Fungal Biostarter Effect on the Quality of Dry-Aged Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.1.1. Sample Collection and Preparation
2.1.2. Fungal Biostarter
2.2. Research Methods
2.2.1. Aging Conditions
2.2.2. The pH Determination
2.2.3. Color Measurement
2.2.4. Glucose and Lactate Concentration in Tissue, and Muscle Glycolytic Potential
2.2.5. Water-Holding Capacity and Meat Plasticity
2.2.6. Meat Composition
2.2.7. Determination of the Shear Force
2.2.8. The Content of Malondialdehyde
2.2.9. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.2.10. Consumer Evaluation of the Sensory Quality of Dry-Aged Beef
2.2.11. Fungal Biostarter Taxonomic Identification
2.2.12. Bacterial DNA Extraction
2.2.13. Bacterial Metabarcoding
2.2.14. Statistical Analysis
3. Results
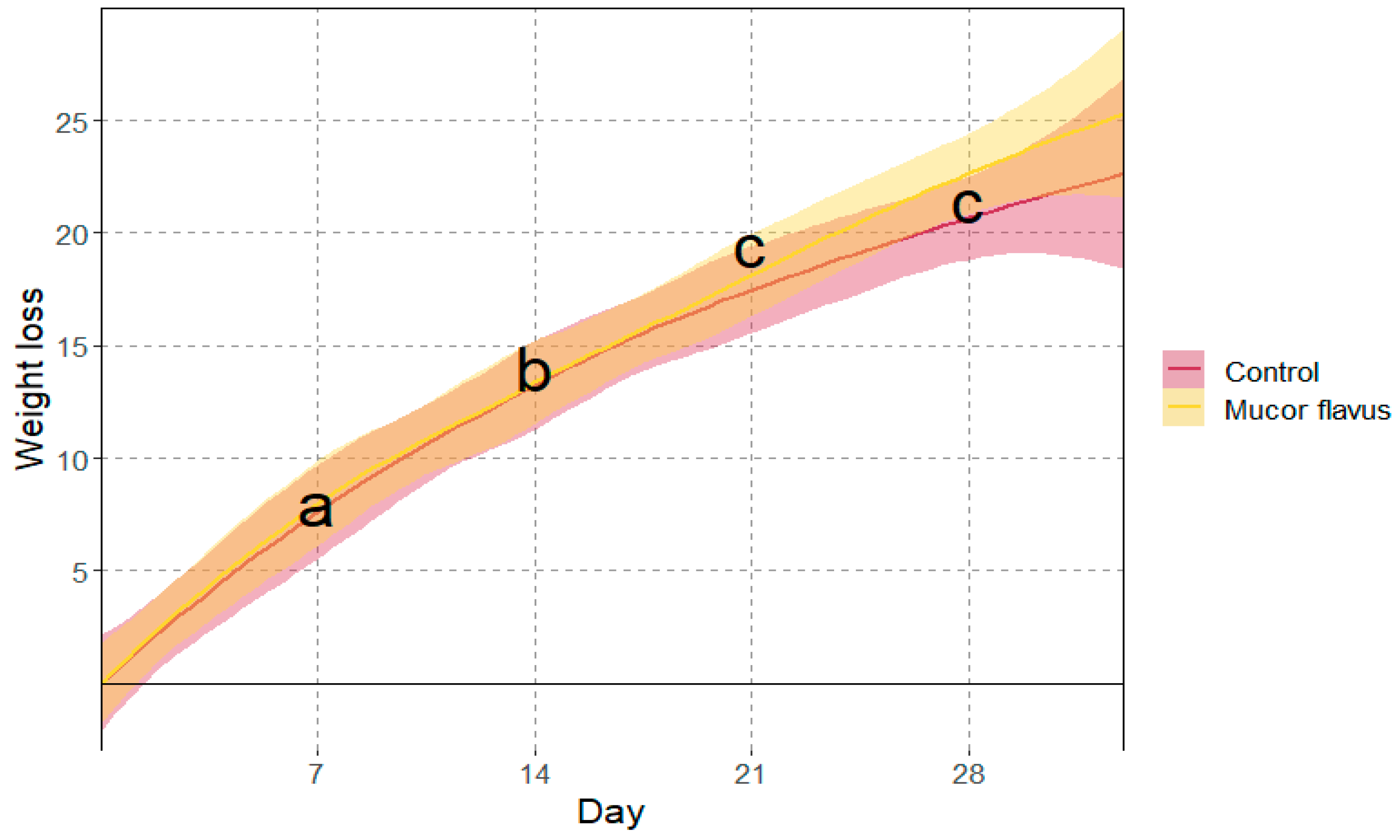
3.1. Physicochemical Changes of DAB
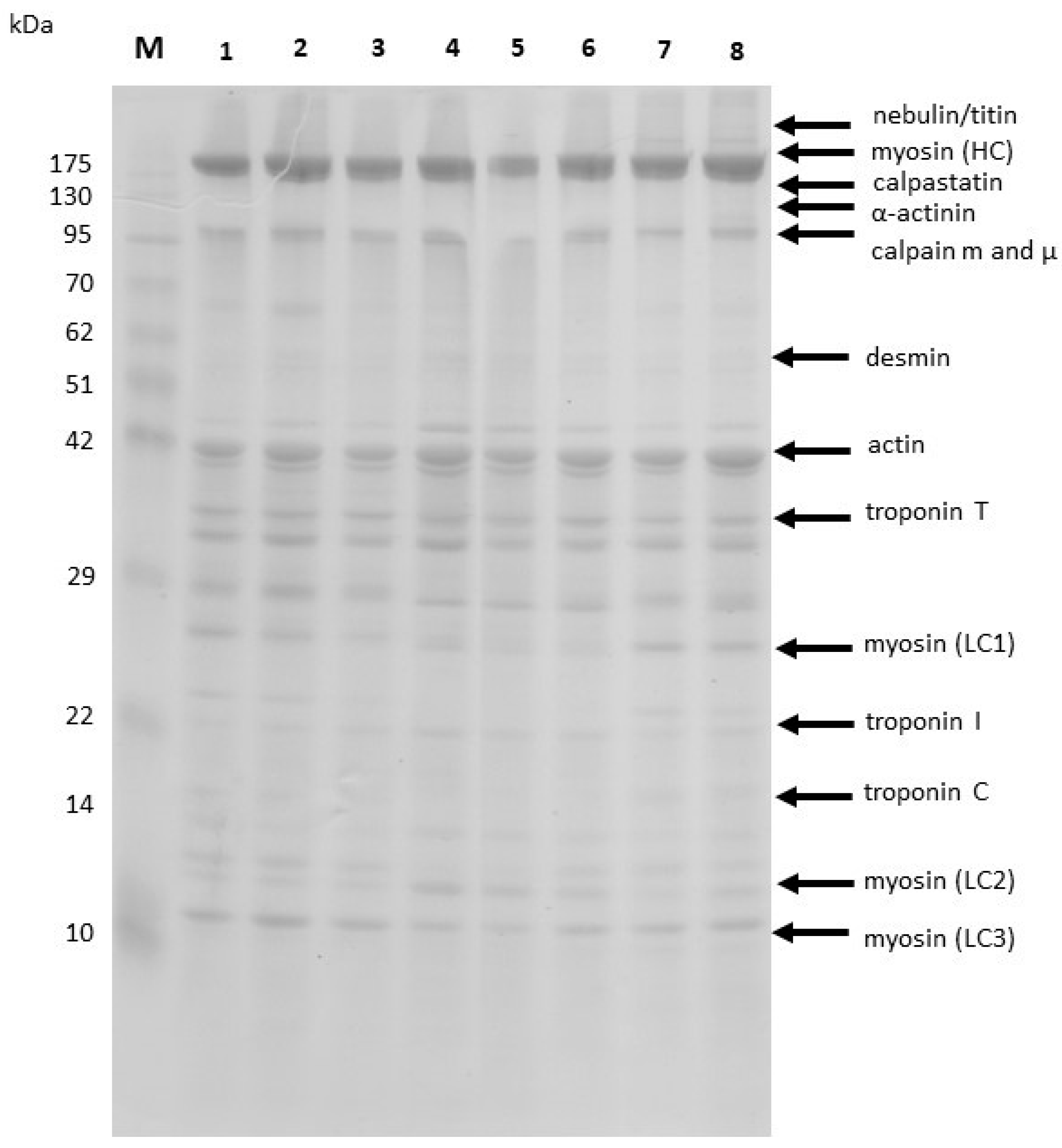
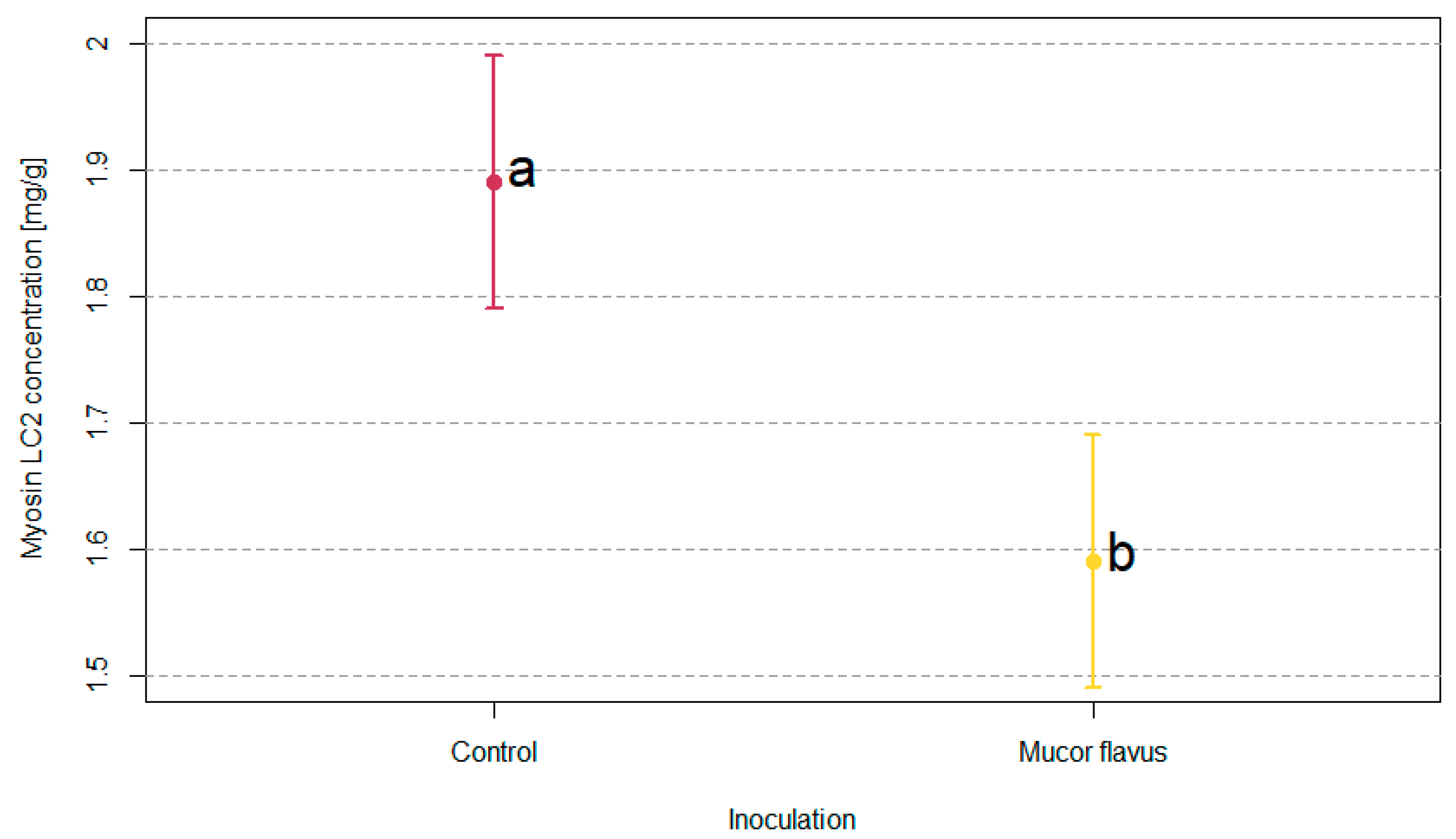
3.2. Myofibrillar Proteins Profiles of DAB
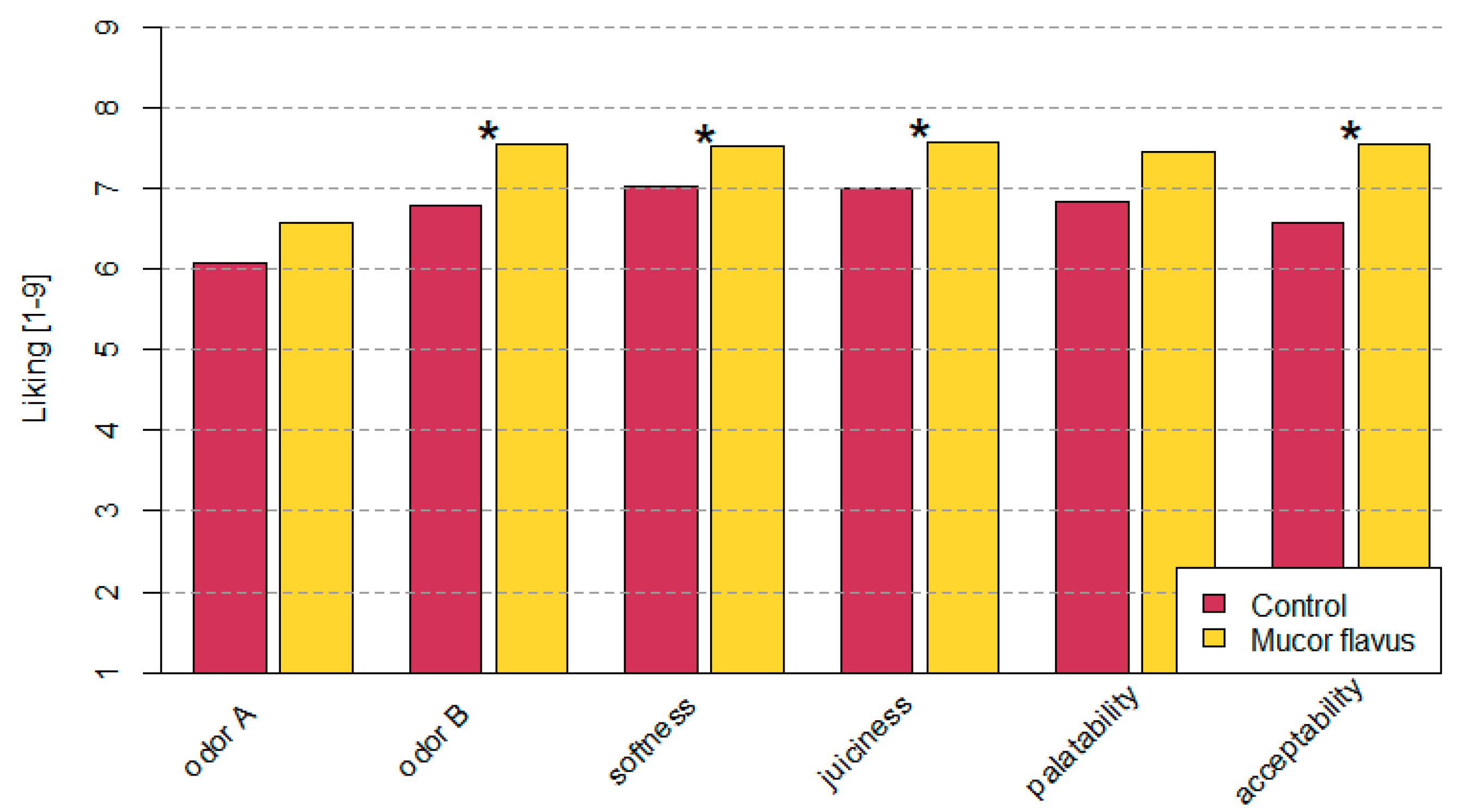
3.3. Changes in Sensory Quality of Dry-Aged Beef
3.4. Bacterial Diversity of DAB
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hocquette, J.F.; Bauchart, D.; Micol, D.; Polkinghorne, R.; Picard, B. Beff quality. In Meat Quality Genetic and Environmental Factors; Przybylski, W., Hopkins, D., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2016; pp. 333–363. [Google Scholar]
- Hocquette, J.F.; Wezemael, L.V.; Chriki, S.; Legrand, I.; Verbeke, W.; Farmer, L.; Scollan, N.D.; Polkinghorne, R.; Rødbotten, R.; Allen, P.; et al. Modelling of beef sensory quality for a better prediction of palatability. Meat Sci. 2014, 97, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ellies-Oury, M.P.; Chriki, S.; Legrand, I.; Pogorzelski, G.; Wierzbicki, J.; Farmer, L.; Troy, D.; Polkinghorne, R.; Hocquette, J.F. Contributions of tenderness, juiciness and flavor liking to overall liking of beef in Europe. Meat Sci. 2020, 168, 108190. