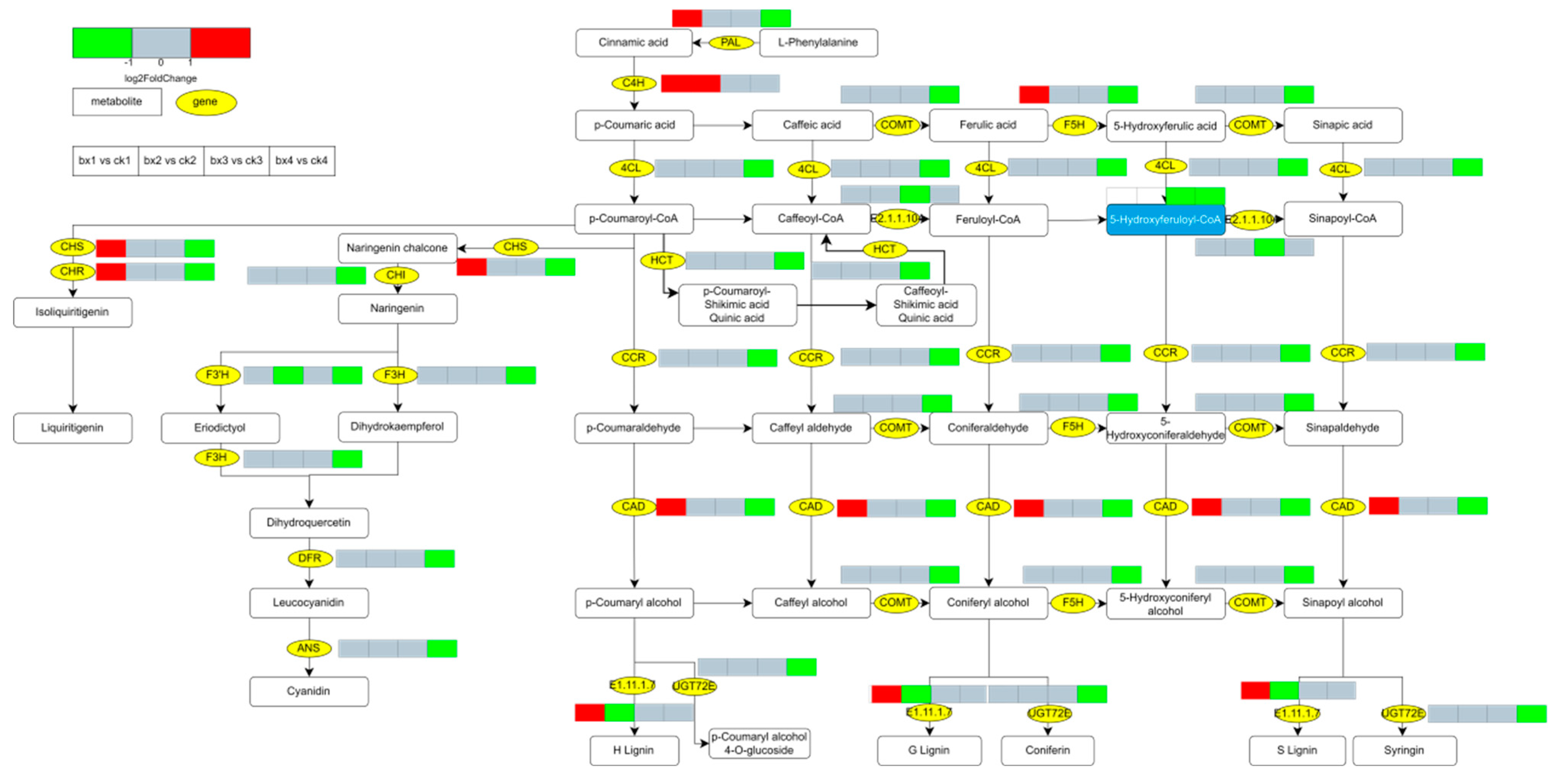
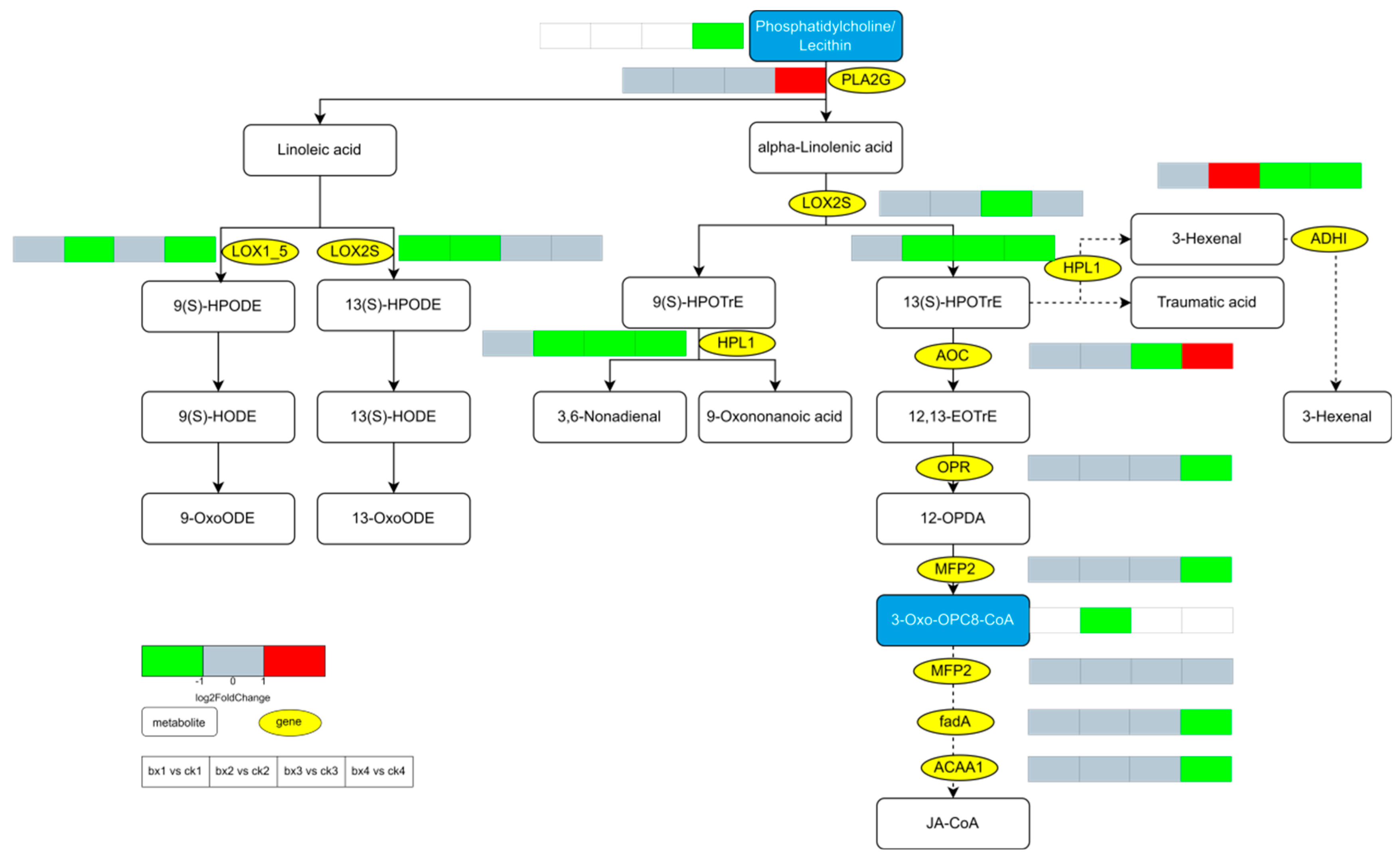
3.10. Changes of Metabolites and Genes
4. Discussion
5. Conclusions
- Fresh-cut Lanzhou lily bulbs have the lowest browning index, a good quality and good appearance under the conditions of MAP of 10% O2 + 5% CO2 + 85% N2 and 4 °C.
- MAP reduces the activity of PAL, PPO, POD and the content of MDA.
