Foliar Selenate and Zinc Oxide Separately Applied to Two Pea Varieties: Effects on Growth Parameters and Accumulation of Minerals and Macronutrients in Seeds under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Design and Sample Preparation
2.3. Growth Measures
2.4. Measured Soluble Solids and Protein
2.5. Concentration of Minerals
2.6. Statistical Analysis
3. Results
3.1. General Effects
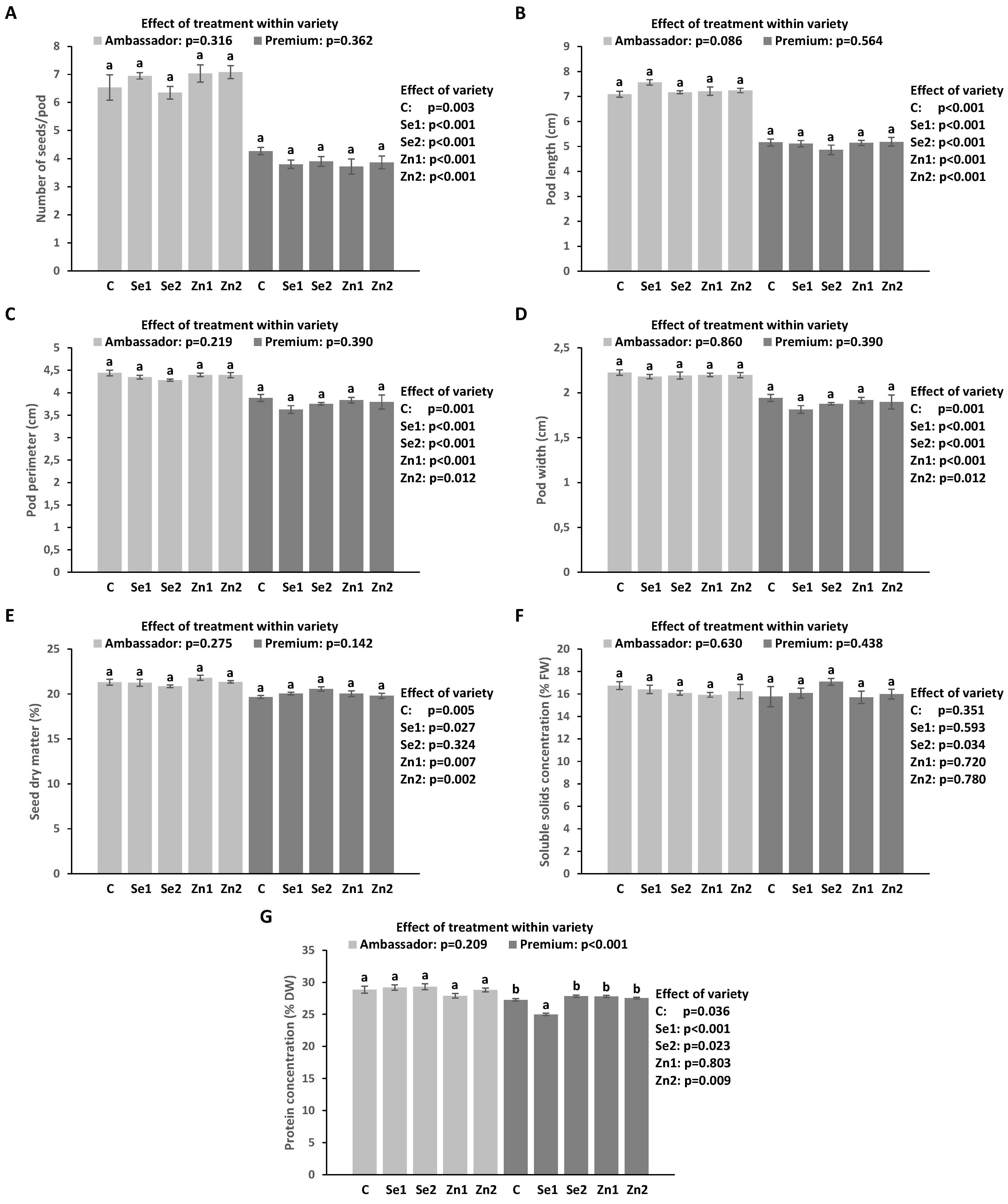
3.2. Growth Parameters
3.3. Soluble Solid Levels (SSC) in Seeds
3.4. Protein Levels in Seeds
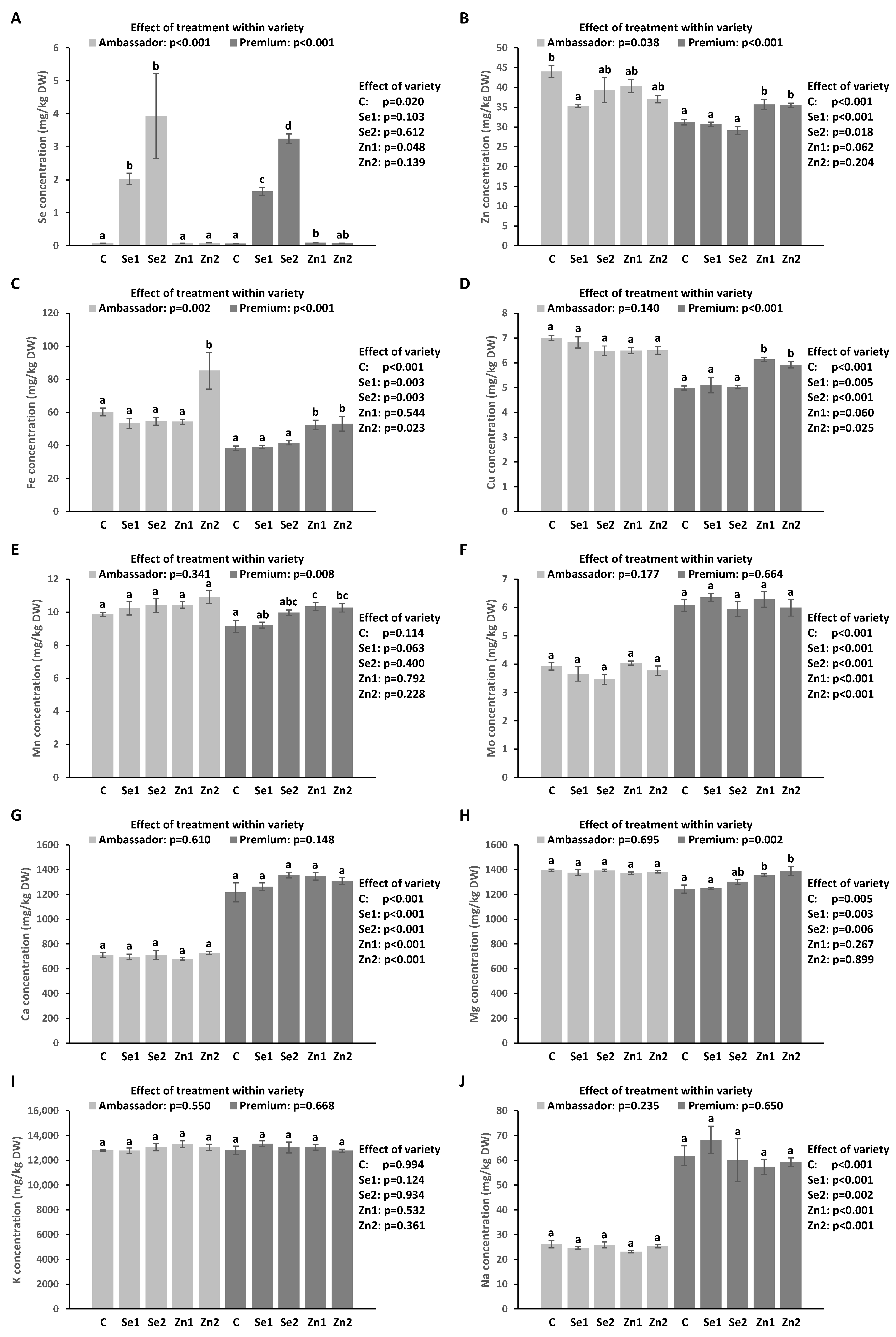
3.5. Concentration of Trace Elements in Seeds
3.6. Concentration of Macro Elements in Seeds
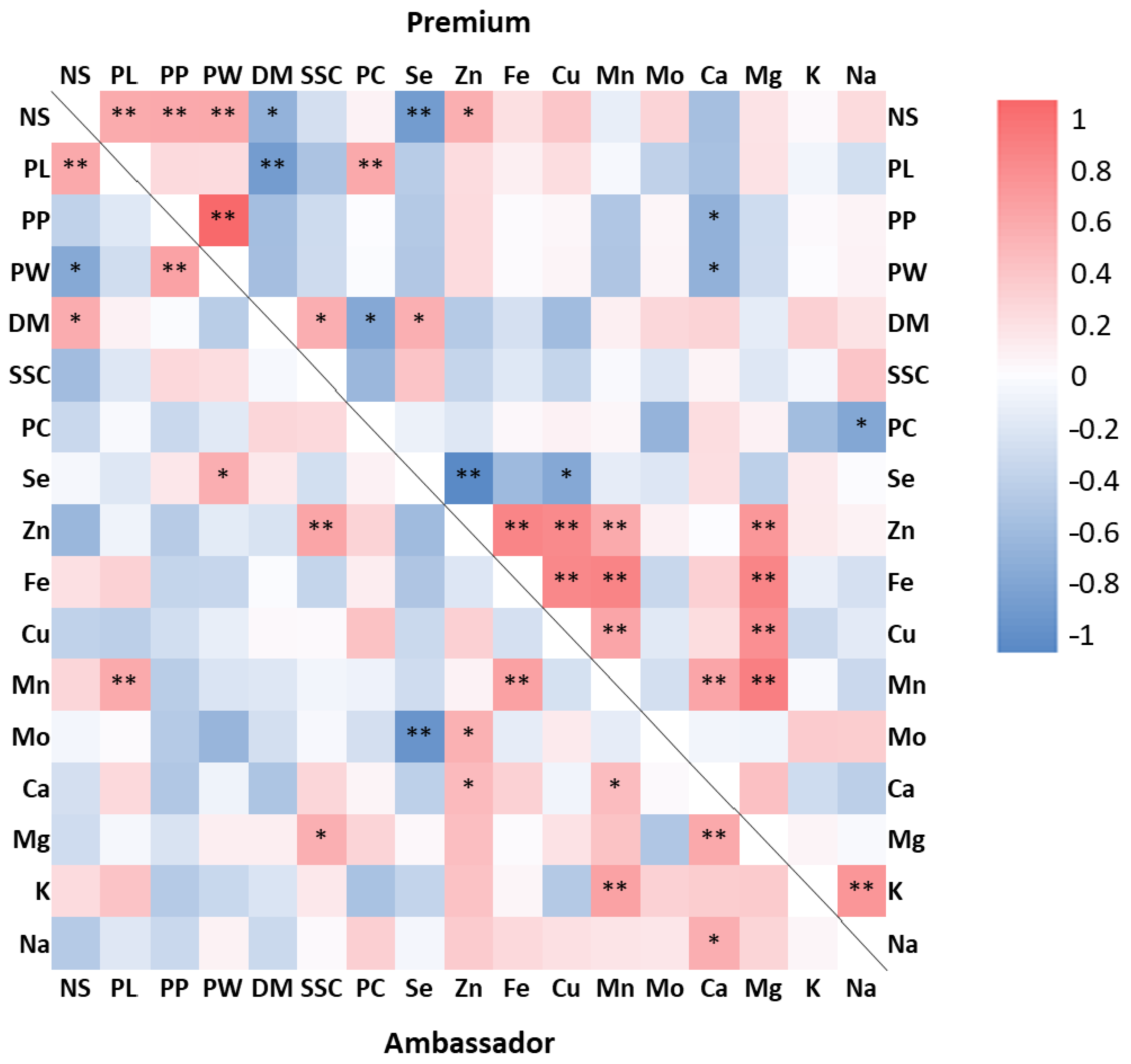
3.7. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R.S. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The Metal of Life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Du Laing, G.; Petrovic, M.; Lachat, C.; De Boevre, M.; Klingenberg, G.J.; Sun, Q.; De Saeger, S.; De Clercq, J.; Ide, L.; Vandekerckhove, L.; et al. Course and Survival of COVID-19 Patients with Comorbidities in Relation to the Trace Element Status at Hospital Admission. Nutrients 2021, 13, 3304. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium Intake, Status, and Health: A Complex Relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Agnew, U.M.; Slesinger, T.L. Zinc Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shreenath, A.P.; Ameer, M.A.; Dooley, J. Selenium Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kumssa, D.B.; Joy, E.J.M.; Ander, E.L.; Watts, M.J.; Young, S.D.; Walker, S.; Broadley, M.R. Dietary Calcium and Zinc Deficiency Risks Are Decreasing but Remain Prevalent. Sci. Rep. 2015, 5, 10974. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of Heavy Metals in the Soils of the European Union and Proposed Priority Areas for Detailed Assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association: Brussels, Belgium; International Fertilizer Industry Association: Paris, France, 2008. [Google Scholar]