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Terlouw, E.M.; Mullen, A.M.; Franco, D.; Warner, R.D.; Lorenzo, J.M.; Purslow, P.P.; Gerrard, D.; Hopkins, D.L.; Troy, D.; et al. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi-platform proteomics studies. Meat Sci. 2021, 172, 108311. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.B.; Ma, D.; Setyabrata, D.; Farouk, M.M.; Lonergan, S.M.; Huff-Lonergan, E.; Hunt, M.C. Understanding postmortem biochemical processes and post-harvest aging factors to develop novel smart-aging strategies. Meat Sci. 2018, 144, 74–90. [Google Scholar] [CrossRef]
- Smith, R.D.; Nicholson, K.L.; Nicholson, J.D.W.; Harris, K.B.; Miller, R.K.; Griffin, D.B.; Savell, J.W. Dry versus Wet aging of beef: Retail cutting yields and consumer palatability evaluations of steaks from US Choice and US Select short loins. Meat Sci. 2008, 79, 631–639. [Google Scholar] [CrossRef]
- Dashdorj, D.; Tripathi, V.K.; Cho, S.; Kim, Y.; Hwang, I. Dry aging of beef; Review. J. Anim. Sci. Techn. 2016, 58, 20. [Google Scholar] [CrossRef]
- Domaradzki, P.; Florek, M.; Litwińczuk, Z. Shaping flavour profile of beef meat during dry ageing process. Food Sci. Technol. Qual. 2020, 27, 5–30. [Google Scholar]
- Domaradzki, P.; Florek, M.; Litwińczuk, Z. Dry ageing of beef–technological aspects. Food Sci. Technol. Qual. 2019, 26, 17–37. [Google Scholar]
- Lee, H.J.; Yoon, J.W.; Kim, M.; Oh, H.; Yoon, Y.; Jo, C. Changes in microbial composition on the crust by different air flow velocities and their effect on sensory properties of dry-aged beef. Meat Sci. 2019, 153, 152–158. [Google Scholar] [CrossRef]
