Dietary Grape Pomace Supplementation in Lambs Affects the Meat Fatty Acid Composition, Volatile Profiles and Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Design and Sample Collection
2.2. Evaluation of Meat Color
2.3. Drip Loss, Cooking Loss, and the Chemical Composition of Meat Samples
2.4. Fatty Acid Profiles of Meat and Lipid Oxidation
2.5. Analysis of Volatile Compounds
2.6. Lipid Oxidation
2.7. Statistical Analysis
3. Results
3.1. Fatty Acid Profiles, Polyphenol Content and Antioxidant Activity in the GP and Standard Diets
3.2. The Physical and Chemical Composition of the Meat
3.3. Fatty Acid Profiles
3.4. Identification of Volatile Compounds in Meat Samples
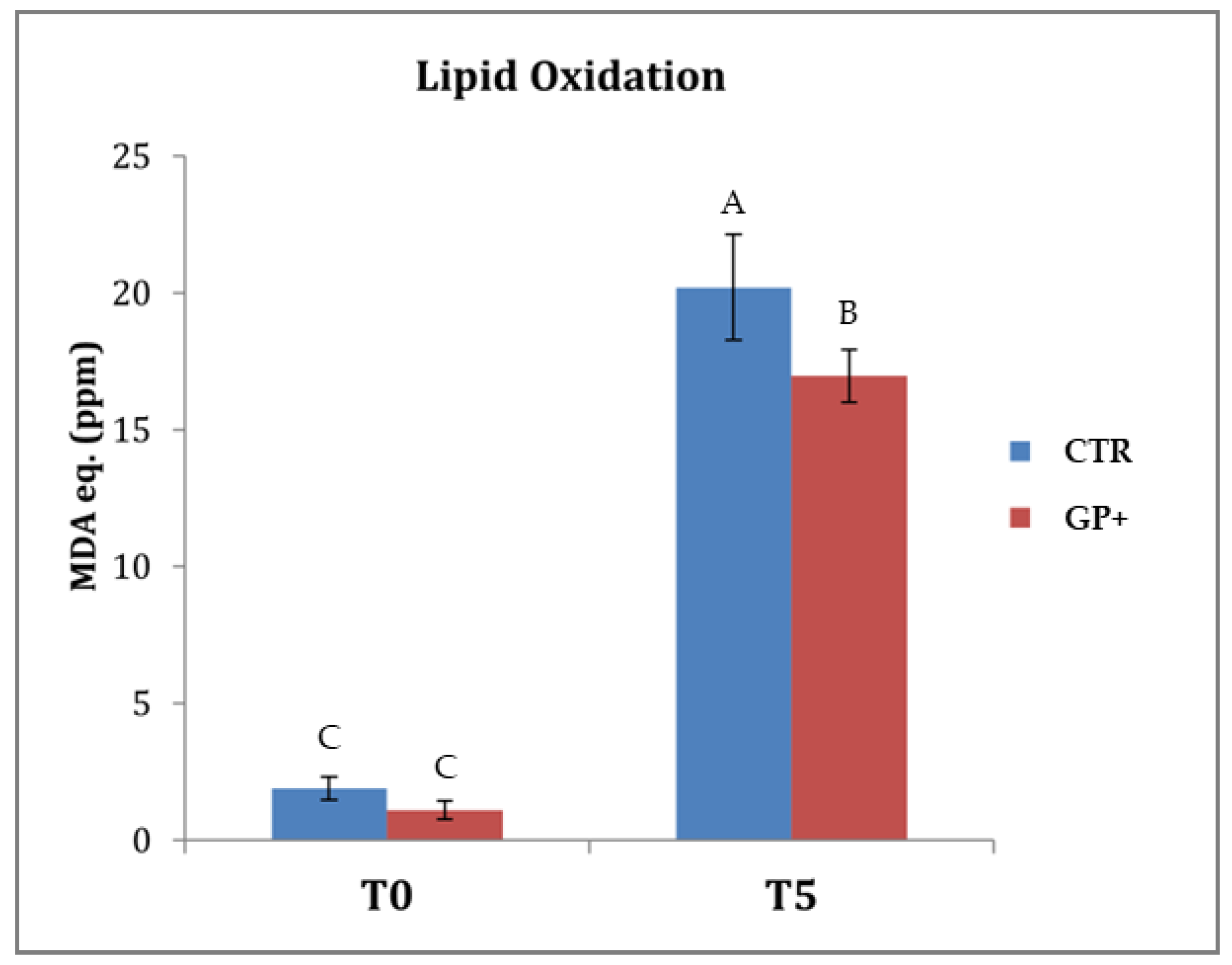
3.5. Oxidative Stability of Cooked Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of 431 bioactive compounds: Extraction, characterization, and biotechnological applications of 432 phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Flores, D.R.M.; da Fonseca, A.F.P.; Schmitt, J.; Tonetto, C.J.; Junior, A.G.R.; Hammerschmitt, R.K.; Facco, D.B.; Brunetto, G.; Nörnberg, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on meat quality. Small Rum. Res. 2020, 195, 106234. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Guerra, C.; Gallardo, B.; Lavín, P.; Mantecón, A.R.; de la Fuente, M.A.; Manso, T. Grape pomace in ewes diet: Effects on meat quality and the fatty acid profile of their suckling lambs. Food Res. Int. 2018, 113, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Petrotos, K.; Goulas, P.; Kouretas, D. Effects of dietary grape pomace supplementation on performance, carcass traits and meat quality of lambs. In Vivo 2018, 32, 807–812. [Google Scholar] [CrossRef]
- Ianni, A.; Di Luca, A.; Martino, C.; Bennato, F.; Marone, E.; Grotta, L.; Cichelli, A.; Martino, G. Dietary Supplementation of Dried Grape Pomace Increases the Amount of Linoleic Acid in Beef, Reduces the Lipid Oxidation and Modifies the Volatile Profile. Animals 2019, 9, 578. [Google Scholar] [CrossRef]
- Cashman, K.D.; Hayes, A. Red meat’s role in addressing ‘nutrients of public health concern’. Meat Sci. 2017, 132, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Li, Q.; Zhang, R.X.; Liu, W.Z.; Ren, Y.S.; Zhang, C.X. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim. Feed Sci. Technol. 2018, 236, 76–85. [Google Scholar] [CrossRef]
- NRC National Research Council (US); Committee on Nutrient Requirements of Small Ruminants; Board on Agriculture; Division on Earth, & Life Studies. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; China Legal Publishing House: Beijing, China, 2007.
