Visualization of Sugar Content Distribution of White Strawberry by Near-Infrared Hyperspectral Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. White Strawberry Samples
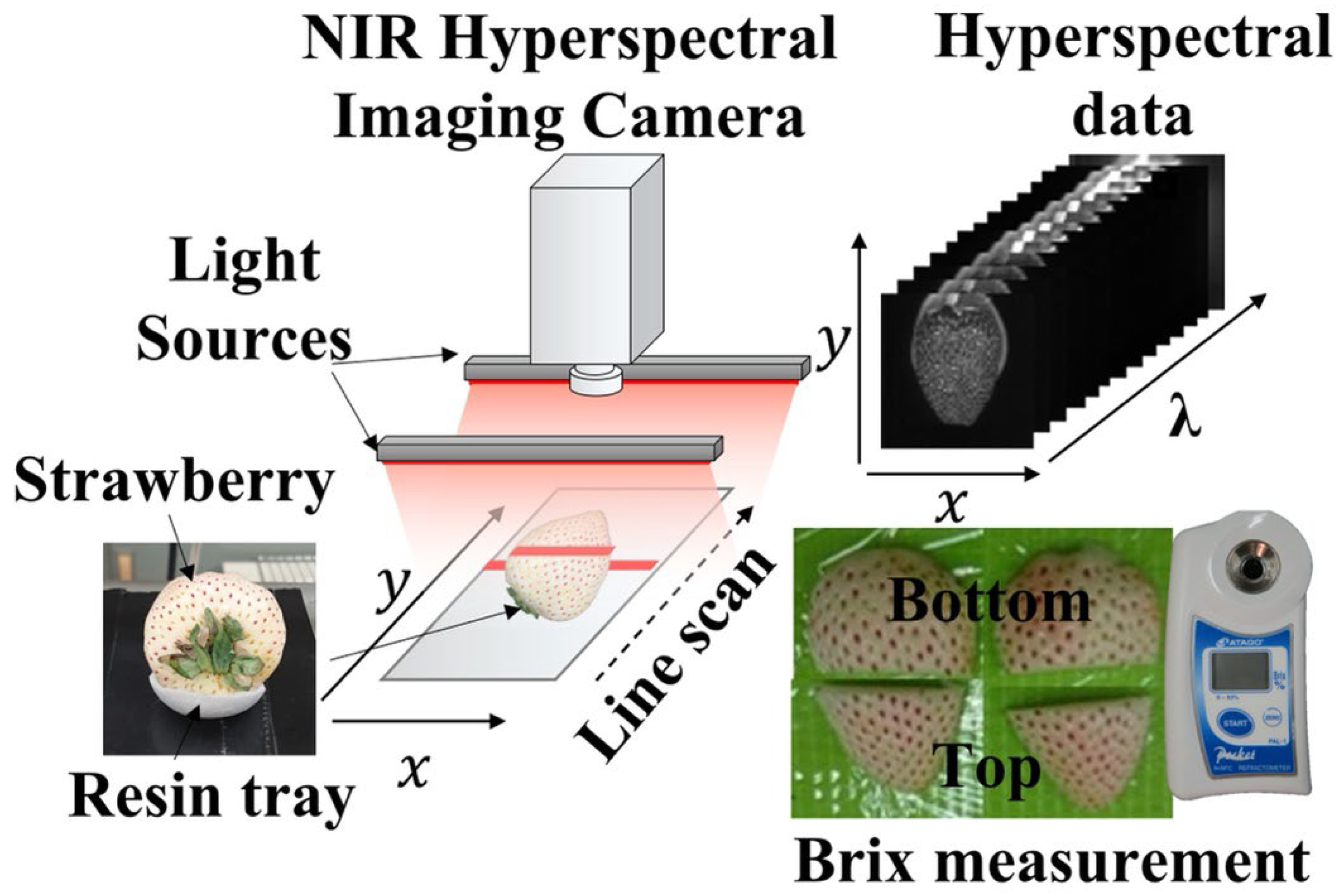
2.2. NIR Hyperspectral Images and