- Ryu, S.; Park, M.R.; Maburutse, B.E.; Lee, W.J.; Park, D.J.; Cho, S.; Park, D.J.; Cho, S.; Hwang, I.; Oh, S.; et al. Diversity and Characteristics of the Meat Microbiological Community on Dry Aged Beef. J. Microbiol. Biotechnol. 2018, 28, 105–108. [Google Scholar] [CrossRef]
- Sunesen, L.O.; Stahnke, L.H. Mould starter cultures for dry sausages–selection, application and effects. Meat Sci. 2003, 65, 935–948. [Google Scholar] [CrossRef]
- Oh, H.; Lee, H.J.; Lee, J.; Jo, C.; Yoon, Y. Identification of Microorganisms Associated with the Quality Improvement of Dry-Aged Beef Through Microbiome Analysis and DNA Sequencing, and Evaluation of Their Effects on Beef Quality. J. Food Sci. 2019, 84, 2944–2954. [Google Scholar] [CrossRef]
- Hanagasaki, T.; Asato, N. Changes in free amino acid content and hardness of beef while dry-aging with Mucor flavus. J. Anim. Sci. Technol. 2018, 60, 19. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Zhu, Y.P.; Hu, Q.; Li, L.T.; Saito, M.; Zhang, S.X.; Yin, L.J. Transformation of Isoflavones During Sufu (A Traditional Chinese Fermented Soybean Curd) Production by Fermentation With Mucor flavus at Low Temperature. Int. J. Food Prop. 2011, 14, 629–639. [Google Scholar] [CrossRef]
- Morin-Sardin, S.; Nodet, P.; Coton, E.; Jany, J.L. Mucor: A Janus-faced fungal genus with human health impact and industrial applications. In Fungal Biology Reviews; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldrá, F. Handbook of Muscle Foods Analysis; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2009. [Google Scholar]
- Przybylski, W.; Sionek, B.; Jaworska, D.; Santé-Lhoutellier, V. The application of biosensors for drip loss analysis and glycolytic potential evaluation. Meat Sci. 2016, 117, 7–11. [Google Scholar] [CrossRef]
