Improvement of Color and Oxidative Stabilities in Nellore Bull Dark Meat in High-Oxygen Package by Lactate and Rosemary Oil Extract
Abstract
:1. Introduction
2. Materials and Methods
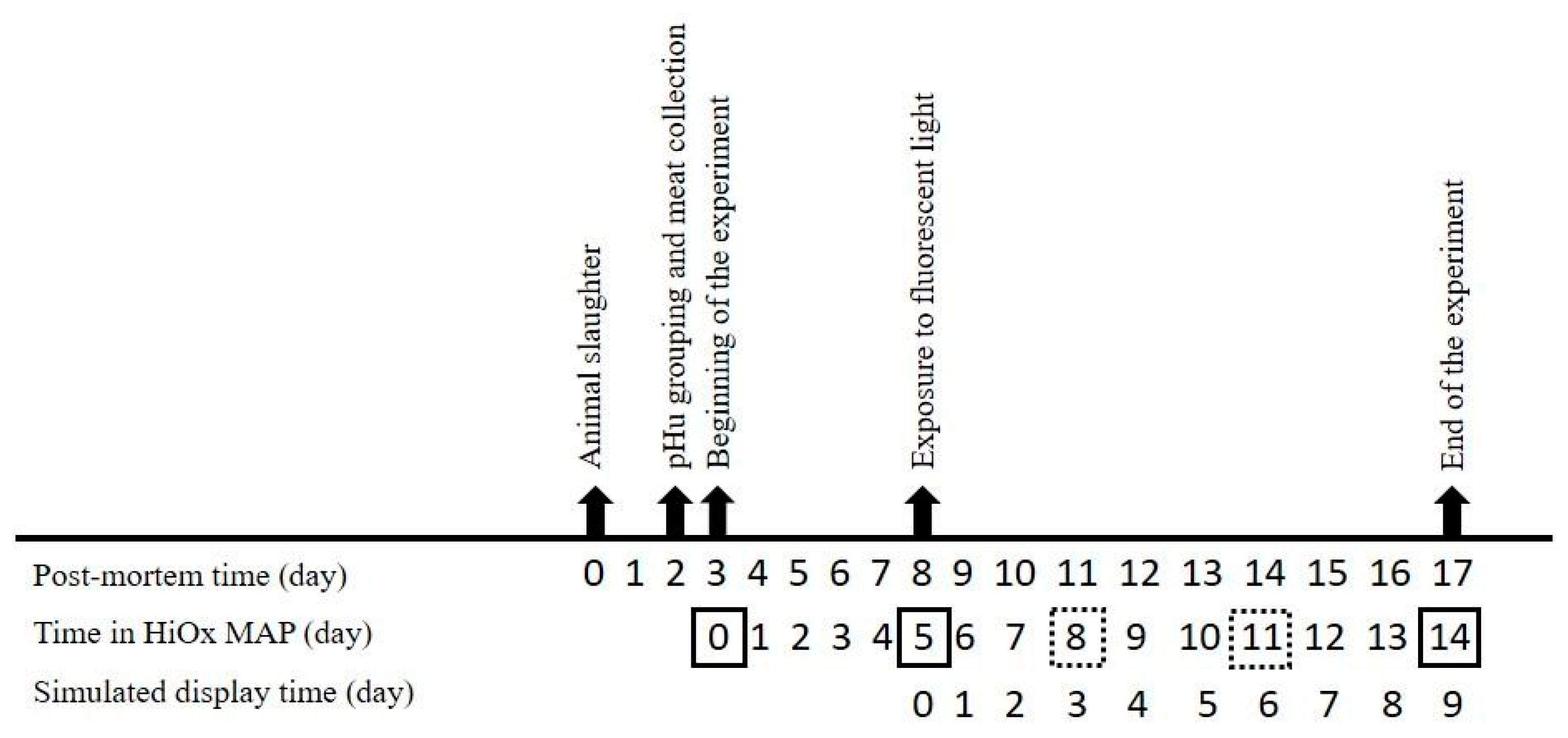
2.1. Raw Materials, Processing, and Store/Display Conditions
2.2. Headspace Composition and pH
2.3. Instrumental Color
2.4. Myoglobin Redox States, Nitric Oxide Metmyoglobin-Reducing Ability and Oxygen Consumption
2.5. Lipid Oxidation
2.6. Experimental Design and Statistical Analysis
3. Results
3.1. Muscle pH and Headspace Gas Composition
3.2. Surface Redness
3.3. Oxidative Stability (Surface MetMb, MRA, and TBARS)
4. Discussion
4.1. Muscle pH and Headspace Composition
4.2. Color Development and Stability of Antioxidant-Enhanced Steaks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brazilian Beef Exporters Association (ABIEC). Beef Report 2022. Available online: https://www.abiec.com.br/en/publicacoes/beef-report-2022-2/ (accessed on 27 December 2022).