- Bennato, F.; Ianni, A.; Florio, M.; Grotta, L.; Pomilio, F.; Saletti, M.A.; Martino, G. Nutritional Properties of Milk from Dairy Ewes Fed with a Diet Containing Grape Pomace. Foods 2022, 11, 1878. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 15th ed.; AOAC International: Washington, DC, USA, 1990. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Boil. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Grotta, L.; Castellani, F.; Palazzo, F.; Haouet, M.N.; Martino, G. Treatment Optimisation and Sample Preparation for the Evaluation of Lipid Oxidation in Various Meats Through TBARs Assays before Analysis. Food Anal. Methods 2017, 10, 1870–1880. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Flores, D.R.M.; da Fonseca, P.A.F.; Schmitt, J.; Tonetto, C.J.; Garcia Rosado Junior, A.; Hammerschmitt, R.K.; Facco, D.B.; Brunetto, G.; Nornber, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on productive performance, carcass characteristics, and blood parameters. Livest. Sci. 2020, 240, 104–169. [Google Scholar] [CrossRef]
- Cheah, K.S.; Cheah, A.M.; Krausgrill, D.I. Effect of dietary supplementation of vitamin E on pig meat quality. Meat Sci. 1995, 39, 255–264. [Google Scholar] [CrossRef]
- Castellini, C.; Dal Bosco, A.; Bernardini, M.; Cyril, H.W. Effect of Dietary Vitamin E on the Oxidative Stability of Raw and Cooked Rabbit Meat. Meat Sci. 1998, 50, 153–161. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, G.H.; Xu, X.L.; Zhao, G.M.; Li, C.B. Phospholipase A2 and antioxidant enzyme activities in normal and PSE pork. Meat Sci. 2010, 84, 143–146. [Google Scholar] [CrossRef]
- Tsao, F.H.; Culver, B.J.; Pierre, J.F.; Shanmuganayagam, D.; Patten, C.C.; Meyer, K.C. Effect of prophylactic supplementation with grape polyphenolics on endotoxin-induced serum secretory phospholipase A2 activity in rats. Comp. Med. 2012, 62, 271–278. [Google Scholar]
- Chikwanha, O.C.; Moelich, E.; Gouws, P.; Muchenje, V.; Nolte, J.V.E.; Dugan, M.E.; Mapiye, C. Effects of feeding increasing levels of grape (Vitis vinifera cv. Pinotage) pomace on lamb shelf-life and eating quality. Meat Sci. 2019, 157, 107887. [Google Scholar] [CrossRef] [PubMed]
- Massaro Junior, F.L.; Bumbieris Junior, V.H.; Pereira, E.S.; Zanin, E.; Horst, E.H.; Prado Calixto, O.P.; Peixoto, E.L.T.; Galbeiro, S.; Mizubuti, I.Y. Grape pomace silage on growth performance, carcass, and meat quality attributes of lambs. Sci. Agric. 2021, 70, e20200343. [Google Scholar] [CrossRef]
