The Hepatoprotective Effect of Two Date Palm Fruit Cultivars’ Extracts: Green Optimization of the Extraction Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Date Palm Fruits
2.2. Conventional Extraction of the Date Palm Fruit Cultivars via Ethanol
2.3. Green Extraction of Date Palm Fruits Using Water Bath-Assisted Extraction (WAE)
2.3.1. The Experimental Design
2.3.2. Preparation of Date Palm Fruits for WAE
2.3.3. Water Bath-Assisted Extraction (WAE) Method
2.4. Determination of Experimental “Response” Parameters
2.4.1. Total Phenolic Content (TPC) Determination
2.4.2. Total Flavonoid Content (TFC) Determination
2.4.3. Ferric Reducing Antioxidant Power Assay (FRAP)
2.4.4. Radical-Scavenging Antioxidant Ability by ABTS
2.5. The Hepatoprotective Activity Assessment of Date Palm Fruit Cultivars
2.5.1. Animals and Ethical Approval
2.5.2. Extraction of Date Palm Fruits for In Vivo Experiments
2.5.3. Experimental Design
2.5.4. General Health and Body Weight Changes
2.5.5. Collection of Blood Samples and Hepatic Tissues
2.5.6. Liver Toxicity Evaluation
Histopathological Examinations
Determination of Hydroxyproline
2.5.7. Determination of Liver Function
2.5.8. Enzyme-Linked Immunosorbent Assay Evaluations
2.5.9. Apoptosis Assay via the Detection of DNA Fragmentation
2.6. Statistical Analysis
3. Results
3.1. Ethanol and Water Conventional Extraction Date Palm Fruits Cultivars
3.2. Production of Date Palm Fruit Extract Applying the WAE Approach
3.2.1. Effect of WAE the Date Palm Fruit Varieties on TPC
3.2.2. Effect of WAE the Date Palm Fruit Varieties on TFC
3.2.3. Effect of WAE the Date Palm Fruit Varieties on FRAP
3.2.4. Effect of WAE the Date Palm Fruit Varieties on Scavenging Activity (ABTS Assay)
3.2.5. WAE Optimization
3.3. The Hepatoprotective Activity of Date Palm Fruit Cultivars
3.3.1. General Health and Body Weight Changes (%)
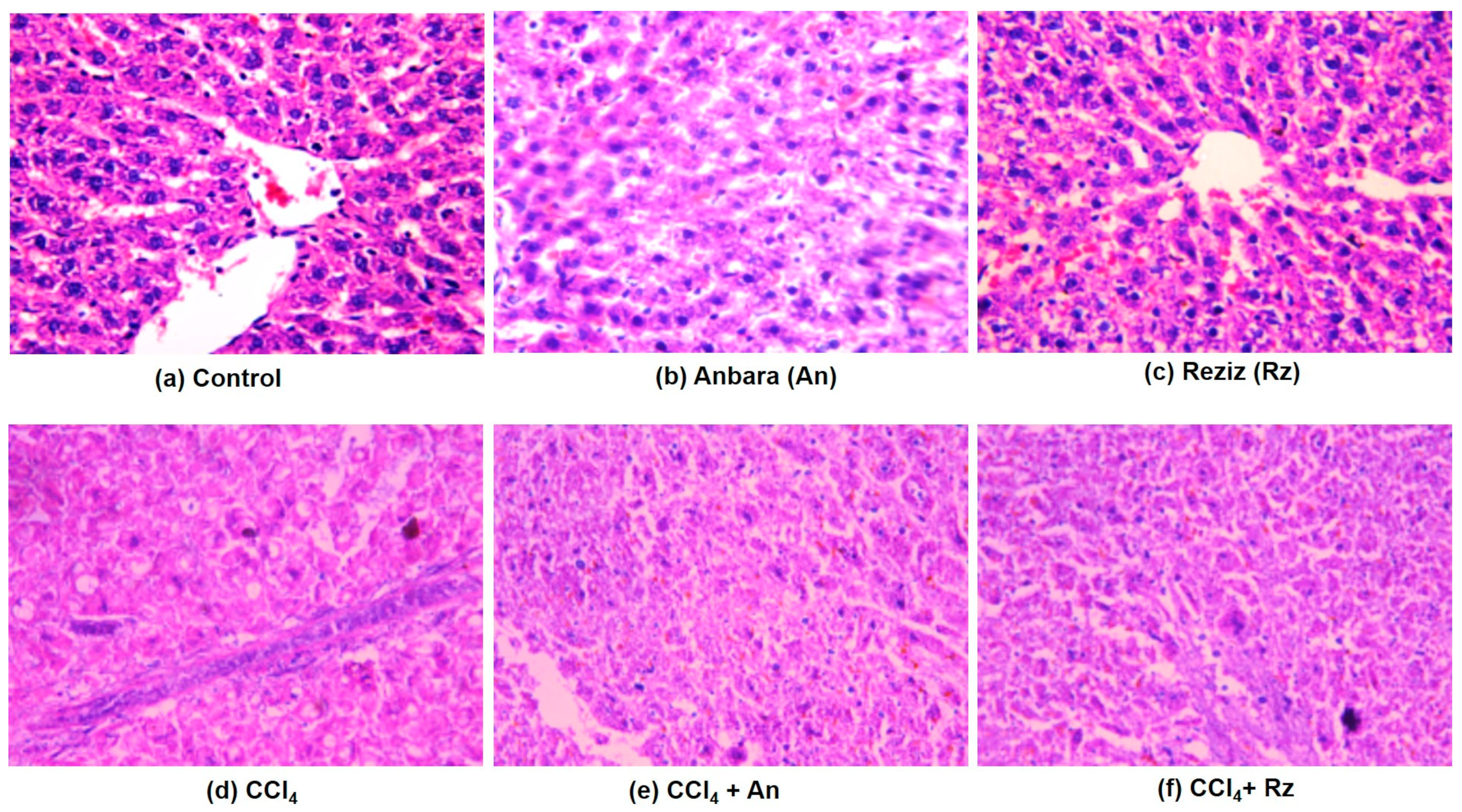
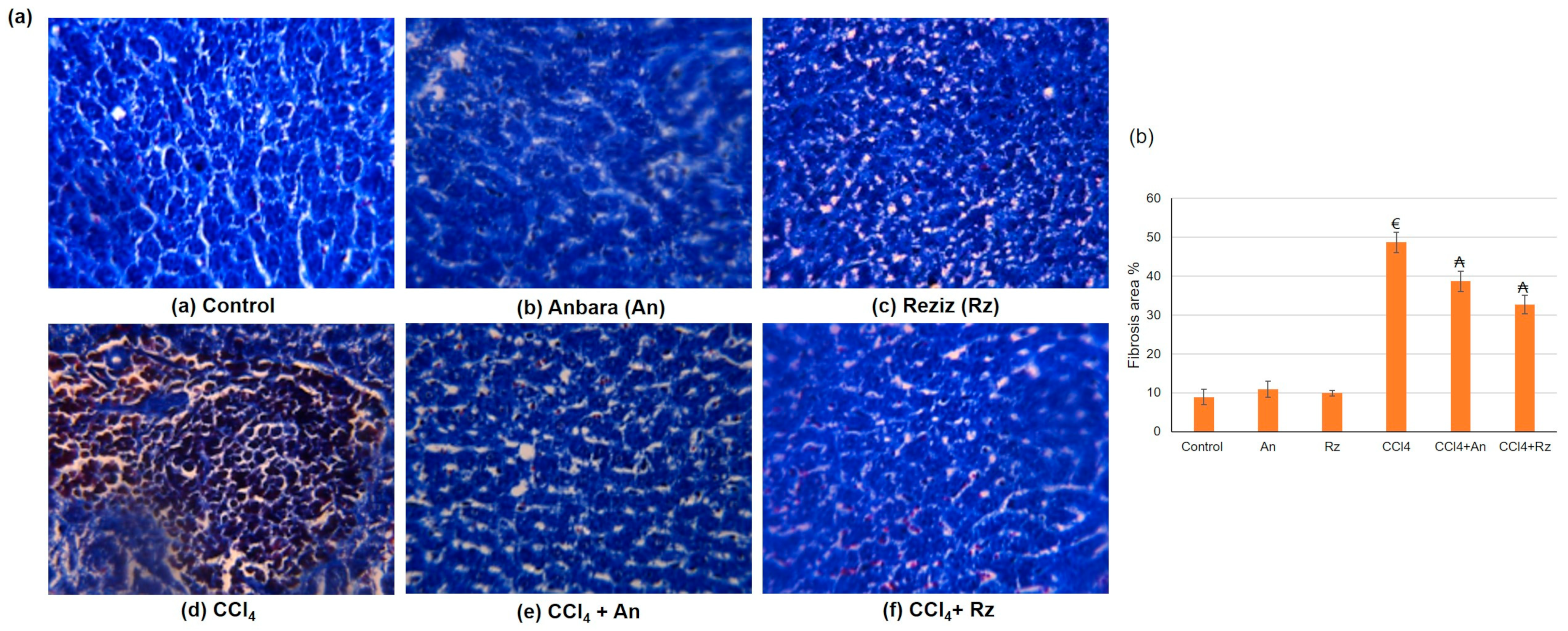
3.3.2. The Histological Studies
Histological Examination Using H&E
Histological Studies Using Masson’s Trichrome (MT) Stain
3.3.3. Effect of An and Rz Extract Administration on Liver Enzymes in CCl4-Induced Hepatotoxicity
3.3.4. Effect of An and Rz on Hydroxyproline Content in CCl4-Induced Hepatotoxicity
3.3.5. Effect of An and Rz on Serum Level of Alpha-Fetoprotein (AFP) in CCl4-Induced Hepatotoxicity
3.3.6. Effect of An and Rz Administration on the Hepatic Content of Adiponectin (ADP) in CCl4-Induced Hepatotoxicity
| Animal Group | Hydroxyproline (nmol/L) | α-Fetoprotein (AFP) (ng/mL) | Adiponectin (ADP) (ng/mL) |
|---|---|---|---|
| Control | 23.43 ± 0.21 | 0.53 ± 0.25 | 8.07 ± 0.25 |
| An | 19.25 ± 0.21 | 0.54 ± 0.05 | 7.50 ± 0.14 |
| Rz | 22.67 ± 0.21 | 0.52 ± 0.03 | 7.70 ± 0.20 |
| CCl4 | 75.23 ± 0.15 € | 3.37 ± 0.42 € | 3.87 ± 0.15 € |