- Monin, G.; Sellier, P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: The case of the hampshire breed. Meat Sci. 1985, 13, 49–63. [Google Scholar] [CrossRef]
- Hamm, R. Functional properties of the myofibrillar system and their measurement. In Muscle as Food; Bechtel, P.J., Ed.; Academic Press: London, UK, 1986; pp. 143–147. [Google Scholar]
- Shahidi, F. The 2-tiobarbituric acid (TBA) methodology for the evaluation of warmed-over flavour and rancidity in meat products. In Proceedings of the 36th ICoMST, Havana, Cuba, 28 August–1 September 1990; Available online: https://digicomst.ie/1990-3/1990_09_11/ (accessed on 22 January 2023).
- Bollag, D.M.; Edestein, S.J. Protein Methods; Wiley–Liss A John Wiley and Sons, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Lim, J. Hedonic scaling: A review of methods and theory. Food Qual. Prefer. 2011, 22, 733–747. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; Taylor & Francis: Abingdon, UK, 2006; ISBN 9780849338397. [Google Scholar]
- Baryłko-Pikielna, N.; Matuszewska, I. Sensory Food Research; Wydawnictwo Naukowe PTTZ: Kraków, Poland, 2014; ISBN 978-83-935421-3-0. [Google Scholar]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Siedlecki, I.; Gorczak, M.; Okrasińska, A.; Wrzosek, M. Chance or Necessity—The Fungi Co−Occurring with Formica polyctena Ants. Insects 2021, 12, 204. [Google Scholar] [CrossRef]
- Ryu, S.; Shin, M.; Cho, S.; Hwang, I.; Kim, Y.; Oh, S. Molecular Characterization of Microbial and Fungal Communities on Dry-Aged Beef of Hanwoo Using Metagenomic Analysis. Foods 2020, 9, 1571. [Google Scholar] [CrossRef]
- Gumińska, N.; Płecha, M.; Walkiewicz, H.; Hałakuc, P.; Zakryś, B.; Milanowski, R. Culture purification and DNA extraction procedures suitable for next-generation sequencing of euglenids. J. Appl. Phycol. 2018, 30, 3541–3549. [Google Scholar] [CrossRef]