- Özkan, M.; Kirca, A.; Cemeroğlu, B. Effect of moisture content on CIE color values in dried apricots. Eur. Food Res. Technol. 2003, 216, 217–219. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Khliji, S.; van de Ven, R.; Lamb, T.A.; Lanza, M.; Hopkins, D.L. Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Sci. 2010, 85, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Luciano, G.; Monahan, F.J.; Vasta, V.; Biondi, L.; Lanza, M.; Priolo, A. Dietary tannins improve lamb meat colour stability. Meat Sci. 2009, 81, 120–125. [Google Scholar] [CrossRef]
- Lock, A.L.; Bauman, D.E. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 2004, 39, 1197–1206. [Google Scholar] [CrossRef]
- Yu, L. Free radical scavenging properties of conjugated linoleic acids. J. Agric. Food Chem. 2001, 49, 3452–3456. [Google Scholar] [CrossRef]
- Song, H.J.; Grant, I.; Rotondo, D.; Mohede, I.; Sattar, N.; Heys, S.D.; Wahle, K.W.J. Effect of CLA supplementation on immune function in young healthy volunteers. Eur. J Clin. Nutr. 2005, 59, 508–517. [Google Scholar] [CrossRef]
- Evans, M.E.; Brown, J.M.; McIntosh, M.K. Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism. J. Nutr. Biochem. 2002, 13, 508–516. [Google Scholar] [CrossRef]
- Platt, I.; Rao, L.G.; El-Sohemy, A. Isomer-specific effects of conjugated linoleic acid on mineralized bone nodule formation from human osteoblast-likecells. Exp. Biol. Med. 2007, 232, 246–252. [Google Scholar]
- Lock, A.L.; Corl, B.A.; Barbano, D.M.; Bauman, D.E.; Ip, C. The anticarcinogenic effect of trans-11 18:1 is dependent on its conversion to cis-9, trans-11 CLA by Δ9-desaturase in rats. J. Nutr. 2004, 134, 2698–2704. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Mottram, D.S.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Pegg, R.B. Hexanal as an indicator of meat flavor deterioration. J. Food Lipids 1994, 1, 177–186. [Google Scholar] [CrossRef]
- Ventanas, S.; Puolanne, E.; Tuorila, H. Temporal changes of flavour and texture in cooked bologna type sausages as affected by fat and salt content. Meat Sci. 2010, 85, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cui, H.; Yuan, X.; Liu, L.; Liu, X.; Wang, Y.; Ding, J.; Xiang, H.; Zhang, X.; Liu, J.; et al. Identification of the main aroma compounds in Chinese local chicken high-quality meat. Food Chem. 2021, 359, 129930. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhang, L.; He, L.; Zhang, Z.; Zhang, T.; Mu, W.; Lin, J.; Liu, F. Toxicological effects of the fungal volatile compound 1-octen-3-ol against the red flour beetle, Tribolium castaneum (Herbst). Ecotoxicol. Environ. Saf. 2021, 208, 111597. [Google Scholar] [CrossRef]
- Xiong, C.; Li, Q.; Li, S.; Chen, C.; Chen, Z.; Huang, W. In vitro antimicrobial activities and mechanism of 1-octen-3-ol against food-related bacteria and pathogenic fungi. J. Oleo Sci. 2017, 66, 1041–1049. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