| CCl4 + An | 30.73 ± 0.21 ₳ | 1.45 ± 0.05 ₳ | 6.04 ± 0.05 ₳ |
| CCl4 + Rz | 39.33 ± 0.21 ₳ | 1.53 ± 0.03 ₳ | 5.57 ± 0.25 ₳ |
3.3.7. Effect of An and Rz on Nitrosative and Oxidative Stress and Lipid Peroxidation in CCl4-Induced Hepatotoxicity
3.3.8. Effect of An and Rz on IFN-γ, TNF-α, and NF-κB in CCl4-Induced Hepatotoxicity
3.3.9. The Effect of An and Rz on Apoptosis Using DNA Fragmentation Assays in CCl4-Induced Hepatotoxicity
4. Discussion
4.1. Optimization of An and Rz Cultivars of Date Palm Fruit Extraction Using WAE
4.2. The Hepatoprotective Activity of An and Rz Cultivar Extract
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Dashti, Y.A.; Holt, R.R.; Keen, C.L.; Hackman, R.M. Date Palm Fruit (Phoenix dactylifera): Effects on Vascular Health and Future Research Directions. Int. J. Mol. Sci. 2021, 22, 4665. [Google Scholar] [CrossRef]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Chaira, N.; Smaali, M.I.; Martinez-Tomé, M.; Mrabet, A.; Murcia, M.A.; Ferchichi, A. Simple phenolic composition, flavonoid contents and anti-oxidant capacities in water-methanol extracts of Tunisian common date cultivars (Phoenix dactylifera L.). Int. J. Food Sci. Nutr. 2009, 60, 316–329. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Aldosari, S.A.; Vidyasagar, P.S.; Nair, K.M.; Nair, M.G. Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J. Agric. Food Chem. 2013, 61, 5834–5840. [Google Scholar] [CrossRef]
- Al-Qarawi, A.; Abdel-Rahman, H.; Ali, B.; Mousa, H.; El-Mougy, S. The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2005, 98, 313–317. [Google Scholar] [CrossRef]
- El Arem, A.; Thouri, A.; Zekri, M.; Saafi, E.B.; Ghrairi, F.; Zakhama, A.; Achour, L. Nephroprotective effect of date fruit extract against dichloroacetic acid exposure in adult rats. Food Chem. Toxicol. 2014, 65, 177–184. [Google Scholar] [CrossRef]
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.M.; Emeka, P.M.; Badger-Emeka, L.I.; Matsunami, K.; Shehata, T.M.; Elsewedy, H.S. Date Palm Extract (Phoenix dactylifera) PEGylated Nanoemulsion: Development, Optimization and Cytotoxicity Evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef]
- Baliga, M.S.; Baliga, B.R.V.; Kandathil, S.M.; Bhat, H.P.; Vayalil, P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res. Int. 2011, 44, 1812–1822. [Google Scholar] [CrossRef]
- Eid, N.; Osmanova, H.; Natchez, C.; Walton, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P. Impact of palm date consumption on microbiota growth and large intestinal health: A randomised, controlled, cross-over, human intervention study. Br. J. Nutr. 2015, 114, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Alalwan, T.A.; Perna, S.; Mandeel, Q.A.; Abdulhadi, A.; Alsayyad, A.S.; D’Antona, G.; Negro, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; et al. Effects of Daily Low-Dose Date