- The mechanism by which MAP inhibits the browning of fresh-cut Lanzhou lily bulbs may be that it retards the reduction in the ratio of unsaturated fatty acids to saturated fatty acids in the cell membrane of the bulbs. Specifically, MAP inhibits the lipid peroxidation of the membrane to maintain the integrity of the cell membrane, and probably inhibits the metabolic pathways of ‘Phenylpropanoid biosynthesis’, ‘Flavonoid biosynthesis’ and ‘Stilbenoid, diarylheptanoid and gingerol biosynthesis’ and the expression of their key enzyme genes, thus inhibiting the oxidation of phenolic substances.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4CL | 4-coumarate:CoA ligase |
| ACAA1 | Acetyl-CoA acyltransferase 1 |
| ADH | Alcohol dehydrogenase |
| ANS | Anthocyanidin synthase |
| AOC | Allene oxide cyclase |
| BD | Browning index |
| BP | Biological process |
| C4H | Cinnamic acid 4-hydroxylase |
| CAD | Cinnamoyl alcohol dehydrogenase |
| CC | Cellular component |
| CCR | Cinnamoyl CoA reductase |
| CHI | CHS-chalcone isomerase |
| CHR | Chalcone reductase |
| CHS | Chalcone synthase |
| COMT | Caffeic acid O-methyltransferase |
| CWC | Chinese water chestnut |
| DEGs | Differentially expressed genes |
| DFR | Dihydroflavonol 4-reductase |
| DMs | Differential metabolites |
| EUG | Eugenol emulsions |
| F3′H | Flavanone 3′-hydroxylase |
| F3H | Flavanone 3 hydroxylase |
| F5H | Flavanone 5 hydroxylase |
| FFV | Fresh-cut fruits and vegetables |
| HCT | Hydroxycinnamoyl transferase |
| HPCD | High-pressure carbon dioxide |
| HPL1 | Hydroperoxide lyase l |
| LOX | Lipoxygenase |
| MAP | Modified atmosphere packaging |
| MDA | Malonaldehyde |
| MF | Molecular function |
| MFP2 | Enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase |
| OPLS-DA | Orthogonal partial least-squares discriminant analysis |
| OPR | 12-oxo-phytodienoic acid reductase |
| PAL | Phenylalanine ammonia lyase |
| PCA | Principal component analysis |
| PLA2G | Phospholipase A2 Group |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| PVP | Polyvinylpyrrolidone |
| ROS | Reactive oxygen species |
References
- Li, W.; Wang, Y.; Wei, H.; Zhang, Y.; Guo, Z.; Qiu, Y.; Wen, L.; Xie, Z. Structural characterization of Lanzhou lily (Lilium davidii var. unicolor) polysaccharides and determination of their associated antioxidant activity. J. Sci. Food Agric. 2020, 100, 5603–5616. [Google Scholar] [CrossRef]
- Li, X.; Luan, B.L. Discovery of Al2O3 particles incorporation mechanism in plasma electrolytic oxidation of AM60B magnesium alloy. Mater. Lett. 2012, 86, 88–91. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Yan, L.; Guo, Y.; Niu, L. Phenolic Compounds and Antioxidant Activity of Bulb Extracts of Six Lilium Species Native to China. Molecules 2012, 17, 9361–9378. [Google Scholar] [CrossRef]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K.; Yildiz, F. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1033141. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Rojas-Graü, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Munuera, I.P.; Fiszman, S.; Martín-Belloso, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Cantos, E.; Tudela, J.A.; Gil, M.I.; Espín, J.C. Phenolic Compounds and Related Enzymes Are Not Rate-Limiting in Browning Development of Fresh-Cut Potatoes. J. Agric. Food Chem. 2002, 50, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Higley, N.A.; Bush, R.K. Sulfites in foods: Uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv. Food Res. 1986, 30, 1–76. [Google Scholar] [CrossRef] [PubMed]