| GP | CTR | GP+ | |
|---|---|---|---|
| Fatty acids 1 (%) | |||
| C16:0 | 11.31 ± 1.26 | 19.64 ± 0.40 | 18.94 ± 2.37 |
| C18:0 | 3.90 ± 0.80 | 3.34 ± 0.06 | 3.74 ± 0.35 |
| C18:1 c9 | 15.10 ± 2.87 | 22.87 ± 0.08 | 22.48 ± 0.57 |
| C18:2 c9, c12 | 66.75 ± 4.05 | 51.62 ± 0.51 | 52.36 ± 1.46 |
| C18:3 c9, c12, c15 | 2.79 ± 2.06 | 2.50 ± 0.02 | 2.44 ± 0.04 |
| SFA | 15.21 ± 0.87 | 22.99 ± 0.47 | 23.68 ± 2.02 |
| MUFA | 15.10 ± 0.23 | 22.87 ± 0.08 | 22.48 ± 0.57 |
| PUFA | 69.54 ± 0.68 | 54.12 ± 0.53 | 53.81 ± 1.43 |
| DM (%) | 33.95 ± 1.50 | 89.00 ± 0.20 | 88.00 ± 0.15 |
| TPCs 2 (mg GAE g−1) | 73.36 ± 7.66 | 2.16 ± 0.13 B | 2.44 ± 0.11 A |
| AOA 2 (µmol TEAC g−1) | 496.12 ± 19.23 | 45.09 ± 2.15 B | 52.41 ± 3.11 A |
| Physical Trait (%) | CTR | GP+ | p |
|---|---|---|---|
| Drip loss (DL) | 1.16 ± 0.50 | 1.08 ± 0.30 | ns |
| Cooking loss (CL) | 35.04 ± 2.11 | 33.93 ± 2.79 | ns |
| Chemical composition (%) | |||
| Dry matter (DM) | 20.12 ± 2.04 | 21.16 ± 1.32 | ns |
| Total lipids 1 | 10.56 ± 4.59 | 10.21 ± 4.51 | ns |
| Coordinates | T2 | T7 | ||||
|---|---|---|---|---|---|---|
| CTR | GP+ | p | CTR | GP+ | p | |
| L* | 43.4 ± 4.79 | 41.56 ± 3.37 | ns | 41.15 ± 4.50 | 39.71 ± 3.59 | ns |
| a* | 16.13 ± 1.39 A | 15.92 ± 2.61 A | ns | 8.53 ± 1.23 B | 7.77 ± 1.56 B | ns |
| b* | 5.34 ± 1.08 | 4.74 ± 1.49 | ns | 6.47 ± 1.06 | 6.36 ± 1.73 | ns |
| Fatty Acid 1 | CTR | GP+ | p |
|---|---|---|---|
| C8:0 | 0.05 ± 0.01 | 0.04 ± 0.01 | ns |
| C10:0 | 0.27 ± 0.09 | 0.21 ± 0.08 | ns |
| C12:0 | 0.44 ± 0.12 | 0.38 ± 0.14 | ns |
| C14:0 | 5.12 ± 0.83 | 4.16 ± 0.78 | ns |
| C16:0 | 26.76 ± 2.73 | 24.61 ± 1.51 | ns |
| C18:0 | 16.79 ± 2.19 | 19.54 ± 1.81 | * |
| C20:0 | 0.14 ± 0.03 | 0.10 ± 0.02 | ns |
| C22:0 | 0.46 ± 0.10 | 0.45 ± 0.12 | ns |
| C24:0 | 0.43 ± 0.05 | 0.36 ± 0.12 | ns |
| SFA | 50.46 ± 4.59 | 49.85 ± 4.44 | ns |
| C14:1 c9 | 0.66 ± 0.12 | 0.59 ± 0.10 | ns |
| C16:1 c9 | 1.59 ± 0.18 | 1.45 ± 0.24 | ns |
| C18:1 t11 | 1.44 ± 0.23 | 2.32 ± 0.23 | ** |
| C18:1 c9 | 28.72 ± 2.82 | 27.55 ± 3.19 | ns |
| C18:1 c11 | 1.50 ± 0.23 | 1.36 ± 0.21 | ns |
| MUFA | 33.91 ± 3.11 | 33.27 ± 3.04 | ns |
| C18:2 c9, c12 | 10.67 ± 0.93 | 11.97 ± 0.68 | ns |
| C18:3 c9, c12, c15 | 0.67 ± 0.08 | 0.61 ± 0.06 | ns |
| CLA | 1.07 ± 0.09 | 1.22 ± 0.08 | ** |
| PUFA | 12.41 ± 1.21 | 13.8 ± 1.28 | ns |
| Others | 3.22 ± 0.33 | 3.08 ± 0.31 | ns |
| AI | 17.08 ± 4.34 | 17.34 ± 4.67 | ns |
| TI | 9.36 ± 1.51 | 9.51 ± 1.51 | ns |
| VOCs 1 | T0 | T5 | |||||
|---|---|---|---|---|---|---|---|