Consumption on Glycemic Control, Lipid Profile, and Quality of Life in Adults with Pre- and Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2020, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Gad El-Hak, H.N.; Mahmoud, H.S.; Ahmed, E.A.; Elnegris, H.M.; Aldayel, T.S.; Abdelrazek, H.M.A.; Soliman, M.T.A.; El-Menyawy, M.A.I. Methanolic Phoenix dactylifera L. Extract Ameliorates Cisplatin-Induced Hepatic Injury in Male Rats. Nutrients 2022, 14, 1025. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Samir, A.; Elkomy, A.; Aboubaker, M.; Habotta, O.A.; Gaber, A.; Alsanie, W.F.; Abdullah, O.; Elnoury, H.A.; Baioumy, B.; et al. The potential anti-oxidant bioactivity of date palm fruit against gentamicin-mediated hepato-renal injury in male albino rats. Biomed. Pharmacother. 2021, 143, 112154. [Google Scholar] [CrossRef] [PubMed]
- Roshankhah, S.; Abdolmaleki, A.; Salahshoor, M.R. Anti-inflammatory, anti-apoptotic, and anti-oxidant actions of Middle Eastern Phoenix dactylifera extract on mercury-induced hepatotoxicity in vivo. Mol. Biol. Rep. 2020, 47, 6053–6065. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Bahkali, A.H. Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology—Review. Saudi J. Biol. Sci. 2013, 20, 105–120. [Google Scholar] [CrossRef]
- Revathi, S.; Govindarajan, R.K.; Rameshkumar, N.; Hakkim, F.L.; Mohammed, A.-B.; Krishnan, M.; Kayalvizhi, N. Anti-cancer, anti-microbial and anti-oxidant properties of Acacia nilotica and their chemical profiling. Biocatal. Agric. Biotechnol. 2017, 11, 322–329. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Han, H.; Zhao, L.; Liu, X.; Guo, A.; Li, X. Effect of water bath-assisted water extraction on physical and chemical properties of soybean oil body emulsion. Food Sci. Nutr. 2020, 8, 6380–6391. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: Aa comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Hashem, H.; El-Daym, H.; El-Sharnouby, G.; Farghal, S.; Badr, H. The Effect of Extraction Method, Bleaching and Clarification Processes on Quality Second Grade Siwi Date. Dibs. Ind. Eng. 2017, 1, 17–23. [Google Scholar] [CrossRef]
- Hamza, A.A.; Lashin, F.M.; Gamel, M.; Hassanin, S.O.; Abdalla, Y.; Amin, A. Hawthorn Herbal Preparation from Crataegus oxyacantha Attenuates in vivo Carbon Tetrachloride -Induced Hepatic Fibrosis via Modulating Oxidative Stress and Inflammation. Antioxidants 2020, 9, 1173. [Google Scholar] [CrossRef]