- ABCZ. Brazilian Cattle. 2019. Available online: http://www.braziliancattle.com.br/upload/materiais-tecnicos-promocionais/1357876166/Pecu%C3%A1ria%20Brasileira%20CARNE%20E%20LEITE.pdf (accessed on 27 December 2022).
- Ferraz, J.B.S.; Felício, P.E. Production systems–An example from Brazil. Meat Sci. 2010, 84, 238–243. [Google Scholar]
- Rotta, P.P.; Prado, R.M.; Prado, I.N.; Valero, M.V.; Visentainer, J.V.; Silva, R.R. The Effects of Genetic Groups, Nutrition, Finishing Systems and Gender of Brazilian Cattle on Carcass Characteristics and Beef Composition and Appearance: A Review. Asian–Australas. J. Anim. Sci. 2009, 22, 1718–1734. [Google Scholar]
- Ponnampalam, E.N.; Hopkins, D.L.; Bruce, H.; Li, D.; Baldi, G.; Bekhit, A.E.-D.A. Causes and Contributing Factors to “Dark Cutting” Meat: Current Trends and Future Directions: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 400–430. [Google Scholar]
- Ramos, P.M.; dos Santos-Donado, P.R.; de Oliveira, G.M.; Contreras–Castillo, C.J.; Scheffler, T.L.; Silva, S.D.L.E.; Martello, L.S.; Delgado, E.F. Beef of Nellore cattle has limited tenderization despite pH decline in Longissimus lumborum. Sci. Agricola 2022, 79, e202003402022. [Google Scholar]
- Wicks, J.; Beline, M.; Gomez, J.F.M.; Luzardo, S.; Silva, S.L.; Gerrard, D. Muscle Energy Metabolism, Growth, and Meat Quality in Beef Cattle. Agriculture 2019, 9, 195. [Google Scholar]
- McKeith, R.O.; King, D.A.; Grayson, A.L.; Shackelford, S.D.; Gehring, K.B.; Savell, J.; Wheeler, T.L. Mitochondrial abundance and efficiency contribute to lean color of dark cutting beef. Meat Sci. 2016, 116, 165–173. [Google Scholar]
- Tang, J.; Faustman, C.; Hoagland, T.A.; Mancini, R.A.; Seyfert, M.; Hunt, M.C. Postmortem oxygen consumption by mitochondria and its effects on myoglobin form and stability. J. Agric. Food Chem. 2005, 53, 1223–1230. [Google Scholar]
- Barón, C.L.C.; dos Santos-Donado, P.R.; Ramos, P.M.; Donado-Pestana, C.M.; Delgado, E.F.; Contreras-Castillo, C.J. Influence of ultimate pH on biochemistry and quality of Longissimus lumborum steaks from Nellore bulls during ageing. Int. J. Food Sci. Technol. 2021, 56, 3333–3343. [Google Scholar]
- Hughes, J.M.; Clarke, F.; Li, Y.; Purslow, P.; Warner, R. Differences in light scattering between pale and dark beef longissimus thoracis muscles are primarily caused by differences in the myofilament lattice, myofibril and muscle fibre transverse spacings. Meat Sci. 2019, 149, 96–106. [Google Scholar]
- Boito, B.; Lisbinski, E.; Campo, M.D.M.; Guerrero, A.; Resconi, V.; de Oliveira, T.E.; Barcellos, J.O.J. Perception of beef quality for Spanish and Brazilian consumers. Meat Sci. 2021, 172, 108312. [Google Scholar]