- Hernalsteens, S. Chapter 24—Edible Films and Coatings Made Up of Fruits and Vegetables. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 575–588. [Google Scholar]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Wu, P.; Xin, F.; Xu, H.; Chu, Y.; Du, Y.; Tian, H.; Zhu, B. Chitosan inhibits postharvest berry abscission of ‘Kyoho’ table grapes by affecting the structure of abscission zone, cell wall degrading enzymes and SO2 permeation. Postharvest Biol. Technol. 2021, 176, 111507. [Google Scholar] [CrossRef]
- Lobato-Gómez, M.; Hewitt, S.; Capell, T.; Christou, P.; Dhingra, A.; Girón-Calva, P.S. Transgenic and genome-edited fruits: Background, constraints, benefits, and commercial opportunities. Hortic. Res. 2021, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, X.; Ma, Y.; Guan, H.; Liang, H.; Wang, D. Inhibitory effect of modified atmosphere packaging on Escherichia coli O157:H7 in fresh-cut cucumbers (Cucumis sativus L.) and effectively maintain quality during storage. Food Chem. 2022, 369, 130969. [Google Scholar] [CrossRef]
- Ye, J.-J.; Li, J.-R.; Han, X.-X.; Zhang, L.; Jiang, T.-J.; Xia, M. Research article. J. Integr. Agric. 2012, 11, 474–482. [Google Scholar] [CrossRef]
- Bodbodak, S.; Moshfeghifar, M. 2-Advances in Controlled Atmosphere Storage of Fruits and Vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 39–76. [Google Scholar]
- Yang, H.; Tian, T.; Gu, H.; Li, X.; Cai, G.; Sun, J.; Wu, D.; Lu, J. Analysis of factors related to browning of Dangshan pear (Pyrus spp.) wine. Food Chem. 2020, 308, 125665. [Google Scholar] [CrossRef]
- Kan, J.; Xie, W.; Wan, B.; Huo, T.B.; Lin, X.P.; Liu, J.; Jin, C.H. Heat-induced tolerance to browning of fresh-cut lily bulbs (Lilium lancifolium Thunb.) under cold storage. J. Food Biochem. 2019, 43, e12816. [Google Scholar] [CrossRef] [PubMed]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W.B. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.A.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of purslane (Portulaca oleracea L.) extract on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 283, 445–453. [Google Scholar] [CrossRef]
- Toivonen, P.M.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Barros, M.; Saltveit, M.E. Microbial growth in fresh-cut lettuce increases when wound-induced phenolic accumulation is suppressed. Postharvest Biol. Technol. 2013, 83, 34–39. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R.A. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Chen, J.-Y.; He, L.-H.; Jiang, Y.-M.; Kuang, J.-F.; Lu, C.-B.; Joyce, D.C.; Macnish, A.; He, Y.-X.; Lu, W.-J. Expression of PAL and HSPs in fresh-cut banana fruit. Environ. Exp. Bot. 2009, 66, 31–37. [Google Scholar] [CrossRef]