- Singh, D.; Arya, P.V.; Sharma, A.; Aggarwal, V.P.; Dobhal, M.P.; Gupta, R.S. Antioxidant Potential of Plumieride against CCl₄-Induced Peroxidative Damage in Rats. Antioxidants 2014, 3, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Prakash, J.; Qi, S.; Franklin, G. Oral Delivery of Curcumin Polymeric Nanoparticles Ameliorates CCl₄-Induced Subacute Hepatotoxicity in Wistar Rats. Polymers 2018, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Cicek, M.; Sabancilar, İ. Toxicity of carbon tetrachloride, free radicals and role of anti-oxidants. Rev. Environ. Health 2021, 36, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.B.; Kwon, D.; Son, S.W.; Kim, S.H.; Lee, Y.H.; Seo, M.S.; Kim, K.S.; Jung, Y.S. Weaning Mice and Adult Mice Exhibit Differential Carbon Tetrachloride-Induced Acute Hepatotoxicity. Antioxidants 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Arya, P.V.; Aggarwal, V.P.; Gupta, R.S. Evaluation of Antioxidant and Hepatoprotective Activities of Moringa oleifera Lam. Leaves in Carbon Tetrachloride-Intoxicated Rats. Antioxidants 2014, 3, 569–591. [Google Scholar] [CrossRef]
- Sobeh, M.; Hamza, M.S.; Ashour, M.L.; Elkhatieb, M.; El Raey, M.A.; Abdel-Naim, A.B.; Wink, M. A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Anti-oxidant and Hepatoprotective Activities In Vivo. Pharmaceuticals 2020, 13, 84. [Google Scholar] [CrossRef]
- Ogaly, H.A.; Aldulmani, S.A.A.; Al-Zahrani, F.A.M.; Abd-Elsalam, R.M. D-Carvone Attenuates CCl(4)-Induced Liver Fibrosis in Rats by Inhibiting Oxidative Stress and TGF-ß 1/SMAD3 Signaling Pathway. Biology 2022, 11, 739. [Google Scholar] [CrossRef]
- Rahmouni, F.; Badraoui, R.; Ben-Nasr, H.; Bardakci, F.; Elkahoui, S.; Siddiqui, A.J.; Saeed, M.; Snoussi, M.; Saoudi, M.; Rebai, T. Pharmacokinetics and Therapeutic Potential of Teucrium polium against Liver Damage Associated Hepatotoxicity and Oxidative Injury in Rats: Computational, Biochemical and Histological Studies. Life 2022, 12, 1092. [Google Scholar] [CrossRef]
- Abdulhadi, I. Assessing fruit characteristics to standardize quality norms in date cultivars of Saudi Arabia. Indian J. Technol. 2011, 4, 1262–1266. [Google Scholar] [CrossRef]
- Siddeeg, A.; Faisal Manzoor, M.; Haseeb Ahmad, M.; Ahmad, N.; Ahmed, Z.; Kashif Iqbal, K.M.; Aslam Maan, A.; Mahr-Un-Nisa; Zeng, X.-A.; Ammar, A.-F. Pulsed Electric Field-Assisted Ethanolic Extraction of Date Palm Fruits: Bioactive Compounds, Anti-oxidant Activity and Physicochemical Properties. Processes 2019, 7, 585. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Salah Eddine, L. Influence of Extraction Method on Phytochemical Composition and Anti-oxidant Activity from Leaves Extract of Algerian Phoenix dactylifera L. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 84–89. [Google Scholar]
- Hatamnia, A.A.; Abbaspour, N.; Darvishzadeh, R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014, 145, 306–311. [Google Scholar] [CrossRef]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Abas, F.; Lai, O.M.; Ho, C.W.; Tan, C.P. Optimal binary solvent extraction system for phenolic anti-oxidants from mengkudu (Morinda citrifolia) fruit. Molecules 2013, 18, 7004–7022. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef]