- Carpenter, C.E.; Cornforth, D.P.; Whittier, D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001, 57, 359–363. [Google Scholar]
- Lu, X.; Cornforth, D.P.; Carpenter, C.E.; Zhu, L.; Luo, X. Effect of oxygen concentration in modified atmosphere packaging on color changes of the M. longissimus thoraces et lumborum from dark cutting beef carcasses. Meat Sci. 2020, 161, 107999. [Google Scholar]
- Yang, X.; Wang, J.; Holman, B.W.B.; Liang, R.; Chen, X.; Luo, X.; Zhu, L.; Hopkins, D.L.; Zhang, Y. Investigation of the physicochemical, bacteriological, and sensory quality of beef steaks held under modified atmosphere packaging and representative of different ultimate pH values. Meat Sci. 2021, 174, 108416. [Google Scholar]
- Monteiro, P.A.M.; Maciel, I.C.F.; Alvarenga, R.C.; Oliveira, A.L.; Barbosa, F.; Guimarães, S.T.; Souza, F.A.; Lanna, D.P.D.; Rodrigues, B.M.; Lopes, L.S. Carcass traits, fatty acid profile of beef, and beef quality of Nellore and Angus x Nellore crossbred young bulls finished in a feedlot. Livest. Sci. 2022, 256, 104829. [Google Scholar]
- Steele, K.S.; Weber, M.J.; Boyle, E.A.E.; Hunt, M.C.; Lobaton-Sulabo, A.S.; Cundith, C.; Hiebert, Y.H.; Abrolat, K.A.; Attey, J.M.; Clark, S.D.; et al. Shelf life of fresh meat products under LED or fluorescent lighting. Meat Sci. 2016, 117, 75–84. [Google Scholar]
- Zainudin, M.A.M.; Poojary, M.M.; Jongberg, S.; Lund, M.N. Light exposure accelerates oxidative protein polymerization in beef stored in high oxygen atmosphere. Food Chem. 2019, 299, 125132. [Google Scholar]
- Allen, K.; Cornforth, D. Comparison of spice-derived antioxidants and metal chelators on fresh beef color stability. Meat Sci. 2010, 85, 613–619. [Google Scholar]
- Knock, R.C.; Seyfert, M.; Hunt, M.C.; Dikeman, M.E.; Mancini, R.A.; Unruh, J.A.; Higgins, J.J.; Monderen, R.A. Effects of potassium lactate, sodium chloride, sodium tripolyphosphate, and sodium acetate on colour, colour stability, and oxidative properties of injection-enhanced beef rib steaks. Meat Sci. 2006, 74, 312–318. [Google Scholar]
- Ramanathan, R.; Hunt, M.C.; Mancini, R.A.; Nair, M.N.; Denzer, M.L.; Suman, S.P.; Mafi, G.G. Recent Updates in Meat Color Research: Integrating Traditional and High-Throughput Approaches. Meat Muscle Biol. 2020, 4. [Google Scholar] [CrossRef]
- 29Ramanathan, R.; Mancini, R.A.; Naveena, B.M.; Konda, M.K.R. Effects of lactate-enhancement on surface reflectance and absorbance properties of beef longissimus steaks. Meat Sci. 2010, 84, 219–226. [Google Scholar]