- Wang, H.; Qian, Z.; Ma, S.; Zhou, Y.; Patrick, J.W.; Duan, X.; Jiang, Y.; Qu, H. Energy status of ripening and postharvest senescent fruit of litchi (Litchi chinensis Sonn.). BMC Plant Biol. 2013, 13, 55. [Google Scholar] [CrossRef]
- Sheng, L.; Zhou, X.; Liu, Z.-Y.; Wang, J.-W.; Zhou, Q.; Wang, L.; Zhang, Q.; Ji, S.-J. Changed activities of enzymes crucial to membrane lipid metabolism accompany pericarp browning in ‘Nanguo’pears during refrigeration and subsequent shelf life at room temperature. Postharvest Biol. Technol. 2016, 117, 1–8. [Google Scholar] [CrossRef]
- Glew, R.H.; Ayaz, F.A.; Sanz, C.; VanderJagt, D.; Huang, H.; Chuang, L.; Strnad, M. Effect of postharvest period on sugars, organic acids and fatty acids composition in commercially sold medlar (Mespilus germanica’Dutch’) fruit. Eur. Food Res. Technol. 2003, 216, 390–394. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ge, H. The membrane may be an important factor in browning of fresh-cut pear. Food Chem. 2017, 230, 265–270. [Google Scholar] [CrossRef]
- Rui, H.; Cao, S.; Shang, H.; Jin, P.; Wang, K.; Zheng, Y. Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J. Sci. Food Agric. 2010, 90, 1557–1561. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of 1-methylcyclopropene treatment on chilling injury, fatty acid and cell wall polysaccharide composition in loquat fruit. J. Agric. Food Chem. 2009, 57, 8439–8443. [Google Scholar] [CrossRef]
- Saquet, A.; Streif, J.; Bangerth, F. On the involvement of adenine nucleotides in the development of brown heart in’Conference’pears during delayed controlled atmosphere storage. Gartenbauwissenschaft 2001, 66, 140–144. [Google Scholar]
- Lin, Y.; Lin, H.; Lin, Y.; Zhang, S.; Chen, Y.; Jiang, X. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Gao, H.; Chai, H.; Cheng, N.; Cao, W. Effects of 24-epibrassinolide on enzymatic browning and antioxidant activity of fresh-cut lotus root slices. Food Chem. 2017, 217, 45–51. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, L.; Luo, H.; Yu, Z. Establishment of a statistical model for browning of fresh-cut lotus root during storage. Postharvest Biol. Technol. 2014, 92, 164–171. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Sarcheshmeh, M.A.A. Impact of postharvest hot salicylic acid treatment on aril browning and nutritional quality in fresh-cut pomegranate. Hortic. Environ. Biotechnol. 2016, 57, 378–384. [Google Scholar] [CrossRef]
- Wang, H.; Iqbal, A.; Murtaza, A.; Xu, X.; Pan, S.; Hu, W. A Review of Discoloration in Fruits and Vegetables: Formation Mechanisms and Inhibition. Food Rev. Int. 2022, 1–22. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Yu, G.; Wang, Q.; Zeng, Q.; Jiang, Z.; Gao, L. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chem. 2019, 286, 226–233. [Google Scholar]
- Bhushan, B.; Pal, A.; Narwal, R.; Meena, V.S.; Sharma, P.C.; Singh, J. Combinatorial approaches for controlling pericarp browning in Litchi (Litchi chinensis) fruit. J. Food Sci. Technol. 2015, 52, 5418–5426. [Google Scholar] [PubMed]
- Ma, W.; Li, J.; Murtaza, A.; Iqbal, A.; Zhang, J.; Zhu, L.; Xu, X.; Pan, S.; Hu, W. High-pressure carbon dioxide treatment alleviates browning development by regulating membrane lipid metabolism in fresh-cut lettuce. Food Control 2022, 134, 108749. [Google Scholar] [CrossRef]