- Al-Seeni, M.N.; El Rabey, H.A.; Zamzami, M.A.; Alnefayee, A.M. The hepatoprotective activity of olive oil and Nigella sativa oil against CCl(4) induced hepatotoxicity in male rats. BMC Complement. Altern. Med. 2016, 16, 438. [Google Scholar] [CrossRef]
- Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Histotechnology; Mosby: Maryland Heights, MI, USA, 1980. [Google Scholar]
- Kuo, J.-H.S.; Jan, M.-S.; Jeng, J.; Chiu, H.W. Induction of apoptosis in macrophages by air oxidation of dioleoylphosphatidylglycerol. J. Control. Release 2005, 108, 442–452. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Y.X.; Divine, G.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J. Nutr. 2002, 132, 2076–2081. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Honsawek, S.; Poovorawan, Y. Adiponectin as a novel biomarker for liver fibrosis. World J. Hepatol. 2018, 10, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential anti-oxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.L.; Lai, J.T.; Lin, W.C. Inhibitory effect of olive oil on fibrosis induced by carbon tetrachloride in rat liver. Clin. Nutr. 2008, 27, 900–907. [Google Scholar] [CrossRef]
- Mohammed, S.A.A.; Khan, R.A.; El-Readi, M.Z.; Emwas, A.H.; Sioud, S.; Poulson, B.G.; Jaremko, M.; Eldeeb, H.M.; Al-Omar, M.S.; Mohammed, H.A. Suaeda vermiculata Aqueous-Ethanolic Extract-Based Mitigation of CCl(4)-Induced Hepatotoxicity in Rats, and HepG-2 and HepG-2/ADR Cell-Lines-Based Cytotoxicity Evaluations. Plants 2020, 9, 1291. [Google Scholar] [CrossRef]
- Saafi, E.B.; Louedi, M.; Elfeki, A.; Zakhama, A.; Najjar, M.F.; Hammami, M.; Achour, L. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp. Toxicol. Pathol. 2011, 63, 433–441. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; El-Bialy, B.E.; El-Borai, N.B.; AbuBakr, H.O.; Elhadary, A.M.A. Radioprotective effect of Date syrup on radiation- induced damage in Rats. Sci. Rep. 2018, 8, 7423. [Google Scholar] [CrossRef]
- Nakamura, I.; Asumda, F.Z.; Moser, C.D.; Kang, Y.N.N.; Lai, J.P.; Roberts, L.R. Sulfatase-2 Regulates Liver Fibrosis through the TGF-β Signaling Pathway. Cancers 2021, 13, 5279. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Q.L.; Song, Y.N.; Sun, Y.; Wei, B.; Li, X.Y.; Hu, Y.Y.; Liu, P.; Su, S.B. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J. Toxicol. Sci. 2016, 41, 561–572. [Google Scholar] [CrossRef]