- Djenane, D.; Sánchez-Escalante, A.; Beltrán, J.A.; Roncalés, P. Extension of the shelf life of beef steaks packaged in a modified atmosphere by treatment with rosemary and displayed under UV-free lighting. Meat Sci. 2003, 64, 417–426. [Google Scholar]
- Poleti, M.D.; Moncau, C.T.; Silva-Vignato, B.; Rosa, A.F.; Lobo, A.R.; Cataldi, T.R.; Negrão, J.A.; Silva, S.L.; Eler, J.P.; Balieiro, J.C.C. Label-free quantitative proteomic analysis reveals muscle contraction and metabolism proteins linked to ultimate pH in bovine skeletal muscle. Meat Sci. 2018, 145, 209–219. [Google Scholar]
- Rodrigues, R.T.S.; Chizzotti, M.L.; Vital, C.E.; Baracat-Pereira, M.C.; Barros, E.; Busato, K.C.; Gomes, R.A.; Ladeira, M.M.; Martins, T.S. Differences in beef quality between Angus (Bos taurus taurus) and Nellore (Bos taurus indicus) cattle through a proteomic and phosphoproteomic approach. PLoS ONE 2017, 12, e0170294. [Google Scholar]
- American Meat Science Association–International [AMSA]. Meat Color Measurement Guidelines, 1st ed.; AMSA: Champaign, IL, USA, 2012; p. 136. [Google Scholar]
- Selani, M.M.; Shirado, G.A.N.; Margiotta, G.B.; Rasera, M.L.; Marabesi, A.C.; Piedade, S.M.S.; Contreras-Castillo, C.J.; Canniatti-Brazaca, S.G. Pineapple by-product and canola oil as partial fat replacers in low-fat beef burger: Effects on oxidative stability, cholesterol content and fatty acid profile. Meat Sci. 2016, 115, 9–15. [Google Scholar]
- Kim, Y.H.; Hunt, M.C.; Mancini, R.A.; Seyfert, M.; Loughin, T.M.; Kropf, D.H.; Smith, J.S. Mechanism for lactate-color stabilization in injection-enhanced beef. J. Agric. Food Chem. 2006, 54, 7856–7862. [Google Scholar]
- Kim, Y.H.; Keeton, J.T.; Smith, S.B.; Maxim, J.E.; Yang, H.S.; Savell, J.W. Evaluation of antioxidant capacity and colour stability of calcium lactate enhancement on fresh beef under highly oxidising conditions. Food Chem. 2009, 115, 272–278. [Google Scholar]
- Cruzen, S.M.; Kim, Y.B.K.; Lonergan, S.M.; Grubbs, J.K.; Fritchen, A.N.; Huff-Lonergan, E. Effect of early postmortem enhancement of calcium lactate/phosphate on quality attributes of beef round muscles under different packaging systems. Meat Sci. 2015, 101, 63–72. [Google Scholar]
- English, A.R.; Mafi, G.G.; VanOverbeke, D.L.; Ramanathan, R. Effects of extended aging and modified atmospheric packaging on beef top loin steak color. J. Anim. Sci. 2016, 94, 1727–1737. [Google Scholar]
- Bendall, J.R.; Taylor, A.A. Consumption of oxygen by the muscles of beef animals and related species. II. Consumption of oxygen by post-rigor muscle. J. Sci. Food Agric. 1972, 23, 707–719. [Google Scholar]
- Ercolini, D.; Russo, F.; Torrieri, E.; Masi, P.; Villani, F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 2006, 72, 4663–4671. [Google Scholar]