Brix Measurements
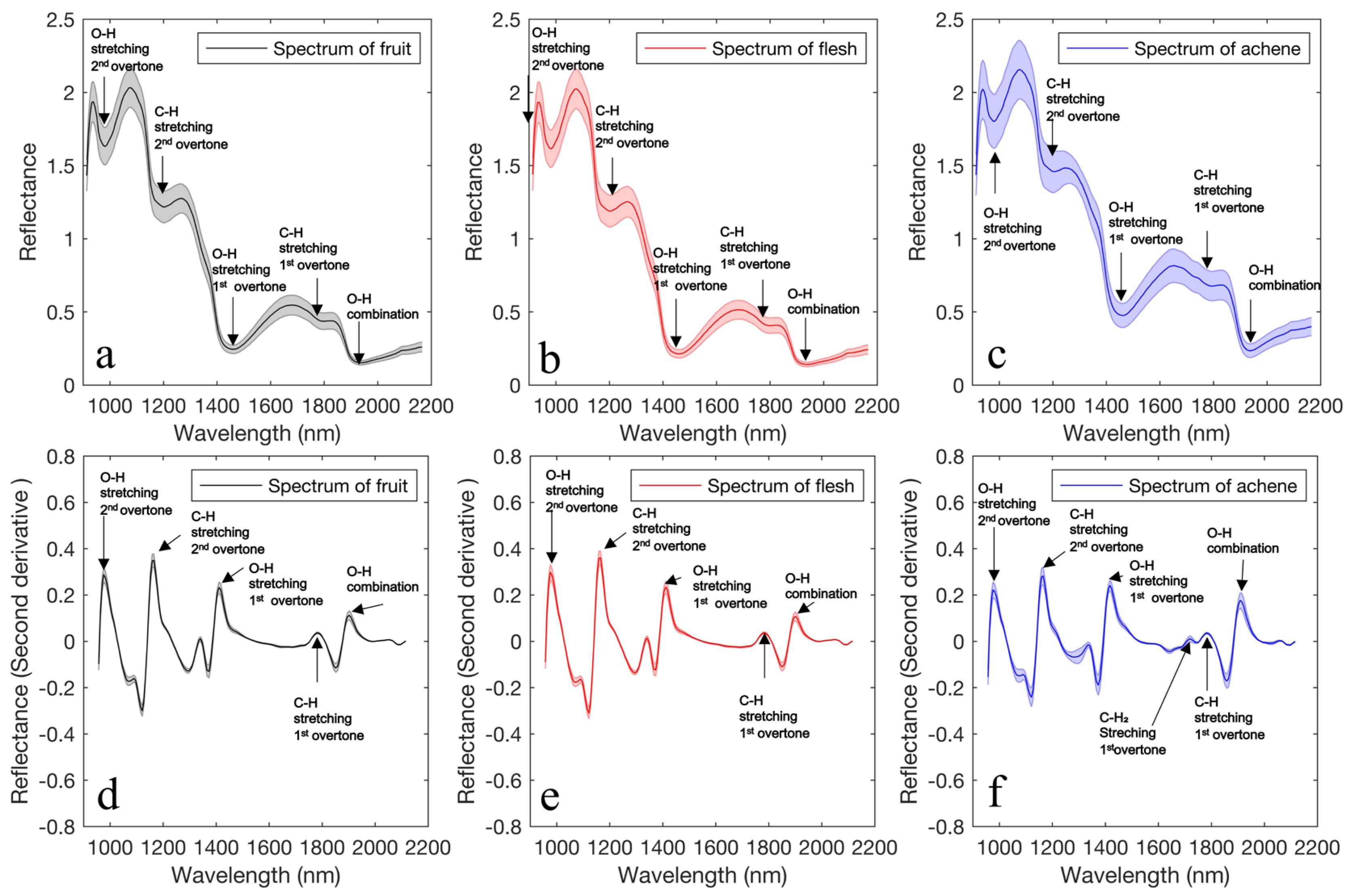
2.3. Preprocessing of Hyperspectral Images