- Yildiz, G.; Aadil, R.M. Comparative analysis of antibrowning agents, hot water and high-intensity ultrasound treatments to maintain the quality of fresh-cut mangoes. J. Food Sci. Technol. 2022, 59, 202–211. [Google Scholar] [PubMed]
- Zheng, J.; Li, S.; Xu, Y.; Zheng, X. Effect of oxalic acid on edible quality of bamboo shoots (Phyllostachys prominens) without sheaths during cold storage. LWT 2019, 109, 194–200. [Google Scholar]
- Qinqin, G.; Shiping, T. Partial characterization of soluble peroxidase in pericarp of litchi fruit. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Jin Zhan 2002, 29, 891–896. [Google Scholar]
- Teng, Y.; Murtaza, A.; Iqbal, A.; Fu, J.; Ali, S.W.; Iqbal, M.A.; Xu, X.; Pan, S.; Hu, W. Eugenol emulsions affect the browning processes, and microbial and chemical qualities of fresh-cut Chinese water chestnut. Food Biosci. 2020, 38, 100716. [Google Scholar]
- Guo, S.; Wang, D.; Ma, Y.; Zhang, Y.; Zhao, X. Combination of RNA-Seq transcriptomics and iTRAQ proteomics reveal the mechanism involved in fresh-cut yam yellowing. Sci. Rep. 2021, 11, 7755. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Gao, M.; Wang, Y.; Tian, X.; Lu, L.; Liu, X. Integrated transcriptomic and metabolomic analysis of cultivar differences provides insights into the browning mechanism of fresh-cut potato tubers. Postharvest Biol. Technol. 2022, 188, 111905. [Google Scholar] [CrossRef]
- Li, X.; Long, Q.; Gao, F.; Han, C.; Jin, P.; Zheng, Y. Effect of cutting styles on quality and antioxidant activity in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Ind. Crops Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme stresses and the impact on nutraceuticals and quality during pre-and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar]
- Hu, W.; Guan, Y.; Feng, K. Biosynthesis of phenolic compounds and antioxidant activity in fresh-cut fruits and vegetables. Front. Microbiol. 2022, 13, 1782. [Google Scholar]
- Angós, I.; Vírseda, P.; Fernández, T. Control of respiration and color modification on minimally processed potatoes by means of low and high O2/CO2 atmospheres. Postharvest Biol. Technol. 2008, 48, 422–430. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, W.; Li, J.; Iqbal, A.; Murtaza, A.; Xu, X.; Pan, S. High-pressure carbon dioxide treatment and vacuum packaging alleviate the yellowing of peeled Chinese water chestnut (Eleocharis tuberosa). Food Packag. Shelf Life 2022, 34, 100927. [Google Scholar] [CrossRef]

| Items | Gruops | 0d | 2d | 4d | 6d | 8d | 10d | 12d | 14d |
|---|---|---|---|---|---|---|---|---|---|
| BD | 4 °C | 0.42 ± 0.077 | 0.46 ± 0.074 * | 0.54 ± 0.056 *& | 0.69 ± 0.038 *& | 0.83 ± 0.074 *& | 0.92 ± 0.17 *& | 1 ± 0.106 *& | 1.08 ± 0.074 *& |
| 10 °C | 0.39 ± 0.082 | 0.48 ± 0.095 * | 0.59 ± 0.091 | 0.78 ± 0.038 * | 0.94 ± 0.21 | 1.06 ± 0.075 * | 1.12 ± 0.094 * | 1.25 ± 0.186 * | |
| 20 °C | 0.4 ± 0.076 | 0.53 ± 0.049 | 0.63 ± 0.058 | 0.92 ± 0.091 | 1.12 ± 0.199 | 1.23 ± 0.058 | 1.28 ± 0.101 | 1.34 ± 0.097 | |
| MDA | 4 °C | 6.25 ± 0.27 | 5.61 ± 0.37 *& | 8.37 ± 0.43 * | 12.99 ± 0.79 * | 10.92 ± 0.99 *& | 11.84 ± 0.67 *& | 14.52 ± 0.81 *& | 16.12 ± 1.32 *& |