- Ramanathan, R.; Nair, M.; Hunt, M.; Suman, S. Mitochondrial functionality and beef colour: A review of recent research. South Afr. J. Anim. Sci. 2019, 49, 9–19. [Google Scholar]
- Mancini, R.A.; Ramanathan, R. Effects of postmortem storage time on color and mitochondria in beef. Meat Sci. 2014, 98, 65–70. [Google Scholar]
- Ramanathan, R.; Mancini, R.A.; Konda, M.R. Effects of Lactate on Beef Heart Mitochondrial Oxygen Consumption and Muscle Darkening. J. Agric. Food Chem. 2009, 57, 1550–1555. [Google Scholar]
- Wu, S.; Luo, X.; Yang, X.; Hopkins, D.L.; Mao, Y.; Zhang, Y. Understanding the development of color and color stability of dark cutting beef based on mitochondrial proteomics. Meat Sci. 2010, 163, 108046. [Google Scholar]
- Ramanathan, R.; Mancini, R.A.; Suman, S.P.; Cantino, M.E. Effects of 4-hydroxy-2-nonenal on beef heart mitochondrial ultrastructure, oxygen consumption, and metmyoglobin reduction. Meat Sci. 2012, 90, 564–571. [Google Scholar]
- Echevarne, C.; Renerre, M.; Labas, R. Metmyoglobin reductase activity in bovine muscles. Meat Sci. 1990, 27, 161–172. [Google Scholar]
- Jacob, R. Implications of the variation in bloom properties of red meat: A review. Meat Sci. 2020, 162, 108040. [Google Scholar]
- Ramanathan, R.; Mancini, R.A.; Konda, M.K.R.; Bailey, K.; More, S.; Mafi, G.G. Evaluating the failure to bloom in dark-cutting and lactate-enhanced beef longissimus steaks. Meat Sci. 2022, 184, 108684. [Google Scholar]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar]
- English, A.R.; Wills, K.M.; Harsh, B.N.; Mafi, G.G.; Vanoverbeke, D.L.; Ramanathan, R. Effects of aging on the fundamental color chemistry of dark-cutting beef. J. Anim. Sci. 2016, 94, 4040–4048. [Google Scholar]
- Abril, M.; Campo, M.M.; Önenç, A.; Sañudo, C.; Albertí, P.; Negueruela, A.I. Beef colour evolution as a function of ultimate pH. Meat Sci. 2001, 58, 69–78. [Google Scholar]
- Loredo-Osti, J.; Sánchez-López, E.; Barreras-Serrano, A.; Figueroa-Saavedra, F.; Pérez-Linares, C.; Ruiz-Albarrán, M.; Dominguez-Muñoz, M.A. An evaluation of environmental, intrinsic and pre-and post-slaughter risk factors associated to dark-cutting beef in a Federal Inspected Type slaughter plant. Meat Sci. 2019, 150, 85–92. [Google Scholar]
- Xiong, Y.L. Role of myofibrillar proteins in water-binding in brine-enhanced meats. Food Res. Int. 2005, 38, 281–287. [Google Scholar]
- Wills, K.M.; Mitacek, R.M.; Mafi, G.G.; Vanoverbeke, D.L.; Jaroni, D.; Jadeja, R.; Ramanathan, R. Improving the lean muscle color of dark-cutting beef by aging, antioxidant-enhancement, and modified atmospheric packaging1. J. Anim. Sci. 2017, 95, 5378–5387. [Google Scholar]