- Andrews, S. FastQC-A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 22 January 2023).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Caporaso, J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Kim, B.R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Isaacson, R.E. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Kemp, R.; Samuelsson, L.M. Effects of dry aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, K.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese black cattle. Meat sci. 2016, 112, 46–51. [Google Scholar] [CrossRef]
- Ribeiro, F.A.; Lau, S.K.; Pflanzer, S.B.; Subbiah, J. Color and lipid stability of dry aged beef during retail display. Meat Sci. 2021, 171, 108274. [Google Scholar] [CrossRef]
- Terjung, N.; Witte, F.; Heinz, V. The dry aging beef paradox: Why dry aging is sometimes not better than wet aging. Meat Sci. 2021, 172, 108355. [Google Scholar] [CrossRef]
- Obuz, E.; Akkaya, L.; Gók, V.; Dikeman, M.E. Effects of blade tenderization, aging method and aging time on meat quality characteristics of longissimus lumborum steaks from cull Holstein cows. Meat Sci. 2014, 96, 1227–1232. [Google Scholar] [CrossRef]
- Gudjónsdóttir, M.; Gacutan, M.D.; Mendes, A.C.; Chronakis, I.S.; Jespersen, L.; Karlsson, A.H. Effects of electrospun chitosan wrapping for dry-ageing of beef, as studied by microbiological, physicochemical and low-field nuclear magnetic resonance analysis. Food Chem. 2015, 184, 167–175. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-C.; Park, S.; Kim, J.; Yoon, Y.; Lee, H. Identification of Microbial Flora in Dry Aged Beef to Evaluate the Rancidity during Dry Aging. Processes 2021, 9, 2049. [Google Scholar] [CrossRef]