| 10 °C | 6.25 ± 0.15 | 9.48 ± 0.77 * | 8.57 ± 0.63 * | 13.43 ± 0.74 * | 13.67 ± 0.87 * | 17.58 ± 1.00 * | 18.03 ± 0.87 * | 20.81 ± 0.79 * | |
| 20 °C | 6.25 ± 0.20 | 11.87 ± 0.59 | 12.41 ± 0.72 | 14.67 ± 0.81 | 18.45 ± 0.64 | 21.46 ± 0.84 | 22.43 ± 1.24 | 25.4 ± 1.42 | |
| PAL | 4 °C | 8.00 ± 0.47 | 7.5 ± 0.68 | 6.28 ± 0.44 | 5.75 ± 0.31 | 4.67 ± 0.31 *& | 4.32 ± 0.49 *& | 4.07 ± 0.21 *& | 3.86 ± 0.48 *& |
| 10 °C | 8.00 ± 0.25 | 7.24 ± 0.37 | 6.79 ± 0.44 | 6.18 ± 0.21 | 5.87 ± 0.48 | 5.53 ± 0.35 | 5.36 ± 0.35 | 4.83 ± 0.42 | |
| 20 °C | 8.00 ± 0.31 | 7.08 ± 0.51 | 6.57 ± 0.48 | 6.35 ± 0.57 | 6.04 ± 0.33 | 5.4 ± 0.28 | 4.97 ± 0.59 | 4.65 ± 0.28 | |
| POD | 4 °C | 2.28 ± 0.29 | 3.21 ± 0.46 * | 3.55 ± 0.27 *& | 4.78 ± 0.63 *& | 6.47 ± 0.76 *& | 9.74 ± 1.06 *& | 8.41 ± 0.74 *& | 7.62 ± 0.69 *& |
| 10 °C | 2.55 ± 0.34 | 3.54 ± 0.55 | 3.94 ± 0.59 | 6.36 ± 0.58 * | 10.69 ± 0.68 * | 12.54 ± 0.94 * | 12.67 ± 0.53 * | 10.48 ± 0.59 * | |
| 20 °C | 2.32 ± 0.29 | 3.87 ± 0.33 | 7.58 ± 0.67 | 12.72 ± 0.48 | 14.56 ± 1.25 | 15.76 ± 0.97 | 16.72 ± 1.02 | 17.46 ± 1.43 | |
| PPO | 4 °C | 13.05 ± 0.15 | 11.42 ± 0.22 * | 10.32 ± 0.57 *& | 8.75 ± 0.46 * | 12.55 ± 0.87 *& | 9.32 ± 0.94 *& | 7.25 ± 0.64 * | 6.45 ± 0.53 * |
| 10 °C | 13.05 ± 0.08 | 11.42 ± 0.15 * | 8.05 ± 0.74 * | 9.00 ± 0.51 * | 9.45 ± 0.58 * | 14.75 ± 0.53 * | 7.45 ± 0.72 * | 6.85 ± 0.62 * | |
| 20 °C | 13.05 ± 0.19 | 14.32 ± 0.28 | 15.27 ± 0.82 | 16.15 ± 0.35 | 16.6 ± 1.04 | 17.4 ± 1.24 | 17.94 ± 0.55 | 18.42 ± 0.47 |
| Items | Gruops | 0d | 2d | 4d | 6d | 8d | 10d | 12d | 14d |
|---|---|---|---|---|---|---|---|---|---|
| BD | CK | 0.88 ± 0.462 | 1.72 ± 0.372 | 2.10 ± 0.400 | 2.64 ± 0.502 | 2.98 ± 0.593 | 3.36 ± 0.383 | 3.72 ± 0.492 | 5.41 ± 0.723 |
| MAP1 | 0.88 ± 0.365 | 1.25 ± 0.271 *$+ | 1.56 ± 0.591 *$+ | 1.75 ± 0.503 *$+ | 1.94 ± 0.375 *&$+ | 2.08 ± 0.499 *&$+ | 2.17 ± 0.294 *+ | 2.23 ± 0.549 *$+ | |
| MAP2 | 0.88 ± 0.275 | 1.33 ± 0.369 *$+ | 1.47 ± 0.306 *$+ | 1.59 ± 0.374 *$+ | 1.64 ± 0.586 *# | 1.82 ± 0.428 *# | 2.06 ± 0.618 *+ | 2.04 ± 0.370 *$+ | |
| MAP3 | 0.88 ± 0.438 | 1.48 ± 0.452 *#& | 1.77 ± 0.169 *#& | 1.86 ± 0.265 *#& | 2.01 ± 0.424 *# | 2.17 ± 0.314 *# | 2.25 ± 0.172 *+ | 2.31 ± 0.220 *#& | |
| MAP4 | 0.88 ± 0.390 | 1.52 ± 0.260 *#& | 1.82 ± 0.311 *#& | 1.97 ± 0.340 *#& | 2.12 ± 0.310 *# | 2.32 ± 0.590 *# | 2.32 ± 0.429 * | 2.43 ± 0.517 *#& | |
| MDA | CK | 1.68 ± 0.68 | 4.96 ± 0.37 | 9.73 ± 0.89 | 13.90 ± 0.23 | 17.18 ± 0.88 | 18.71 ± 0.58 | 21.52 ± 0.79 | 22.47 ± 0.98 |
| MAP1 | 1.68 ± 0.35 | 2.78 ± 0.45 *&$+ | 6.57 ± 0.47 *&$+ | 9.30 ± 0.31 *&$+ | 9.78 ± 0.51 *&$+ | 10.18 ± 0.48 *&$+ | 11.26 ± 0.87 *&+ | 12.36 ± 0.33 *&$+ | |
| MAP2 | 1.68 ± 0.26 | 2.23 ± 0.25 *#+ | 3.96 ± 0.37 *#+ | 5.90 ± 0.53 *#&+ | 7.28 ± 0.25 *#&+ | 8.06 ± 0.62 *#&+ | 9.42 ± 0.77 *#&+ | 10.74 ± 0.54 *#+ | |
| MAP3 | 1.68 ± 033 | 2.38 ± 0.45 *#+ | 3.85 ± 0.44 *#+ | 6.11 ± 0.21 *#+ | 8.40 ± 0.48 *&#+ | 9.13 ± 0.35 *&#+ | 10.52 ± 0.65 *&+ | 12.17 ± 0.42 *#+ | |
| MAP4 | 1.68 ± 0.40 | 4.83 ± 0.47#&$ | 9.42 ± 0.68#&$ | 12.23 ± 0.44 *#&$ | 13.70 ± 0.31 *#&$ | 14.26 ± 0.31 *#&$ | 15.38 ± 0.49 *#&$ | 16.42 ± 0.82 *#&$ | |