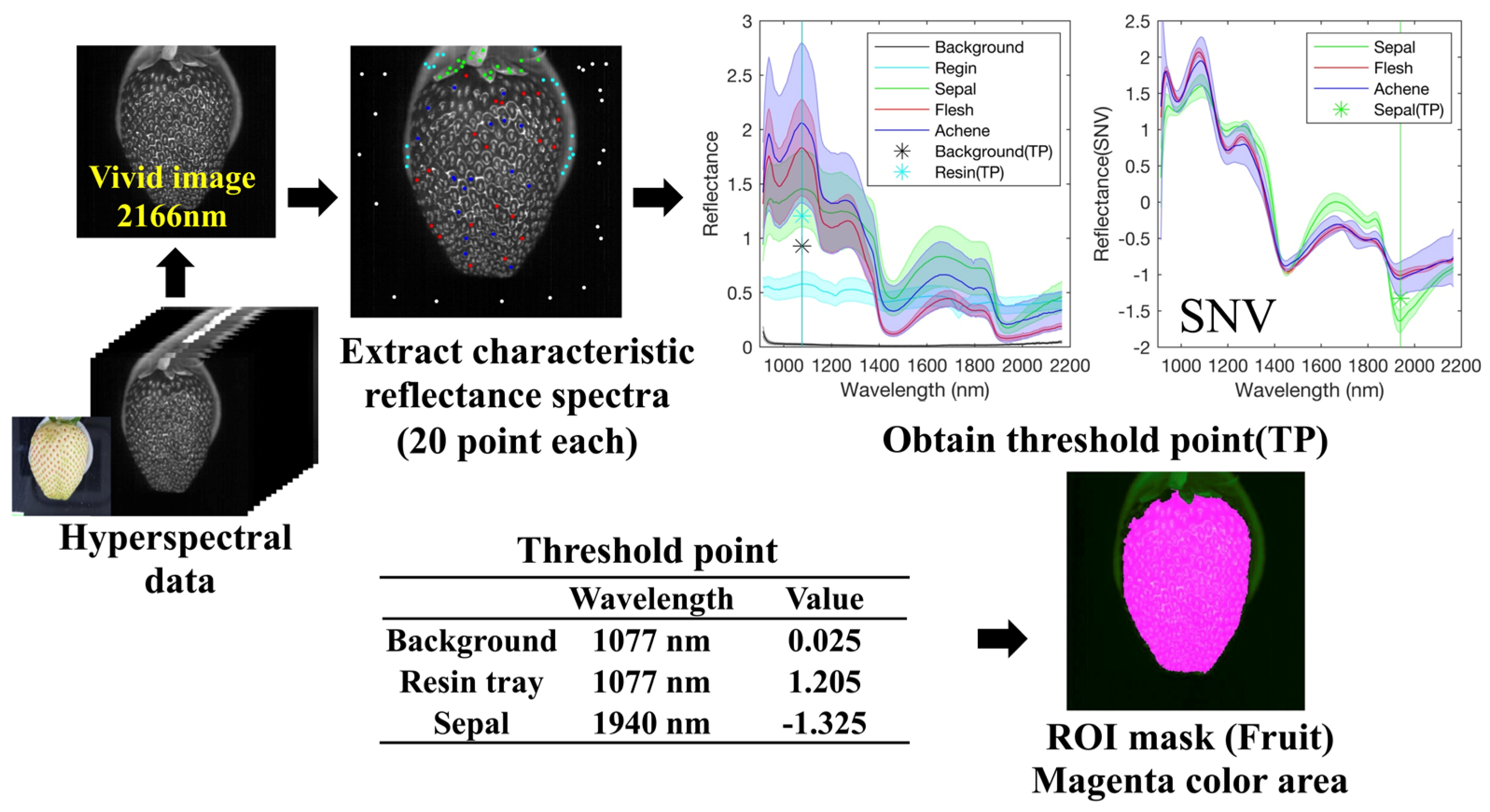
2.3.1. Creating a Fruit Mask Using Thresholding
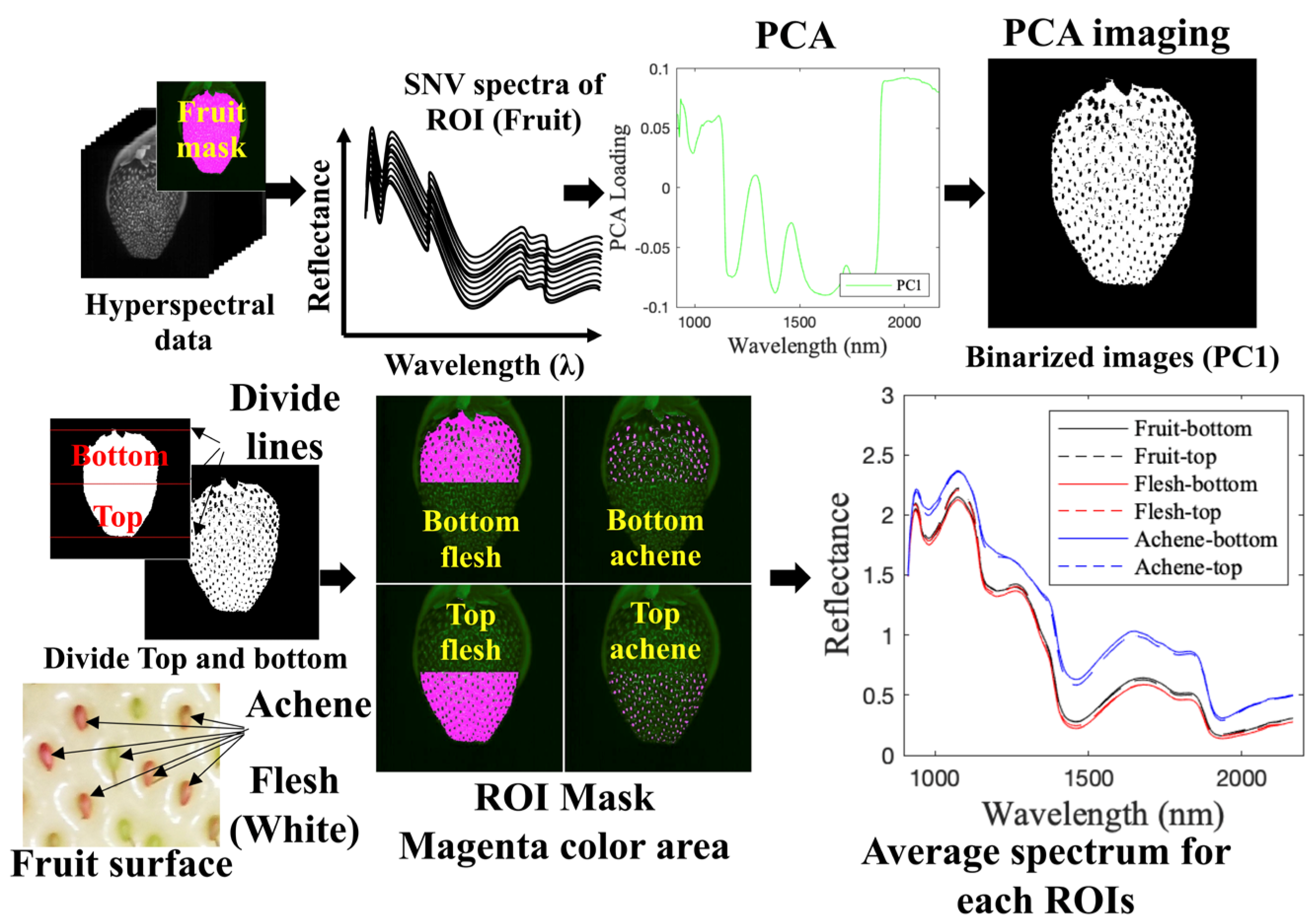