- Chilimba, A.D.C.; Young, S.D.; Black, C.R.; Rogerson, K.B.; Ander, E.L.; Watts, M.J.; Lammel, J.; Broadley, M.R. Maize Grain and Soil Surveys Reveal Suboptimal Dietary Selenium Intake Is Widespread in Malawi. Sci. Rep. 2011, 1, 72. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium Cycling across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, B. A Review of Zinc Nutrition and Plant Breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium Deficiency Risk Predicted to Increase under Future Climate Change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic Biofortification of Cereals with Zinc: A Review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- de Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic Biofortification of Crops to Fight Hidden Hunger in Sub-Saharan Africa. Glob. Food Sec. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Delaqua, D.; Carnier, R.; Berton, R.S.; Corbi, F.C.A.; Coscione, A.R. Increase of Selenium Concentration in Wheat Grains through Foliar Application of Sodium Selenate. J. Food Compos. Anal. 2021, 99, 103886. [Google Scholar] [CrossRef]
- Sattar, A.; Wang, X.; Ul-Allah, S.; Sher, A.; Ijaz, M.; Irfan, M.; Abbas, T.; Hussain, S.; Nawaz, F.; Al-Hashimi, A.; et al. Foliar Application of Zinc Improves Morpho-Physiological and Antioxidant Defense Mechanisms, and Agronomic Grain Biofortification of Wheat (Triticum aestivum L.) under Water Stress. Saudi J. Biol. Sci. 2021, 29, 1699–1706. [Google Scholar] [CrossRef]
- Lyons, G. Biofortification of Cereals with Foliar Selenium and Iodine Could Reduce Hypothyroidism. Front. Plant Sci. 2018, 9, 730. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From Plant Surface to Plant Metabolism: The Uncertain Fate of Foliar-Applied Nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, G. Biofortification of Pulses and Legumes to Enhance Nutrition. Heliyon 2020, 6, e03682. [Google Scholar] [CrossRef]