- dos Santos-Donado, P.R.; Donado-Pestana, C.M.; Tanaka, F.A.O.; Venturini, A.C.; Delgado, E.F.; Contreras-Castillo, C.J. Effects of high-oxygen, carbon monoxide modified atmospheres and vacuum packaging on quality of Longissimus thoracis et lumborum steaks from Nellore cows during ageing. Food Res. Int. 2021, 143, 110226. [Google Scholar]
- Ma, D.; Kim, Y.H.B.; Cooper, B.; Oh, J.-H.; Chun, H.; Choe, J.-H.; Schoonmaker, J.P.; Ajuwon, K.; Min, B. Metabolomics Profiling to Determine the Effect of Postmortem Aging on Color and Lipid Oxidative Stabilities of Different Bovine Muscles. J. Agric. Food Chem. 2017, 65, 6708–6716. [Google Scholar]
- Purslow, P.P.; Gagaoua, M.; Warner, R.D. Insights on meat quality from combining traditional studies and proteomics. Meat Sci. 2021, 174, 108423. [Google Scholar]
- Yang, X.; Zhang, Y.; Zhu, L.; Mao, Y.; Dong, P.; Luo, X. Proteomic Study of the Color Stability of High-Oxygen Modified Atmosphere Packaged Steak during Chilled Storage. Food Sci. 2019, 40, 231–237. [Google Scholar]
- Suman, S.P.; Faustman, C.; Stamer, S.L.; Liebler, D.C. Proteomics of lipid oxidation-induced oxidation of porcine and bovine oxymyoglobins. Proteomics 2007, 7, 628–640. [Google Scholar]
- Bergamaschi, M.; Pizza, A. Effect of pork meat pH on iron release from heme molecule during cooking. J. Life Sci. 2011, 5, 376–380. [Google Scholar]
- Barcellos, V.C.; Mottin, C.; Passetti, R.A.C.; Guerrero, A.; Eiras, C.E.; Prohmann, P.E.F.; Vital, A.C.; Prado, I.N.D. Carcass characteristics and sensorial evaluation of meat from Nellore steers and crossbred Angus vs. Nellore bulls. Acta Sci. Anim. Sci. 2017, 39, 437–448. [Google Scholar]
- Li, Y.; Liu, S. Reducing lipid peroxidation for improving colour stability of beef and lamb: On-farm considerations. J. Sci. Food Agric. 2011, 92, 719–729. [Google Scholar]
- Ramanathan, R.; Mancini, R.A.; Suman, S.P.; Beach, C.M. Covalent binding of 4-hydroxy-2-nonenal to lactate dehydrogenase decreases nadh formation and metmyoglobin reducing activity. J. Agric. Food Chem. 2014, 62, 2112–2117. [Google Scholar]
- Vander Jagt, D.L.; Hunsaker, L.A.; Vander Jagt, T.J.; Gomez, M.S.; Gonzales, D.M.; Deck, L.M.; Royer, R.E. Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes. Biochem Pharmacol. 1997, 53, 1133–1140. [Google Scholar]
| Ingredients | Concentration in the Brine (%) | Final Residual Concentration of the Brine in the Enhanced Muscles (%) § | ||
|---|---|---|---|---|
| Normal pHu (n = 6) | Intermediate pHu (n = 6) | High pHu (n = 6) | ||
| Potassium L-lactate | 27.50 | 2.16 | 2.29 | 2.31 |
| Sodium chloride | 3.30 | 0.26 | 0.27 | 0.28 |