- Bahri, S.; Abdennabi, R.; Mlika, M.; Neji, G.; Jameleddine, S.; Ali, R.B. Effect of Phoenix dactylifera L. Sap Against Bleomycin-Induced Pulmonary Fibrosis and Oxidative Stress in Rats: Phytochemical and Therapeutic Assessment. Nutr. Cancer 2019, 71, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, B.; Iseli, T.; Ramezani-Moghadam, M.; Ho, V.; Wankell, M.; Sun, E.J.; Qiao, L.; George, J.; Hebbard, L.W. The role of AdipoR1 and AdipoR2 in liver fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Su, L.; Esmaili, S.; Iseli, T.J.; Ramezani-Moghadam, M.; Hu, L.; Xu, A.; George, J.; Wang, J. Adiponectin attenuates liver fibrosis by inducing nitric oxide production of hepatic stellate cells. J. Mol. Med. 2015, 93, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Feld, J.J.; Applegate, T.; Matthews, G.V.; Hellard, M.; Sherker, A.; Petoumenos, K.; Zang, G.; Shaw, I.; Yeung, B.; et al. Plasma interferon-gamma-inducible protein-10 (IP-10) levels during acute hepatitis C virus infection. Hepatology 2013, 57, 2124–2134. [Google Scholar] [CrossRef]
- Amer, M.A.; Othman, A.I.; El-Missiry, M.A.; Farag, A.A.; Amer, M.E. Proanthocyanidins attenuated liver damage and suppressed fibrosis in CCl4-treated rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 91127–91138. [Google Scholar] [CrossRef] [PubMed]
- Elgawish, R.A.R.; Rahman, H.G.A.; Abdelrazek, H.M.A. Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicol. Rep. 2015, 2, 1149–1156. [Google Scholar] [CrossRef]
- El Arem, A.; Saafi, E.B.; Ghrairi, F.; Thouri, A.; Zekri, M.; Ayed, A.; Zakhama, A.; Achour, L. Aqueous date fruit extract protects against lipid peroxidation and improves anti-oxidant status in the liver of rats subchronically exposed to trichloroacetic acid. J Physiol. Biochem. 2014, 70, 451–464. [Google Scholar] [CrossRef]
- Al-Shwyeh, H.A. Date Palm (Phoenix dactylifera L.) Fruit as Potential Anti-oxidant and Antimicrobial Agents. J. Pharm. Bioallied. Sci. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa Date Fruit Pulp and Seed in the Management of Diseases through in vitro and in silico Analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Raftar, S.K.A.; Ashrafian, F.; Abdollahiyan, S.; Yadegar, A.; Moradi, H.R.; Masoumi, M.; Vaziri, F.; Moshiri, A.; Siadat, S.D.; Zali, M.R. The anti-inflammatory effects of Akkermansia muciniphila and its derivates in HFD/CCL4-induced murine model of liver injury. Sci. Rep. 2022, 12, 2453. [Google Scholar] [CrossRef]
- Wijaya, R.S.; Read, S.A.; Schibeci, S.; Eslam, M.; Azardaryany, M.K.; El-Khobar, K.; van der Poorten, D.; Lin, R.; Yuen, L.; Lam, V.; et al. KLRG1+ natural killer cells exert a novel anti-fibrotic function in chronic hepatitis B. J. Hepatol. 2019, 71, 252–264. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Ahmed, H.A.; Shaheen, H.M. Dietary Supplementation of Phoenix dactylifera Seeds Enhances Performance, Immune Response, and Antioxidant Status in Broilers. Oxid. Med. Cell Longev. 2016, 2016, 5454963. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahya, M.; Raish, M.; AlSaid, M.S.; Ahmad, A.; Mothana, R.A.; Al-Sohaibani, M.; Al-Dosari, M.S.; Parvez, M.K.; Rafatullah, S. ‘Ajwa’ dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. Phytomedicine 2016, 23, 1240–1248. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; Obaid, W.A.; Mohammedsaleh, Z.M.; Ouchari, W.; Eldahshan, O.A.; Sobeh, M. Ajwa dates (Phoenix dactylifera L.) attenuate cisplatin-induced nephrotoxicity in rats via augmenting Nrf2, modulating NADPH oxidase-4 and mitigating inflammatory/apoptotic mediators. Biomed. Pharmacother. 2022, 156, 113836. [Google Scholar] [CrossRef] [PubMed]