- Rehman, H.M.; Cooper, J.W.; Lam, H.M.; Yang, S.H. Legume Biofortification Is an Underexploited Strategy for Combatting Hidden Hunger. Plant. Cell Environ. 2018, 42, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D.; Ambrose, M.J.; Domoney, C.; Burstin, J. Pea. In Genetics, Genomics and Breeding of Cool Season Grain Legumes; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–49. [Google Scholar]
- Sahruzaini, N.A.; Rejab, N.A.; Harikrishna, J.A.; Khairul Ikram, N.K.; Ismail, I.; Kugan, H.M.; Cheng, A. Pulse Crop Genetics for a Sustainable Future: Where We Are Now and Where We Should Be Heading. Front. Plant Sci. 2020, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Pinto, E.; Vasconcelos, M.W. Legumes as a Cornerstone of the Transition Toward More Sustainable Agri-Food Systems and Diets in Europe. Front. Sustain. Food Syst. 2021, 5, 694121. [Google Scholar] [CrossRef]
- Powers, S.E.; Thavarajah, D. Checking Agriculture’s Pulse: Field Pea (Pisum sativum L.), Sustainability, and Phosphorus Use Efficiency. Front. Plant Sci. 2019, 10, 1489. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the Health Benefits of Peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The Health Benefits, Functional Properties, Modifications, and Applications of Pea (Pisum sativum L.) Protein: Current Status, Challenges, and Perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef] [PubMed]
- Kumari, T.; Deka, S.C. Potential Health Benefits of Garden Pea Seeds and Pods: A Review. Legum. Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Malka, M.; Du Laing, G.; Bohn, T. Separate Effects of Foliar Applied Selenate and Zinc Oxide on the Accumulation of Macrominerals, Macronutrients and Bioactive Compounds in Two Pea (Pisum sativum L.) Seed Varieties. Plants 2022, 11, 2009. [Google Scholar] [CrossRef]
- Malka, M.; Du Laing, G.; Li, J.; Bohn, T. Separate Foliar Sodium Selenate and Zinc Oxide Application Enhances Se but Not Zn Accumulation in Pea (Pisum sativum L.) Seeds. Front. Plant Sci. 2022, 13, 968324. [Google Scholar] [CrossRef] [PubMed]