| PAL | CK | 15.929 ± 0.482 | 14.623 ± 0.429 | 13.228 ± 0.603 | 11.476 ± 0.445 | 10.819 ± 0.483 | 9.741 ± 0.329 | 9.007 ± 0.798 | 7.792 ± 0.535 |
| MAP1 | 15.929 ± 0.577 | 14.149 ± 0.583 | 12.707 ± 0.389 | 9.574 ± 0.886 *$+ | 9.366 ± 0.742 * | 8.64 ± 0.528 *&$+ | 8.136 ± 0.426&$+ | 7.128 ± 0.667&$ | |
| MAP2 | 15.929 ± 0.672 | 13.995 ± 0.378 | 12.667 ± 0.488 | 9.954 ± 0.879 *+ | 8.778 ± 0.973 * | 7.42 ± 0.902 *# | 6.369 ± 0.519 *#& | 5.955 ± 0.385 *#+ | |
| MAP3 | 15.929 ± 0.218 | 14.372 ± 0.462 | 12.642 ± 0.672 | 10.402 ± 0.489#& | 8.739 ± 0.767 * | 7.768 ± 0.484 *# | 7.247 ± 0.619 *#& | 6.329 ± 0.449 *#+ | |
| MAP4 | 15.929 ± 0.569 | 14.282 ± 1.003 | 13.146 ± 0.953 | 10.899 ± 0.731#& | 9.366 ± 0.450 * | 8.105 ± 1.252 *# | 7.477 ± 0.512 *#$ | 7.277 ± 0.930&$ | |
| POD | CK | 5.929 ± 0.362 | 8.623 ± 0.672 | 9.228 ± 0.489 | 14.476 ± 0.367 | 16.819 ± 0.567 | 15.741 ± 0.672 | 14.007 ± 0.378 | 13.792 ± 0.488 |
| MAP1 | 5.929 ± 0.569 | 8.149 ± 1.003 | 8.707 ± 0.886 | 9.574 ± 0.953 *&+ | 9.366 ± 0.731 *$&+ | 6.64 ± 0.450 *&+ | 9.636 ± 0.252 *&+ | 9.128 ± 0.512 *&$ | |
| MAP2 | 5.929 ± 0.484 | 7.995 ± 0.619 * | 6.667 ± 0.449 *#$+ | 5.954 ± 0.472 *#$+ | 5.778 ± 0.282 *#$+ | 5.42 ± 0.429 *#$+ | 7.369 ± 1.003 *#$ | 6.955 ± 0.445 *#+ | |
| MAP3 | 5.929 ± 0.426 | 8.372 ± 0.273 | 8.642 ± 0.902 | 9.402 ± 0.519 *&+ | 7.739 ± 0.385 *#& | 7.105 ± 0.218 *&+ | 9.847 ± 0.390 *&+ | 7.329 ± 0.250 *#+ | |
| MAP4 | 5.929 ± 0.577 | 8.582 ± 0.583 | 9.146 ± 0.289 | 12.899 ± 0.879 *#$& | 8.366 ± 0.483 *#& | 7.768 ± 0.329 *#$& | 7.877 ± 0.298 *#$ | 9.577 ± 0.335 *&$ | |
| PPO | CK | 10.929 ± 0.473 | 13.623 ± 0.402 | 10.228 ± 0.219 | 9.741 ± 0.385 | 11.476 ± 0.218 | 10.819 ± 0.390 | 8.007 ± 0.250 | 7.128 ± 0.426 |
| MAP1 | 10.929 ± 0.369 | 13.149 ± 0.303 *& | 9.707 ± 0.686 *& | 8.792 ± 0.253 *& | 10.574 ± 0.531 *& | 9.369 ± 0.450 *#$ | 8.768 ± 0.252 *&$+ | 6.64 ± 0.512 *& | |
| MAP2 | 10.929 ± 0.484 | 11.795 ± 0.219 *#+ | 9.146 ± 0.449 *# | 7.636 ± 0.772 *#$+ | 9.778 ± 0.282 *#$+ | 8.366 ± 0.429 *#+ | 6.955 ± 0.203 *# | 5.877 ± 0.445 *#+ | |
| MAP3 | 10.929 ± 0.262 | 12.582 ± 0.272 * | 9.642 ± 0.489 * | 8.954 ± 0.173 *& | 10.105 ± 0.567 *& | 7.847 ± 0.772 *#+ | 7.329 ± 0.378 *# | 6.402 ± 0.488 * | |
| MAP4 | 10.929 ± 0.377 | 12.672 ± 0.583 *& | 9.667 ± 0.689 * | 8.739 ± 0.379 *& | 10.899 ± 0.483 *& | 9.366 ± 0.129 *#$ | 7.577 ± 0.298 *# | 6.842 ± 0.335 *& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, C.; Wang, X.; Liu, X.; Zhu, X.; Zhang, J. Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage. Foods 2023, 12, 1335. https://doi.org/10.3390/foods12061335
Li X, Zhang C, Wang X, Liu X, Zhu X, Zhang J. Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage. Foods. 2023; 12(6):1335. https://doi.org/10.3390/foods12061335
Chicago/Turabian StyleLi, Xu, Chaoyang Zhang, Xueqi Wang, Xiaoxiao Liu, Xinliang Zhu, and Ji Zhang. 2023. "Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage" Foods 12, no. 6: 1335. https://doi.org/10.3390/foods12061335
APA StyleLi, X., Zhang, C., Wang, X., Liu, X., Zhu, X., & Zhang, J. (2023). Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage. Foods, 12(6), 1335. https://doi.org/10.3390/foods12061335