| CTR | GP+ | p | CTR | GP+ | p | ||
| Alcohols | 1-Pentanol | 1.63 ± 0.21 A | 1.43 ± 0.19 A | ns | 0.27 ± 0.04 B | 0.25 ± 0.03 B | ns |
| 1-Octen-3-ol | 5.38 ± 0.55 B | 5.87 ± 0.61 B | ns | 6.74 ± 0.65 B | 9.03 ± 0.88 A | ** | |
| 2-Octen-1-ol, (Z)- | 0.39 ± 0.05 B | 0.46 ± 0.06 B | ns | 1.14 ± 0.12 A | 0.99 ± 0.11 A | ns | |
| 1-Octanol | 1.58 ± 0.19 A | 1.72 ± 0.22 A | ns | 0.76 ± 0.09 B | 0.67 ± 0.09 B | ns | |
| Aldehydes | Hexanal | 71.47 ± 5.44 C | 70.08 ± 4.88 C | ns | 79.77 ± 2.03 A | 76.55 ± 1.65 B | * |
| Heptanal | 1.32 ± 0.15 | 1.61 ± 0.17 | ns | 1.19 ± 0.13 | 1.38 ± 0.15 | ns | |
| 4-Pentenal, 2,2-dimethyl- | 0.21 ± 0.08 | 0.12 ± 0.06 | ns | nd | nd | ns | |
| Nonanal | 4.98 ± 0.52 B | 4.25 ± 0.45 B | ns | 5.86 ± 0.46 B | 7.50 ± 0.67 A | * | |
| Octanal | 1.50 ± 0.18 | 1.77 ± 0.20 | ns | 1.46 ± 0.16 | 1.59 ± 0.18 | ns | |
| 2-Decenal, (E)- | 0.39 ± 0.06 | 0.42 ± 0.06 | ns | nd | nd | ns | |
| Aromatic Hydrocarbons | Ethylbenzene | 5.65 ± 0.59 | 6.19 ± 0.63 | ns | 5.08 ± 0.37 | 5.42 ± 0.41 | ns |
| O-xylene | 4.21 ± 0.47 | 4.36 ± 0.49 | ns | 3.78 ± 0.39 | 4.58 ± 0.51 | ns | |
| Benzene, 1,3-dimethyl- | 1.57 ± 0.28 | 2.47 ± 0.31 | ns | 1.29 ± 0.16 | 1.37 ± 0.15 | ns | |
| Benzene, 4-ethyl-1,2-dimethyl- | 0.62 ± 0.11 | 0.69 ± 0.10 | ns | 1.24 ± 0.13 | 1.31 ± 0.15 | ns | |
| Ketones | 2,3-Octanedione | 8.07 ± 0.78 A | 8.06 ± 0.75 A | ns | 0.34 ± 0.05 B | 0.30 ± 0.04 B | ns |
| Esters | Propanoic acid, 2-methyl-, butyl ester | 1.59 ± 0.15 | 1.51 ± 0.17 | ns | nd | nd | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennato, F.; Martino, C.; Ianni, A.; Giannone, C.; Martino, G. Dietary Grape Pomace Supplementation in Lambs Affects the Meat Fatty Acid Composition, Volatile Profiles and Oxidative Stability. Foods 2023, 12, 1257. https://doi.org/10.3390/foods12061257
Bennato F, Martino C, Ianni A, Giannone C, Martino G. Dietary Grape Pomace Supplementation in Lambs Affects the Meat Fatty Acid Composition, Volatile Profiles and Oxidative Stability. Foods. 2023; 12(6):1257. https://doi.org/10.3390/foods12061257
Chicago/Turabian StyleBennato, Francesca, Camillo Martino, Andrea Ianni, Claudia Giannone, and Giuseppe Martino. 2023. "Dietary Grape Pomace Supplementation in Lambs Affects the Meat Fatty Acid Composition, Volatile Profiles and Oxidative Stability" Foods 12, no. 6: 1257. https://doi.org/10.3390/foods12061257
APA StyleBennato, F., Martino, C., Ianni, A., Giannone, C., & Martino, G. (2023). Dietary Grape Pomace Supplementation in Lambs Affects the Meat Fatty Acid Composition, Volatile Profiles and Oxidative Stability. Foods, 12(6), 1257. https://doi.org/10.3390/foods12061257