- Malka, M.; Du Laing, G.; Kurešová, G.; Hegedűsová, A.; Bohn, T. Enhanced Accumulation of Phenolics in Pea (Pisum sativum L.) Seeds upon Foliar Application of Selenate or Zinc Oxide. Front. Nutr. 2023, 10, 1083253. [Google Scholar] [CrossRef]
- Varényiová, M.; Ducsay, L.; Ryant, P. Sulphur Nutrition and Its Effect on Yield and Oil Content of Oilseed Rape (Brassica napus L.). Acta Univ. Agric. Silvic. Mendelianae Brun. 2017, 65, 555–562. [Google Scholar] [CrossRef]
- Ducsay, L.; Ložek, O.; Varga, L. The Influence of Selenium Soil Application on Its Content in Spring Wheat. Plant Soil Environ. 2009, 55, 80–84. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein-Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Kaulmann, A.; Jonville, M.C.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Carotenoids, Polyphenols and Micronutrient Profiles of Brassica Oleraceae and Plum Varieties and Their Contribution to Measures of Total Antioxidant Capacity. Food Chem. 2014, 155, 240–250. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rengel, Z. The Effect of Processing on Pisum Sativum L. Biofortified with Sodium Selenate. J. Plant Nutr. Soil Sci. 2018, 181, 932–937. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rengel, Z. Combined Foliar Selenium and Zinc Biofortification in Field Pea (Pisum sativum): Accumulation and Bioavailability in Raw and Cooked Grains. Crop Pasture Sci. 2017, 68, 265–271. [Google Scholar] [CrossRef]
- Hacisalihoglu, G. Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn. Plants 2020, 9, 1471. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in Plants: Boon or Bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Ros, G.H.; van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium Fertilization Strategies for Bio-Fortification of Food: An Agro-Ecosystem Approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Grela, E.R.; Samolińska, W.; Kiczorowska, B.; Klebaniuk, R.; Kiczorowski, P. Content of Minerals and Fatty Acids and Their Correlation with Phytochemical Compounds and Antioxidant Activity of