- Alkreathy, H.M.; Khan, R.A.; Khan, M.R.; Sahreen, S. CCl4 induced genotoxicity and DNA oxidative damages in rats: Hepatoprotective effect of Sonchus arvensis. BMC Complement. Altern. Med. 2014, 14, 452. [Google Scholar] [CrossRef] [PubMed]
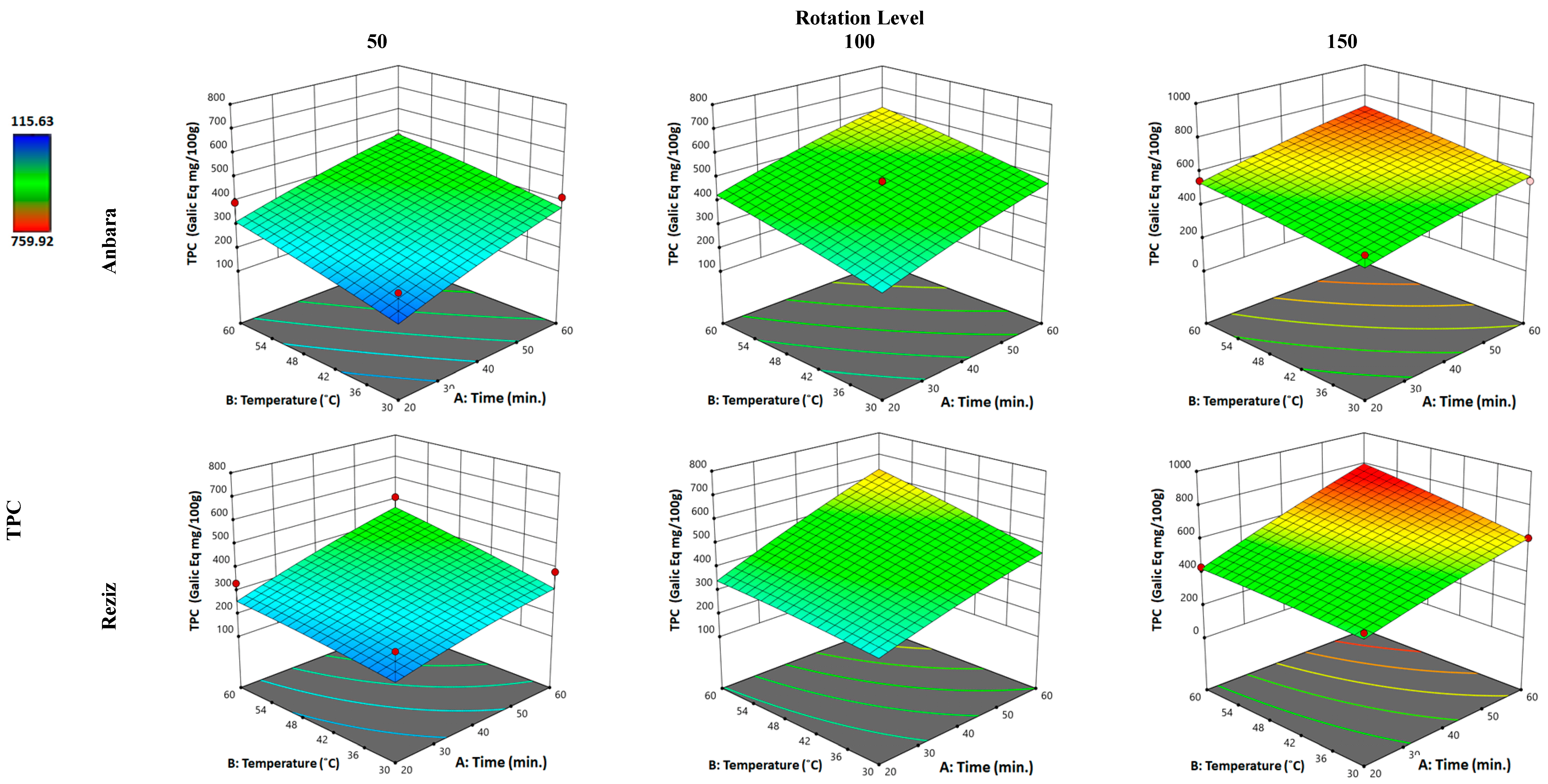
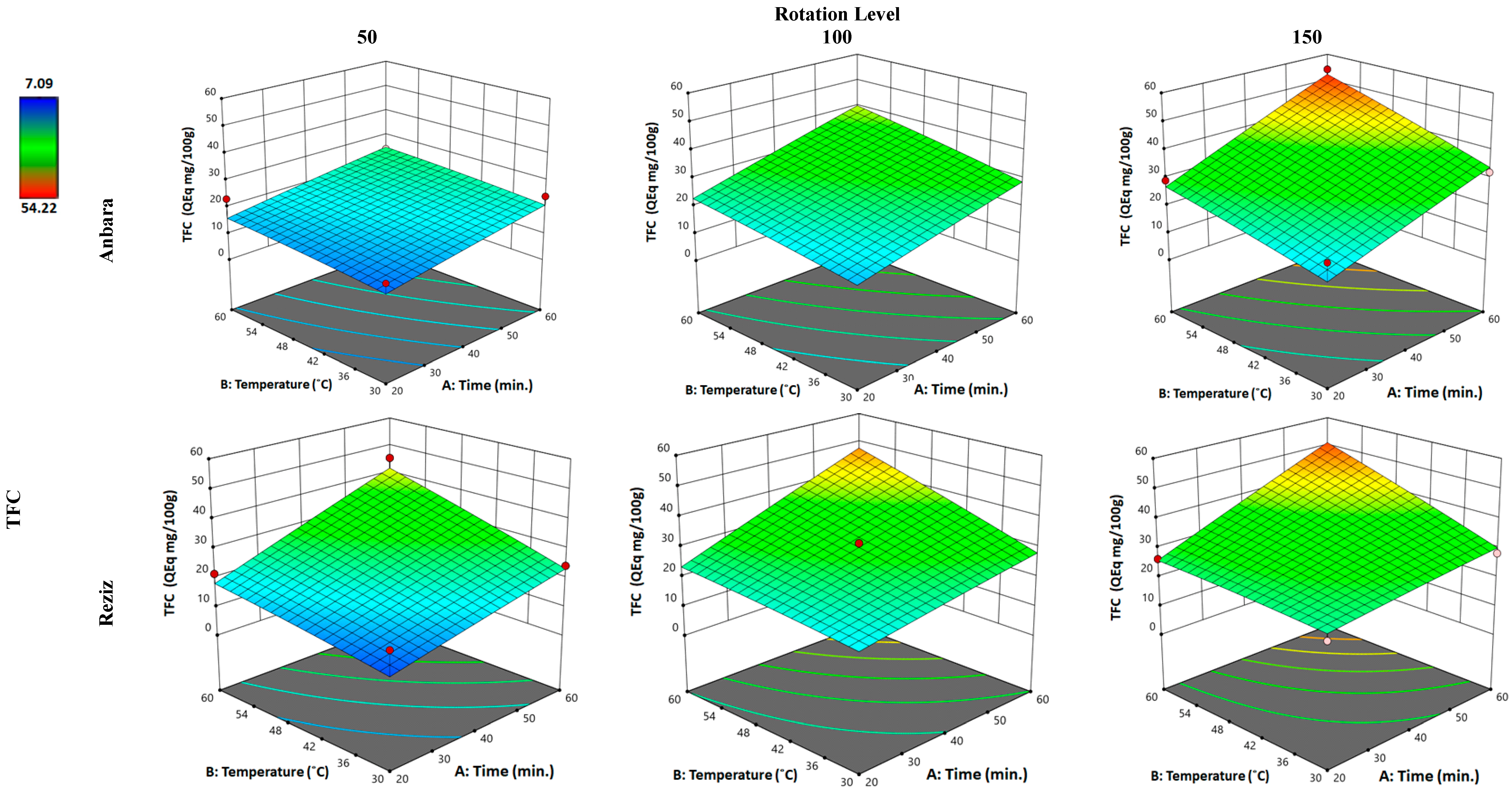
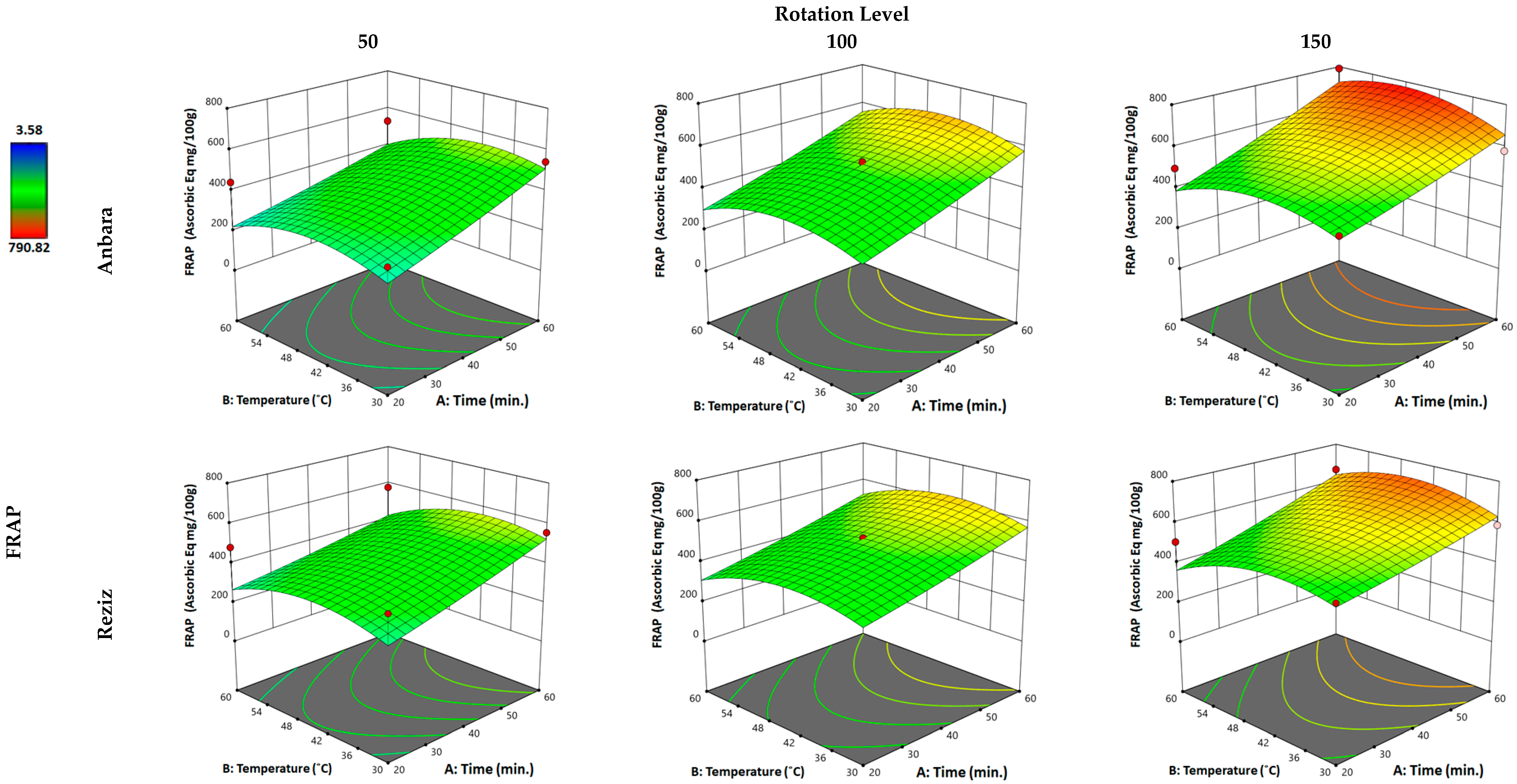
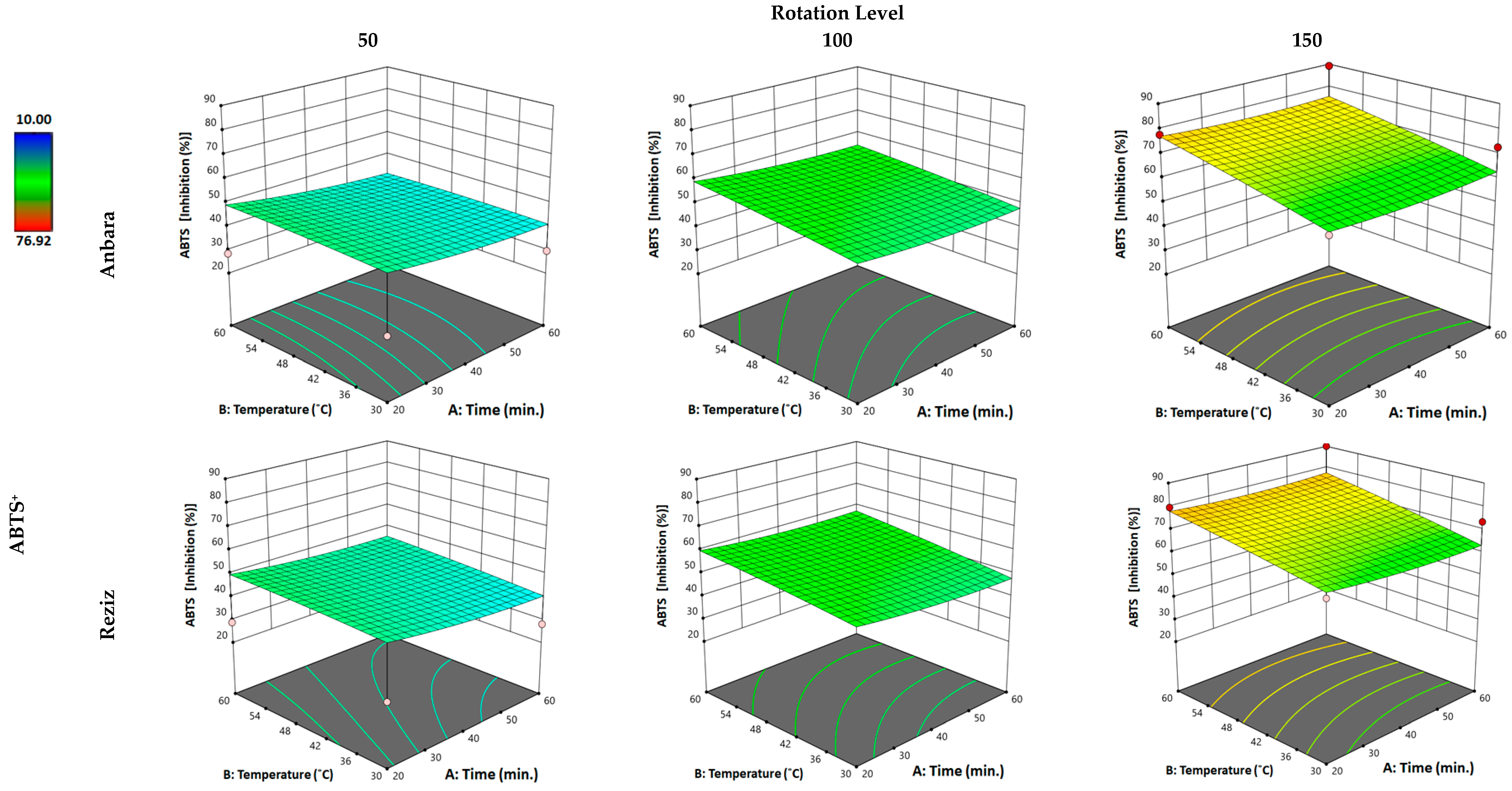

| Independent Variable “Factors” | Unit | Kind of Variable | Level of Variation | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Rotational speed a | (rpm) | Continuous | 50 | 100 | 150 |
| Temperature b | (°C) | Continuous | 30 | 45 | 60 |
| Time | (min.) | Continuous | 20 | 40 | 60 |
| Cultivar | Type | Category | Anbara | Reziz | |
| Dependent “Response” Variable | Abbreviation | Unit | |||
| Total phenolic content | TPC | GAE: mg/100 g fresh sample | |||
| Total flavonoid content | TFC | QE: mg/100 g fresh sample | |||
| Ferric reduces the antioxidant frequency | FRAP | AE: mmol/100 g fresh sample | |||
| The ability of free radical scavenging | ABTS | Inhibition (%) | |||
| Cultivar | Hydro-Ethanol * | Distilled Water | ||||||
|---|---|---|---|---|---|---|---|---|
| TPC ** | TFC # | FRAP & | ABTS @ | TPC ** | TFC # | FRAP & | ABTS @ | |
| An | 894 ± 18.3 | 63.23 ± 3.2 | 995 ± 46.3 | 78.34 ± 2.2 | 265 ± 10.4 | 18.24 ± 2.4 | 350 ± 24.2 | 23.41 ± 1.4 |
| Rz | 788 ± 59.2 | 50.21 ± 2.6 | 953 ± 62.4 | 74.35 ± 5.6 | 189 ± 22.4 | 16.43 ± 1.34 | 455 ± 22.8 | 20.79 ± 1.2 |
| Variables | Measuring Unit | Date Palm Fruit Cultivar | ||
|---|---|---|---|---|
| Anbara | Reziz | |||
| Affecting variables (factors) | Rotation | Rpm | 103 | 108 |
| Temperature | °C | 30 | 38.7 | |
| Time | (min) | 20.44 | 36.624 | |
| Response variables | TPC | (GAE: mg/100 g fresh sample) | 344.322 | 411.295 |
| TFC | QE: mg/100 g fresh sample) | 22.34 | 28.09 | |
| FRAP | (AE: mmol/100 g fresh sample) | 1061.692 | 506.509 | |
| ABTS | (Scavenging effect %) | 48.173 | 54.388 | |
| Animal Groups | Initial Weight (g) | Final Weight | Δ Changing in Body Weight |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| Control | 95.00 ± 5.00 | 158.50 ± 2.50 | 67.64 ± 6.19 |
| An | 103.33 ± 6.03 | 184.67 ± 6.66 | 79.29 ± 4.02 |
| Rz | 98.00 ± 2.29 | 172.00 ± 2.65 | 75.58 ± 1.41 |
| CCl4 | 95.00 ± 5.00 € | 103.67 ± 3.21 € | 9.44 ± 2.38 € |
| CCl4 + An | 102.50 ± 3.54 ₳ | 136.50 ± 2.12 ₳ | 33.39 ± 2.54 ₳ |