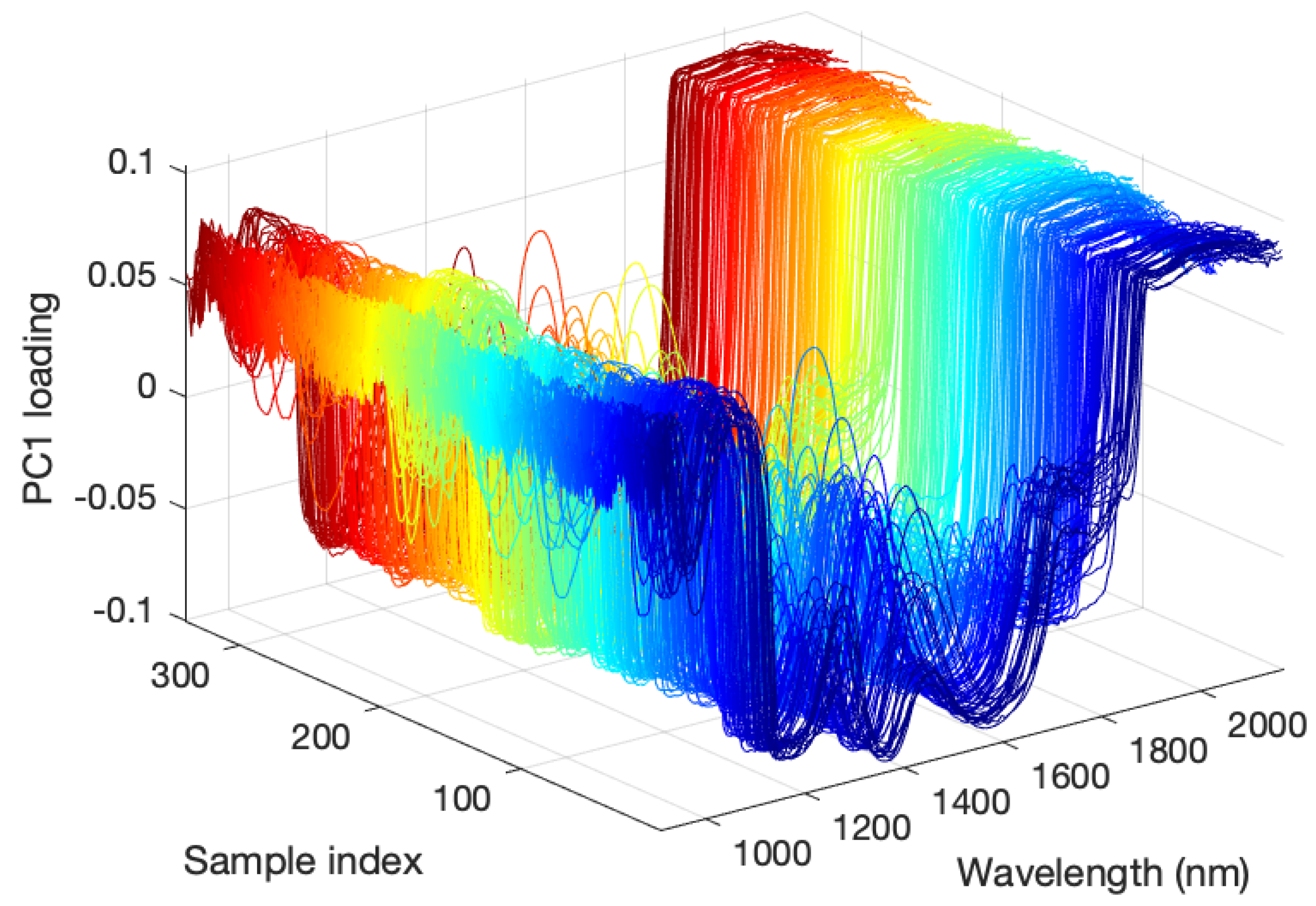
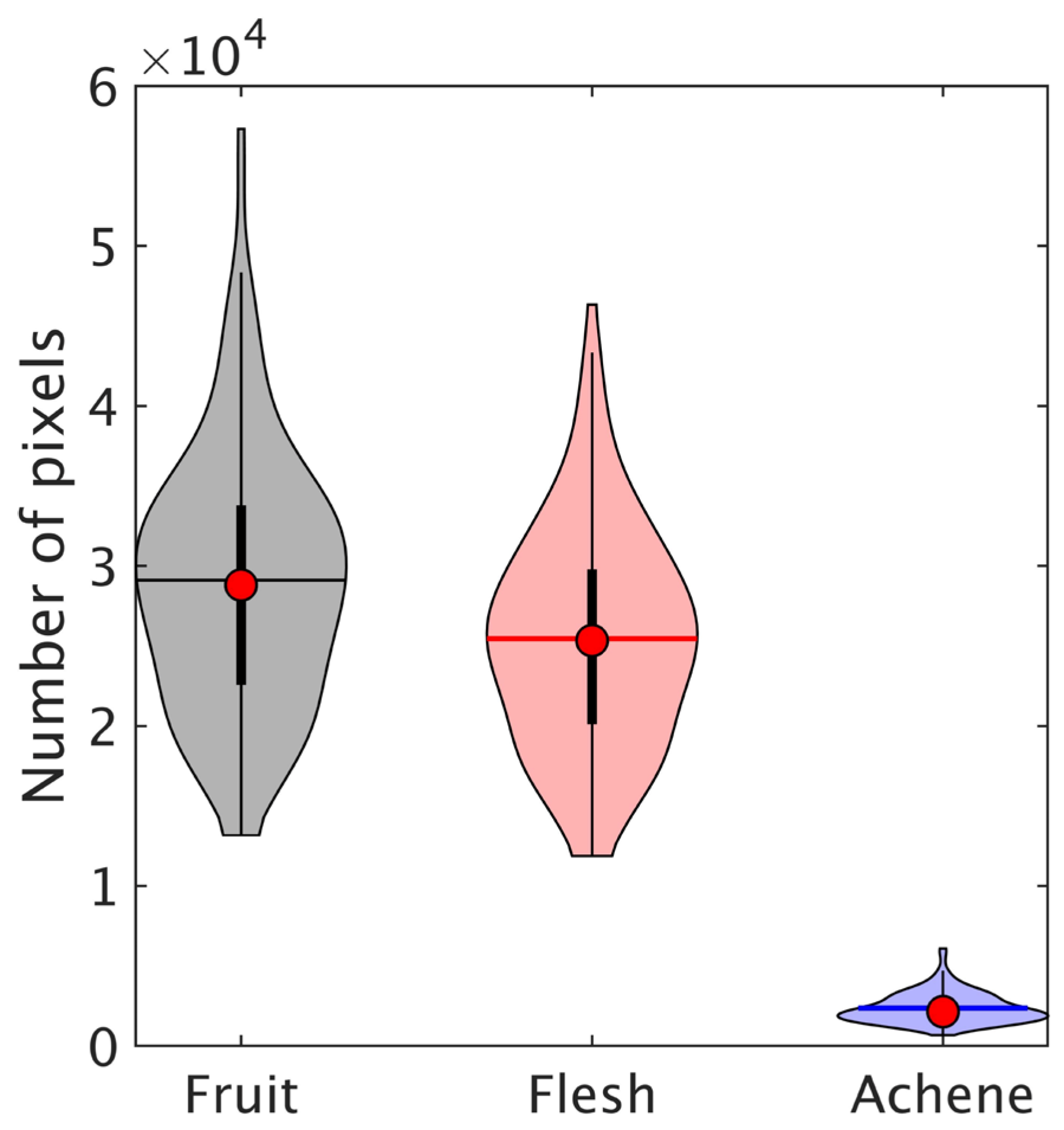
2.3.2. Determination of ROI Corresponding to Flesh Part