| Sodium tripolyphosphate | 3.30 | 0.26 | 0.27 | 0.28 |
| Rosemary oil extract | 0.64 | 0.05 | 0.05 | 0.05 |
| Cold mineral water | 65.26 | 5.13 | 5.43 | 5.49 |
| Total | 100.00 | 7.86 † | 8.32 † | 8.41 † |
| Attribute | pHu | Time in HiOx-MAP (Day) | SE | ||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 8 | 11 | 14 | |||
| pH | Normal | 5.47 ax | 5.58 ax | 5.51 ax | 5.76 bx | 5.82 bx | 0.05 |
| Intermediate | 5.83 aby | 5.87 abcy | 5.77 ay | 5.97 bcy | 6.00 cy | ||
| High | 6.28 abz | 6.36 abz | 6.28 az | 6.23 az | 6.41 bz | ||
| MAP O2 | Normal | 80.4 b | 80.1 b | 78.8 a | 80.2 by | 78.9 ay | 0.3 |
| Intermediate | 81.1 d | 80.3 cd | 78.5 a | 79.8 bcy | 78.9 aby | ||
| High | 81.2 c | 79.7 b | 78.8 ab | 78.8 abx | 78.0 ax | ||
| MAP CO2 | Normal | 19.9 a | 20.7 b | 20.7 bx | 20.4 abx | 20.4 abx | 0.2 |
| Intermediate | 20.0 a | 20.6 ab | 20.7 bx | 20.9 bx | 20.9 bx | ||
| High | 19.8 a | 20.7 b | 21.6 cy | 22.2 cy | 22.2 cy | ||
| L* (Lightness) | a* (Redness) | b* (Yellowness) | Hue Angle | Chroma (Saturation) | DeoxyMb (K/S473/K/S525) | MetMb (K/S572/K/S525) | OxyMb (K/S610/K/S525) | |
|---|---|---|---|---|---|---|---|---|
| Control | 46.50 b | 24.81 b | 17.00 b | 34.30 b | 30.11 b | 0.94 b | 1.23 | 0.21 a |
| Enhanced | 43.28 a | 22.77 a | 15.25 a | 33.72 a | 27.42 a | 0.93 a | 1.23 | 0.30 b |
| SE | 0.263 | 0.261 | 0.211 | 0.179 | 0.324 | 0.002 | 0.004 | 0.005 |
| Attribute | pHu | Time in HiOx-MAP (Day) | SE | ||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 8 | 11 | 14 | |||
| a* (redness) | Normal | 24.78 bz | 24.62 b | 21.17 ax | 20.70 ax | 21.65 ax | 0.74 |
| Intermediate | 22.19 ay | 25.42 b | 24.36 by | 25.10 by | 25.85 by | ||
| High | 18.37 ax | 26.28 b | 25.13 by | 25.68 by | 25.60 by | ||
| b* (yellowness) | Normal | 16.76 z | 16.75 | 15.25 x | 15.46 x | 15.86 x | 0.59 |
| Intermediate | 14.43 ay | 17.61 b | 17.31 by | 17.32 by | 18.23 by | ||
| High | 10.47 ax | 17.21 b | 15.85 bxy | 17.02 bxy | 16.21 bx | ||
| Hue angle | Normal | 33.91 ay | 34.15 axy | 35.64 by | 36.74 by | 36.13 by | 0.45 |
| Intermediate | 32.86 ay | 34.66 by | 35.32 by | 34.61 bx | 35.12 by | ||
| High | 29.54 ax | 33.15 bx | 32.34 bx | 33.49 bx | 32.53 bx | ||
| Chroma (color saturation) | Normal | 29.92 bz | 29.79 b | 26.11 ax | 25.85 ax | 26.85 ax | 0.91 |
| Intermediate | 26.48 ay | 30.93 b | 29.89 by | 30.51 by | 31.65 by | ||
| High | 21.16 ax | 31.42 b | 29.74 by | 30.81 by | 30.37 by | ||
| DeoxyMb (K/S473/K/S525) | Normal | 0.94 bz | 0.93 abx | 0.93 abx | 0.93 abx | 0.92 ax | 0.01 |
| Intermediate | 0.92 ay | 0.94 bx | 0.94 bx | 0.94 by | 0.93 abx | ||
| High | 0.88 ax | 0.95 by | 0.96 by | 0.95 by | 0.94 by | ||