Leguminous Seeds. Biol. Trace Elem. Res. 2017, 180, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Poblaciones, M.J.; Rodrigo, S.; Santamaría, O. Evaluation of the Potential of Peas (Pisum sativum L.) to Be Used in Selenium Biofortification Programs under Mediterranean Conditions. Biol. Trace Elem. Res. 2013, 151, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Gupta, B.; Pathak, G.C. Enhanced Yield and Nutritional Enrichment of Seeds of Pisum Sativum L. through Foliar Application of Zinc. Sci. Hortic. (Amst.) 2013, 164, 474–483. [Google Scholar] [CrossRef]
- Pandey, N.; Gupta, B.; Pathak, G.C. Foliar Application of Zn at Flowering Stage Improves Plant’s Performance, Yield and Yield Attributes of Black Gram. Indian J. Exp. Biol. 2013, 51, 548–555. [Google Scholar]
- Kachinski, W.D.; Ávila, F.W.; dos Reis, A.R.; Muller, M.M.L.; Mendes, M.C.; Petranski, P.H. Agronomic Biofortification Increases Concentrations of Zinc and Storage Proteins in Common Bean (Phaseolus vulgaris L.) Grains. Food Res. Int. 2022, 155, 111105. [Google Scholar] [CrossRef]
- Lara, T.S.; de Lima Lessa, J.H.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium Biofortification of Wheat Grain via Foliar Application and Its Effect on Plant Metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rengel, Z. Soil and Foliar Zinc Biofortification in Field Pea (Pisum sativum L.): Grain Accumulation and Bioavailability in Raw and Cooked Grains. Food Chem. 2016, 212, 427–433. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Reis, A.R. The Molecular Genetics of Zinc Uptake and Utilization Efficiency in Crop Plants. In Plant Micronutrient Use Efficiency; Academic Press: Cambridge, MA, USA, 2018; pp. 87–108. ISBN 9780128122433. [Google Scholar]