- Warmink, J.A.; Nazir, R.; Corten, B.; van Elsas, J.D. Hitchhikers on the fungal highway: The helper effect for bacterial migration via fungal hyphae. Soil Biol. Biochem. 2011, 43, 760–765. [Google Scholar] [CrossRef]
- Bonfante, P.; Desirò, A. Who lives in a fungus? The diversity, origins and functions of fungal endobacteria living in Mucoromycota. ISME J. 2017, 11, 1727–1735. [Google Scholar] [CrossRef]
- Picard, B.; Berri, C.; Lefaucheur, L.; Molette, C.; Sayd, T.; Terlouw, C. Skeletal muscle proteomics in livestock production. Brief. Funct. Genom. 2010, 9, 259–278. [Google Scholar] [CrossRef]
- Sierra, V.; Gonzáles-Blanco, L.; Diñeiro, Y.; Diaz, F.; Garcia-Espina, M.J.; Coto-Montes, A.; Gagaoua, M.; Oliván, M. New insigths on the impact of cattle handling on post-mortem myofibrillar muscle proteome and meat tenderization. Foods 2021, 10, 3115. [Google Scholar] [CrossRef]
- Ding, Z.; Wei, Q.; Liu, C.; Zhang, H.; Huang, F. The quality changes and proteomic analysis of cattle muscle postmortem during rigor mortis. Foods 2022, 11, 217. [Google Scholar] [CrossRef]
- Li, P.; Wang, T.; Mao, Y.; Zhang, Y.; Niu, L.; Liang, R.; Zhu, L.; Luo, X. Effect of ultimate pH on post mortem myofibrillar protein degradation and meat quality characteristics of Chinese yellow crossbreed cattle. Sci. World J. 2014, 2014, 174253. [Google Scholar] [CrossRef]
- Ribeiro, F.A.; Lau, S.K.; Furbeck, R.A.; Herrera, N.J.; Henriot, M.L.; Bland, N.A.; Fernando, S.C.; Subbiah, J.; Sullivan, G.A.; Calkins, C.R. Ultimate pH effects on dry-aged beef quality. Meat Sci. 2021, 172, 108365. [Google Scholar] [CrossRef]
- Patrignani, F.; Iucci, L.; Vallicelli, M.; Guerzoni, E.; Garnidi, F.; Lanciotti, R. Role of surface-inoculated Debaryomyces hansenii and Yarrowia lipolytica strains in dried fermented sausage manufacture. Part 1: Evaluation of their effects on microbial evolution, lipolytic and proteolytic patterns. Meat Sci. 2007, 75, 676–686. [Google Scholar] [CrossRef]