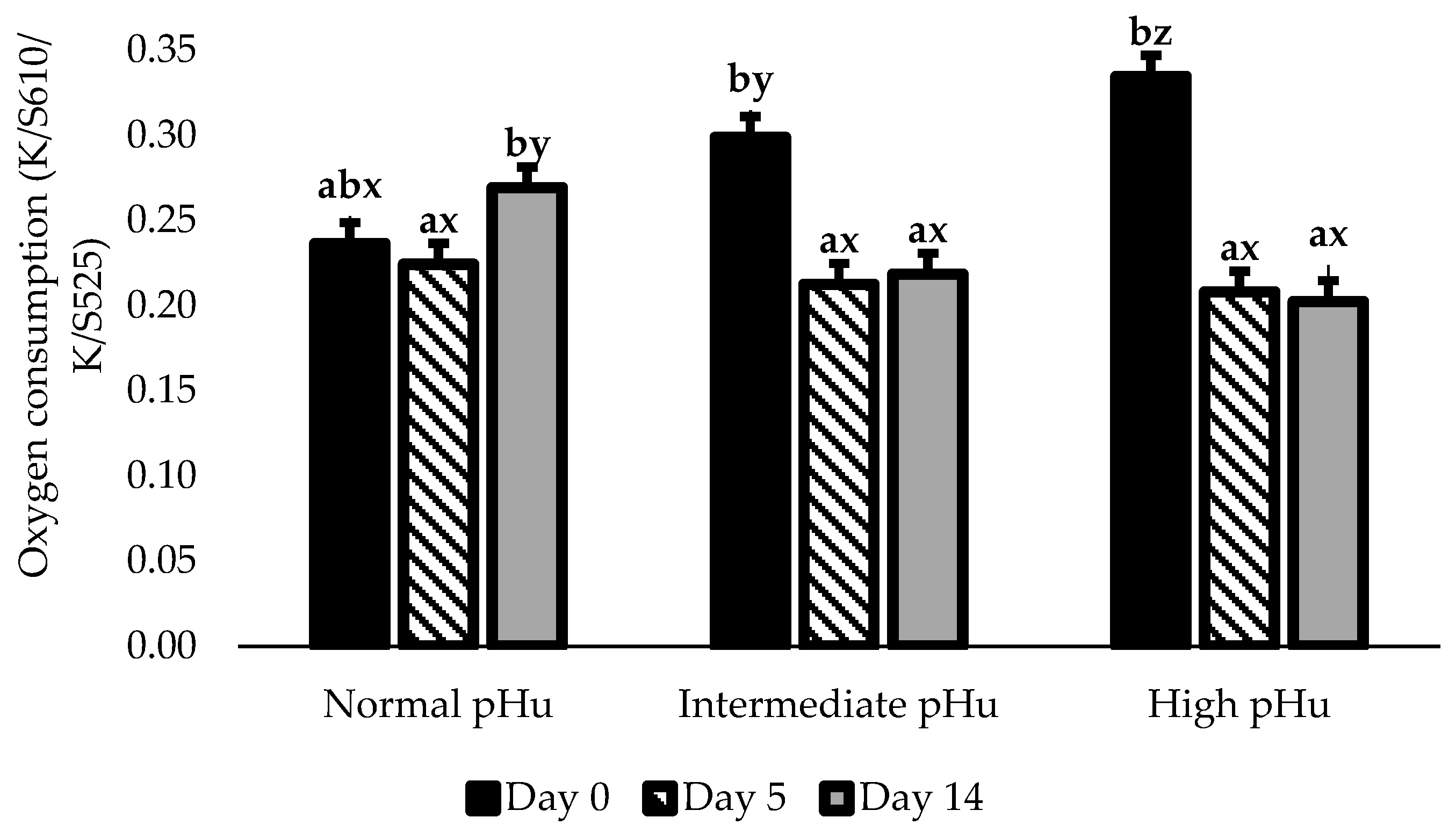
| OxyMb (K/S610/K/S525) | Normal | 0.25 abx | 0.23 a | 0.27 bcy | 0.29 cy | 0.27 bcy | 0.01 |
| Intermediate | 0.30 by | 0.22 a | 0.24 ax | 0.23 ax | 0.22 ax | ||
| High | 0.39 bz | 0.22 a | 0.25 axy | 0.23 ax | 0.23 ax | ||
| MetMb (K/S572/K/S525) | Normal | 1.29 cy | 1.22 bx | 1.17 ax | 1.14 ax | 1.15 ax | 0.01 |
| Intermediate | 1.28 cxy | 1.25 bcxy | 1.21 ay | 1.21 ay | 1.22 aby | ||
| High | 1.25 abx | 1.27 by | 1.26 abz | 1.26 abz | 1.24 ay | ||
| Attribute | pHu | Group | Time in HiOx-MAP (Day) | SE | ||
|---|---|---|---|---|---|---|
| 0 | 5 | 14 | ||||
| MRA (NO-MetMb, K/S572/K/S525) | Normal | Control | 0.88 bw | 0.87 bw | 0.75 aw | 0.02 |
| Enhanced | 0.89 aw | 0.89 aw | 0.89 ax | |||
| Intermediate | Control | 0.90 aw | 0.85 aw | 0.89 ax | ||
| Enhanced | 0.93 awx | 0.95 ax | 0.90 ax | |||
| High | Control | 0.96 ax | 0.95 ax | 1.01 ay | ||
| Enhanced | 0.98 ax | 1.01 ay | 1.01 ay | |||
| TBARS (mg malonaldehyde/kg steak) | Normal | Control | 0.15 ay | 0.21 bz | 0.33 cy | 0.01 |
| Enhanced | 0.10 awx | 0.18 byz | 0.22 bx | |||
| Intermediate | Control | 0.08 aw | 0.17 bxy | 0.22 cx | ||
| Enhanced | 0.12 axy | 0.12 aw | 0.12 aw | |||
| High | Control | 0.09 aw | 0.15 bwxy | 0.15 bw | ||
| Enhanced | 0.13 axy | 0.14 awx | 0.16 aw | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, C.C.d.S.; Guimarães, K.A.; Delgado, E.F.; Balieiro, J.C.d.C.; Venturini, A.C.; Castillo, C.J.C. Improvement of Color and Oxidative Stabilities in Nellore Bull Dark Meat in High-Oxygen Package by Lactate and Rosemary Oil Extract. Foods 2023, 12, 1302. https://doi.org/10.3390/foods12061302
Ribeiro CCdS, Guimarães KA, Delgado EF, Balieiro JCdC, Venturini AC, Castillo CJC. Improvement of Color and Oxidative Stabilities in Nellore Bull Dark Meat in High-Oxygen Package by Lactate and Rosemary Oil Extract. Foods. 2023; 12(6):1302. https://doi.org/10.3390/foods12061302
Chicago/Turabian StyleRibeiro, Caio César de Sousa, Kathelyn Araújo Guimarães, Eduardo Francisquine Delgado, Júlio César de Carvalho Balieiro, Anna Cecilia Venturini, and Carmen Josefina Contreras Castillo. 2023. "Improvement of Color and Oxidative Stabilities in Nellore Bull Dark Meat in High-Oxygen Package by Lactate and Rosemary Oil Extract" Foods 12, no. 6: 1302. https://doi.org/10.3390/foods12061302
APA StyleRibeiro, C. C. d. S., Guimarães, K. A., Delgado, E. F., Balieiro, J. C. d. C., Venturini, A. C., & Castillo, C. J. C. (2023). Improvement of Color and Oxidative Stabilities in Nellore Bull Dark Meat in High-Oxygen Package by Lactate and Rosemary Oil Extract. Foods, 12(6), 1302. https://doi.org/10.3390/foods12061302