- Smrkolj, P.; Germ, M.; Kreft, I.; Stibilj, V. Respiratory Potential and Se Compounds in Pea (Pisum sativum L.) Plants Grown from Se-Enriched Seeds. J. Exp. Bot. 2006, 57, 3595–3600. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Selenium in Soils and Crops, Its Deficiencies in Livestock and Humans: Implications for Management. Commun. Soil Sci. Plant Anal. 2000, 31, 1791–1807. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in Soils, Water and Food Crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- De Groote, H.; Tessema, M.; Gameda, S.; Gunaratna, N.S. Soil Zinc, Serum Zinc, and the Potential for Agronomic Biofortification to Reduce Human Zinc Deficiency in Ethiopia. Sci. Rep. 2021, 11, 8770. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, L.; Liu, M.; Zhou, J.; Pan, W.; Fu, H.; Zhang, X.; Ma, Q.; Wu, L. Glycine-Chelated Zinc Rather than Glycine-Mixed Zinc Has Lower Foliar Phytotoxicity than Zinc Sulfate and Enhances Zinc Biofortification in Waxy Corn. Food Chem. 2022, 370, 131031. [Google Scholar] [CrossRef] [PubMed]
- Kayan, N.; Gulmezoglu, N.; Kaya, M.D. The Optimum Foliar Zinc Source and Level for Improving Zn Content in Seed of Chickpea. Legum. Res. 2015, 38, 826–831. [Google Scholar] [CrossRef]
| pH (H2O) | pH (KCl) | N | P | K | Ca | Mg | Zn | Se | Cox (%) | Humus (%) | Soil Type |
| mg kg−1 | |||||||||||
| 7.55 | 6.36 | 13.3 | 253 | 285 | 5630 | 364 | 2.47 | 0.08 | 1.39 | 4.01 | Gleyic fluvisol |
| Average air temperature (°C) and total rainfall (mm) in brackets | |||||||||||
| April | May | June | |||||||||
| 12.4 (48.9) | 15.2 (57.6) | 19.3 (52.5) | |||||||||
| Treatment | Variety | Treatment × Variety | |
|---|---|---|---|
| DF | 4 | 1 | 4 |
| Number of seeds/pod | NS | <0.001 | NS |
| Pod length (cm) | NS | <0.001 | NS |
| Pod perimeter (cm) | NS | <0.001 | NS |
| Pod width (cm) | NS | <0.001 | NS |
| Seed dry matter (%) | NS | <0.001 | NS |
| Soluble solids (% FW) | NS | NS | NS |
| Protein (% DW) | 0.002 | <0.001 | <0.001 |
| Se (mg/kg DW) | <0.001 | NS | NS |
| Zn (mg/kg DW) | 0.002 | <0.001 | 0.002 |
| Fe (mg/kg DW) | <0.001 | <0.001 | 0.011 |
| Cu (mg/kg DW) | 0.007 | <0.001 | <0.001 |
| Mn (mg/kg DW) | 0.004 | 0.004 | NS |
| Mo (mg/kg DW) | NS | <0.001 | NS |
| Ca (mg/kg DW) | NS | <0.001 | NS |
| Mg (mg/kg DW) | 0.004 | <0.001 | 0.001 |