- Martin, A.; Asensio, M.A.; Bermudez, M.E.; Cordoba, M.G.; Aranda, E.; Cordoba, J.J. Proteolytic activity of Penicillium chrysogenum and Debaryomyces hansenii during controlled ripening of pork loins. Meat Sci. 2002, 62, 129–137. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Hu, Q.; Li, L.T.; Saito, M.; Yin, L.J. Production of sufu, a traditional Chinese fermented soyabean food, by fermentation with Mucor flavus at low temperature. Food Sci. Technol. Res. 2009, 15, 347–352. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Toldra, F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998, 49, S101–S110. [Google Scholar] [CrossRef] [PubMed]
- Farouk, M.M.; Beggan, M.; Hurst, S.; Stuart, A.; Dobbie, P.M.; Bekhit, A.E.D. Meat quality attributes of chilled venison and beef. J. Food Qual. 2007, 30, 1023–1039. [Google Scholar] [CrossRef]
- Mikami, N.; Toyotome, T.; Yamashiro, Y.; Sugo, K.; Yoshitomi, K.; Takaya, M.; Shimada, K. Dry-aged beef manufactured in Japan: Microbiota identification and their effects on product characteristics. Food Res. Int. 2021, 140, 110020. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]

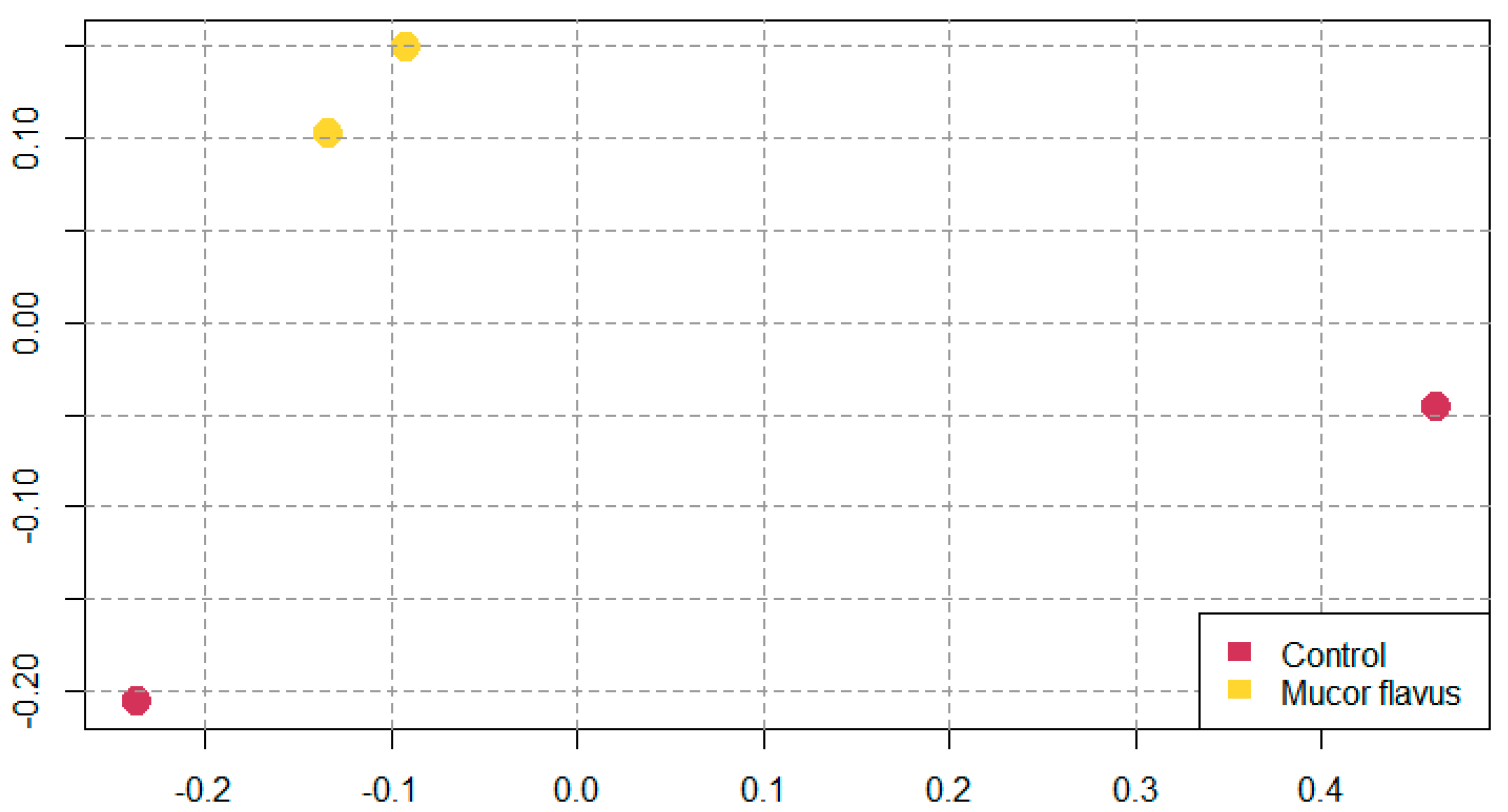
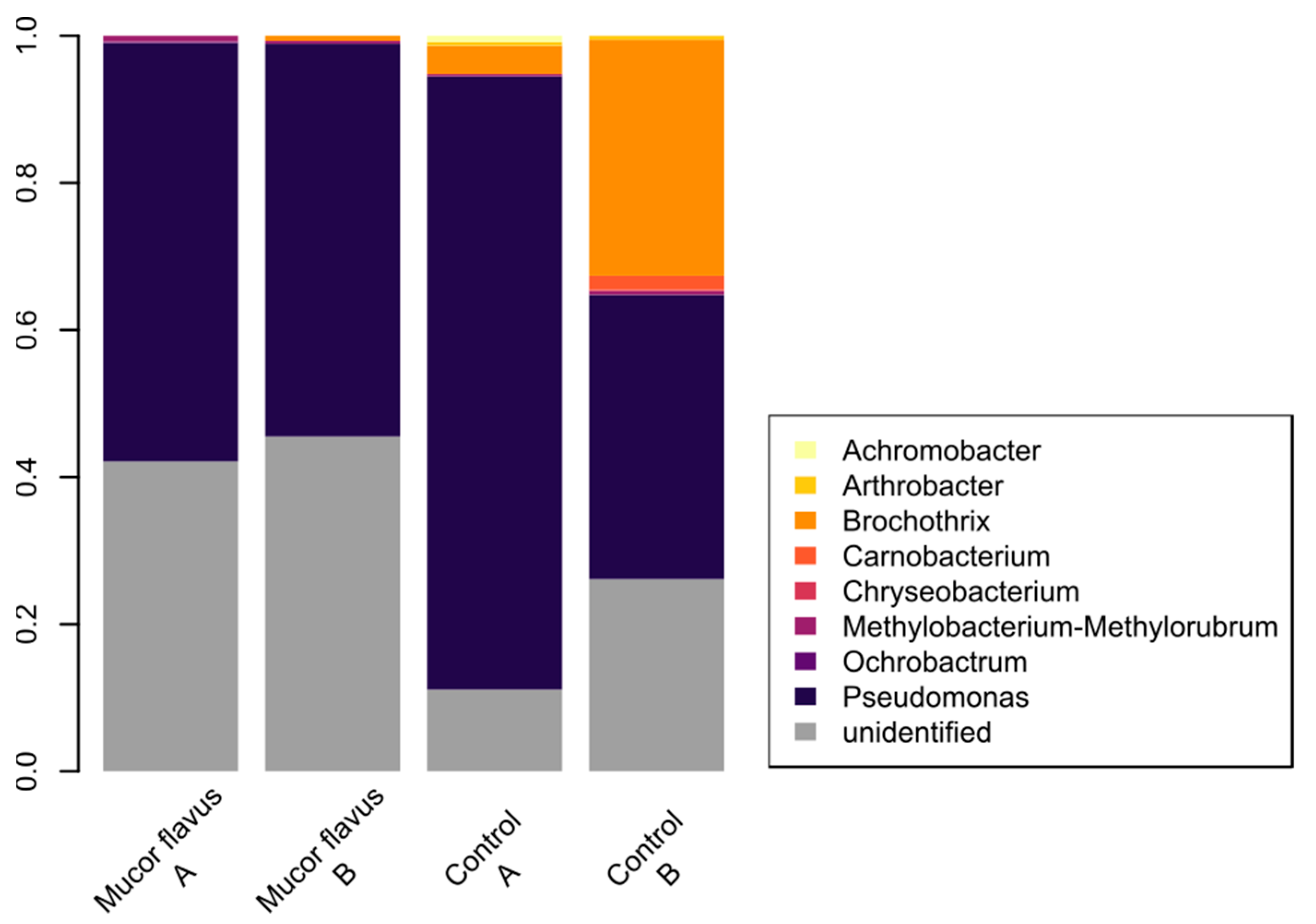
| Traits | Group | SEM | |
|---|---|---|---|
| Biostarter− | Biostarter+ | ||
| pH | 5.61 | 5.60 | 0.05 |
| Color parameters: L* | 33.03 | 34.11 | 1.93 |
| a* | 19.32 | 19.74 | 1.69 |
| b* | 16.50 | 16.27 | 1.64 |
| Glucose (mmol/L) | 5.83 | 5.29 | 0.70 |
| Lactate (mmol/L) | 57.24 | 63.81 | 5.98 |
| Glycolytic potential (mmol/L) | 68.90 | 74.40 | 6.75 |
| WHC (cm2/g) | 13.49 | 14.00 | 1.68 |
| Plasticity (cm2) | 3.37 | 3.30 | 0.21 |
| Moisture content (%) | 66.84 | 68.97 | 1.41 |
| Fat content (%) | 9.16 | 8.80 | 1.38 |