| CCl4 + Rz | 100.00 ± 5.00 ₳ | 136.00 ± 1.00 ₳ | 36.71 ± 5.84 ₳ |
| Animal Group | ALT (U/L) | AST (U/L) | ALP (U/L) | γGT (U/L) | LDH (U/L) |
|---|---|---|---|---|---|
| Control | 23.33 ± 2.52 | 163.67 ± 2.52 | 43.33 ± 4.04 | 1.53 ± 0.55 | 79.00 ± 4.00 |
| An | 29.50 ± 0.71 | 209.50 ± 0.71 | 51.00 ± 1.41 | 6.35 ± 0.50 | 1064.50 ± 3.54 |
| Rz | 32.60 ± 0.53 | 211.63 ± 0.55 | 53.67 ± 1.53 | 8.50 ± 0.46 | 1181.67 ± 2.08 |
| CCl4 | 60.00 ± 3.61 € | 293.00 ± 2.65 € | 269.00 ± 4.00 € | 21.00 ± 4.58 € | 1332.67 ± 2.52 € |
| CCl4 + An | 36.47 ± 0.50 | 250.33 ± 1.53 ₳ | 71.33 ± 2.52 ₳ | 12.33 ± 1.53 ₳ | 1267.33 ± 2.08 ₳ |
| CCl4 + Rz | 38.00 ± 1.00 | 273.33 ± 1.53 ₳ | 72.33 ± 2.52 ₳ | 15.00 ± 1.00 ₳ | 1280.67 ± 2.08 ₳ |
| Animal Group | (GSH) (μmol/L) | (GPX) (μmol/L) | (MDA) (nmol/mL) | (NO) (µM/mL) |
|---|---|---|---|---|
| Control | 57.03 ± 0.15 | 152.40 ± 5.36 | 32.53 ± 0.21 | 2.37 ± 0.15 |
| An | 53.50 ± 0.85 | 156.75 ± 3.70 | 122.50 ± 0.14 | 7.70 ± 0.28 |
| Rz | 54.23 ± 0.15 | 149.87 ± 2.15 | 69.53 ± 0.15 | 3.33 ± 0.04 |
| CCl4 | 21.47 ± 0.15 € | 68.47 ± 4.15 € | 143.50 ± 0.10 € | 2.85 ± 0.05 € |
| CCl4 + An | 46.50 ± 0.20 ₳ | 101.30 ± 3.20 ₳ | 61.47 ± 0.15 ₳ | 4.33 ± 0.15 ₳ |
| CCl4 + Rz | 39.40 ± 0.10 ₳ | 118.70 ± 3.68 ₳ | 48.60 ± 0.10 ₳ | 4.04 ± 0.05 ₳ |
| Animal Group | IFN-γ (pg/mL) | NFκB (ng/mL) | TNF-α (pg/mL) |
|---|---|---|---|
| Control | 96.20 ± 0.20 | 68.13 ± 0.15 | 22.37 ± 0.15 |
| An | 98.55 ± 0.07 | 71.53 ± 0.15 | 20.73 ± 0.15 |
| Rz | 90.67 ± 0.21 | 73.67 ± 0.21 | 23.23 ± 0.03 |
| CCl4 | 15.17 ± 0.15 € | 127.85 ± 0.07 € | 73.65 ± 0.35 € |
| CCl4 + An | 46.73 ± 0.21 ₳ | 90.63 ± 0.15 ₳ | 27.60 ± 0.30 ₳ |
| CCl4 + Rz | 65.33 ± 0.21 ₳ | 82.33 ± 0.21 ₳ | 25.33 ± 0.21 ₳ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, N.K.; Mohamed, H.A.; Moawad, M.E.; Younis, N.S.; Mohamed, M.E. The Hepatoprotective Effect of Two Date Palm Fruit Cultivars’ Extracts: Green Optimization of the Extraction Process. Foods 2023, 12, 1229. https://doi.org/10.3390/foods12061229
Alqahtani NK, Mohamed HA, Moawad ME, Younis NS, Mohamed ME. The Hepatoprotective Effect of Two Date Palm Fruit Cultivars’ Extracts: Green Optimization of the Extraction Process. Foods. 2023; 12(6):1229. https://doi.org/10.3390/foods12061229
Chicago/Turabian StyleAlqahtani, Nashi K., Hisham A. Mohamed, Mahmoud E. Moawad, Nancy S. Younis, and Maged E. Mohamed. 2023. "The Hepatoprotective Effect of Two Date Palm Fruit Cultivars’ Extracts: Green Optimization of the Extraction Process" Foods 12, no. 6: 1229. https://doi.org/10.3390/foods12061229
APA StyleAlqahtani, N. K., Mohamed, H. A., Moawad, M. E., Younis, N. S., & Mohamed, M. E. (2023). The Hepatoprotective Effect of Two Date Palm Fruit Cultivars’ Extracts: Green Optimization of the Extraction Process. Foods, 12(6), 1229. https://doi.org/10.3390/foods12061229