| K (mg/kg DW) | NS | NS | NS |
| Na (mg/kg DW) | NS | <0.001 | NS |
| Se | ||||
| Variety | Se Treatment (g of Se/ha) | Se Intake from 100 g (μg/day) | % RDA from 100 g (USDA *) | % RDA from 100 g (EFSA *) |
| Ambassador | ||||
| 0 | 8 | 15 | 12 | |
| 50 | 203 | 369 | 290 | |
| 100 | 393 | 715 | 562 | |
| Premium | ||||
| 0 | 7 | 12 | 10 | |
| 50 | 165 | 300 | 235 | |
| 100 | 325 | 591 | 464 | |
| Zn | ||||
| Variety | Zn Treatment (g of Zn/ha) | Zn Intake from 100 g (mg/day) | % RDA from 100 g (USDA *) | % RDA from 100 g (EFSA *) |
| Ambassador | ||||
| 0 | 4.40 | 46 | 38 | |
| 375 | 4.04 | 42 | 35 | |
| 750 | 3.71 | 39 | 32 | |
| Premium | ||||
| 0 | 3.13 | 33 | 27 | |
| 375 | 3.57 | 38 | 31 | |
| 750 | 3.55 | 37 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malka, M.; Laing, G.D.; Hegedűsová, A.; Bohn, T. Foliar Selenate and Zinc Oxide Separately Applied to Two Pea Varieties: Effects on Growth Parameters and Accumulation of Minerals and Macronutrients in Seeds under Field Conditions. Foods 2023, 12, 1286. https://doi.org/10.3390/foods12061286
Malka M, Laing GD, Hegedűsová A, Bohn T. Foliar Selenate and Zinc Oxide Separately Applied to Two Pea Varieties: Effects on Growth Parameters and Accumulation of Minerals and Macronutrients in Seeds under Field Conditions. Foods. 2023; 12(6):1286. https://doi.org/10.3390/foods12061286
Chicago/Turabian StyleMalka, Maksymilian, Gijs Du Laing, Alžbeta Hegedűsová, and Torsten Bohn. 2023. "Foliar Selenate and Zinc Oxide Separately Applied to Two Pea Varieties: Effects on Growth Parameters and Accumulation of Minerals and Macronutrients in Seeds under Field Conditions" Foods 12, no. 6: 1286. https://doi.org/10.3390/foods12061286
APA StyleMalka, M., Laing, G. D., Hegedűsová, A., & Bohn, T. (2023). Foliar Selenate and Zinc Oxide Separately Applied to Two Pea Varieties: Effects on Growth Parameters and Accumulation of Minerals and Macronutrients in Seeds under Field Conditions. Foods, 12(6), 1286. https://doi.org/10.3390/foods12061286