| Protein content (%) | 21.61 | 20.97 | 0.51 |
| Connective tissue (%) | 0.87 | 0.84 | 0.14 |
| Ash content (%) | 0.66 | 0.90 | 0.14 |
| Traits | Group | SEM | |
|---|---|---|---|
| Biostarter− | Biostarter+ | ||
| pH | 5.75 | 5.84 | 0.07 |
| Color parameters: L* | 28.76 | 26.63 | 1.57 |
| a* | 25.34 | 24.61 | 1.72 |
| b* | 24.38 | 22.67 | 1.67 |
| Shear force after grilling (N) | 75.35 | 65.91 | 4.27 |
| Penetration force after grilling (mm) | 12.82 | 12.11 | 0.55 |
| The content of malondialdehyde (mg/kg) | 0.98 | 0.52 | 0.43 |
| Measure | Biostarter− (Sample 1) | Biostarter− (Sample 2) | Biostarter+ (Sample 1) | Biostarter+ (Sample 2) |
|---|---|---|---|---|
| Chao1 | 14 | 17 | 10 | 10 |
| Shannon | 0.93 | 2.25 | 1.21 | 1.23 |
| Simpson | 0.37 | 0.86 | 0.59 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylski, W.; Jaworska, D.; Płecha, M.; Dukaczewska, K.; Ostrowski, G.; Sałek, P.; Sawicki, K.; Pawłowska, J. Fungal Biostarter Effect on the Quality of Dry-Aged Beef. Foods 2023, 12, 1330. https://doi.org/10.3390/foods12061330
Przybylski W, Jaworska D, Płecha M, Dukaczewska K, Ostrowski G, Sałek P, Sawicki K, Pawłowska J. Fungal Biostarter Effect on the Quality of Dry-Aged Beef. Foods. 2023; 12(6):1330. https://doi.org/10.3390/foods12061330
Chicago/Turabian StylePrzybylski, Wiesław, Danuta Jaworska, Magdalena Płecha, Karina Dukaczewska, Grzegorz Ostrowski, Piotr Sałek, Krzysztof Sawicki, and Julia Pawłowska. 2023. "Fungal Biostarter Effect on the Quality of Dry-Aged Beef" Foods 12, no. 6: 1330. https://doi.org/10.3390/foods12061330
APA StylePrzybylski, W., Jaworska, D., Płecha, M., Dukaczewska, K., Ostrowski, G., Sałek, P., Sawicki, K., & Pawłowska, J. (2023). Fungal Biostarter Effect on the Quality of Dry-Aged Beef. Foods, 12(6), 1330. https://doi.org/10.3390/foods12061330