and Achene Part Using a Combination of PCA and Image Processing
2.4. PLSR Modeling
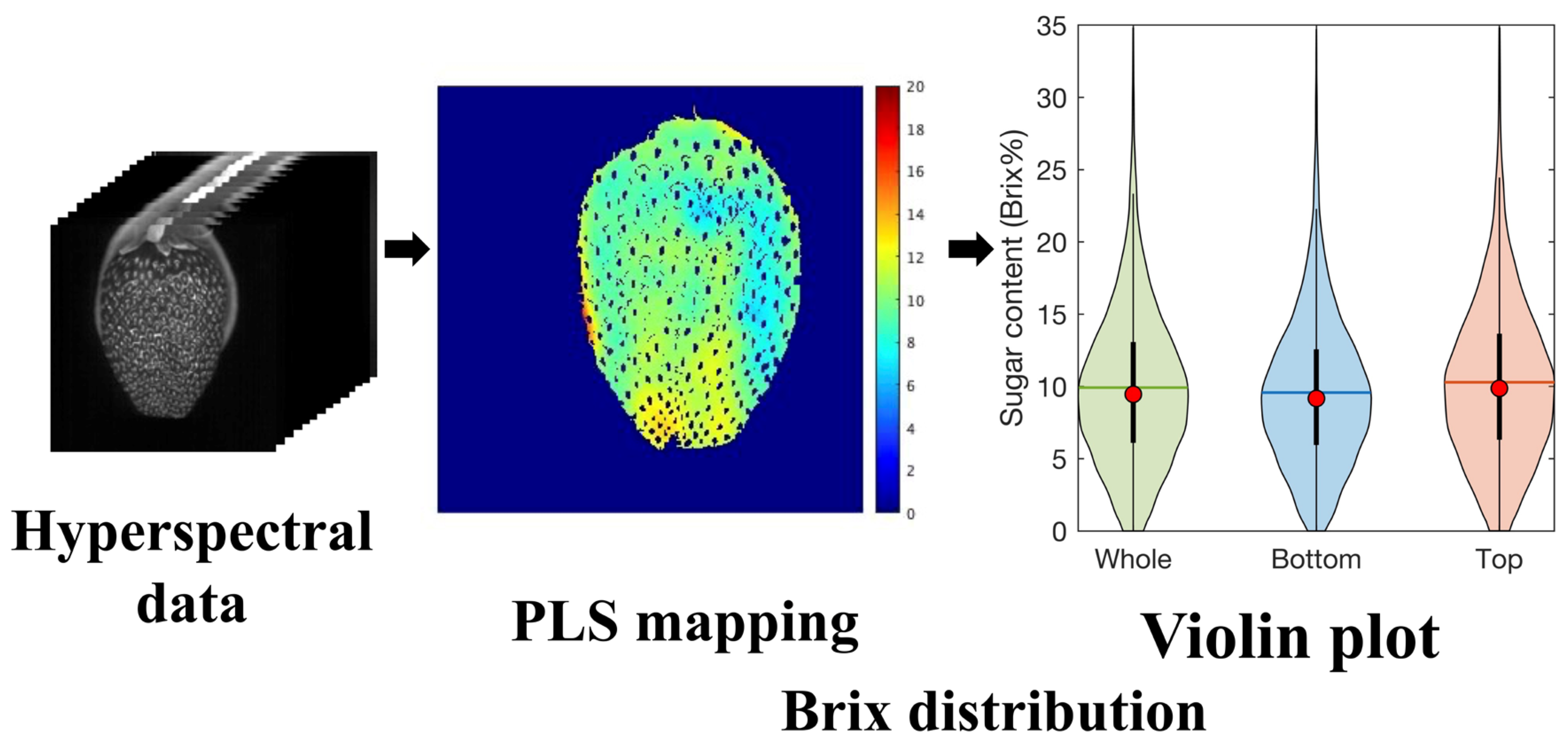
2.5. Visualization of the Sugar Content Distribution
3. Results and Discussion
3.1. Preprocessing of Hyperspectral Images
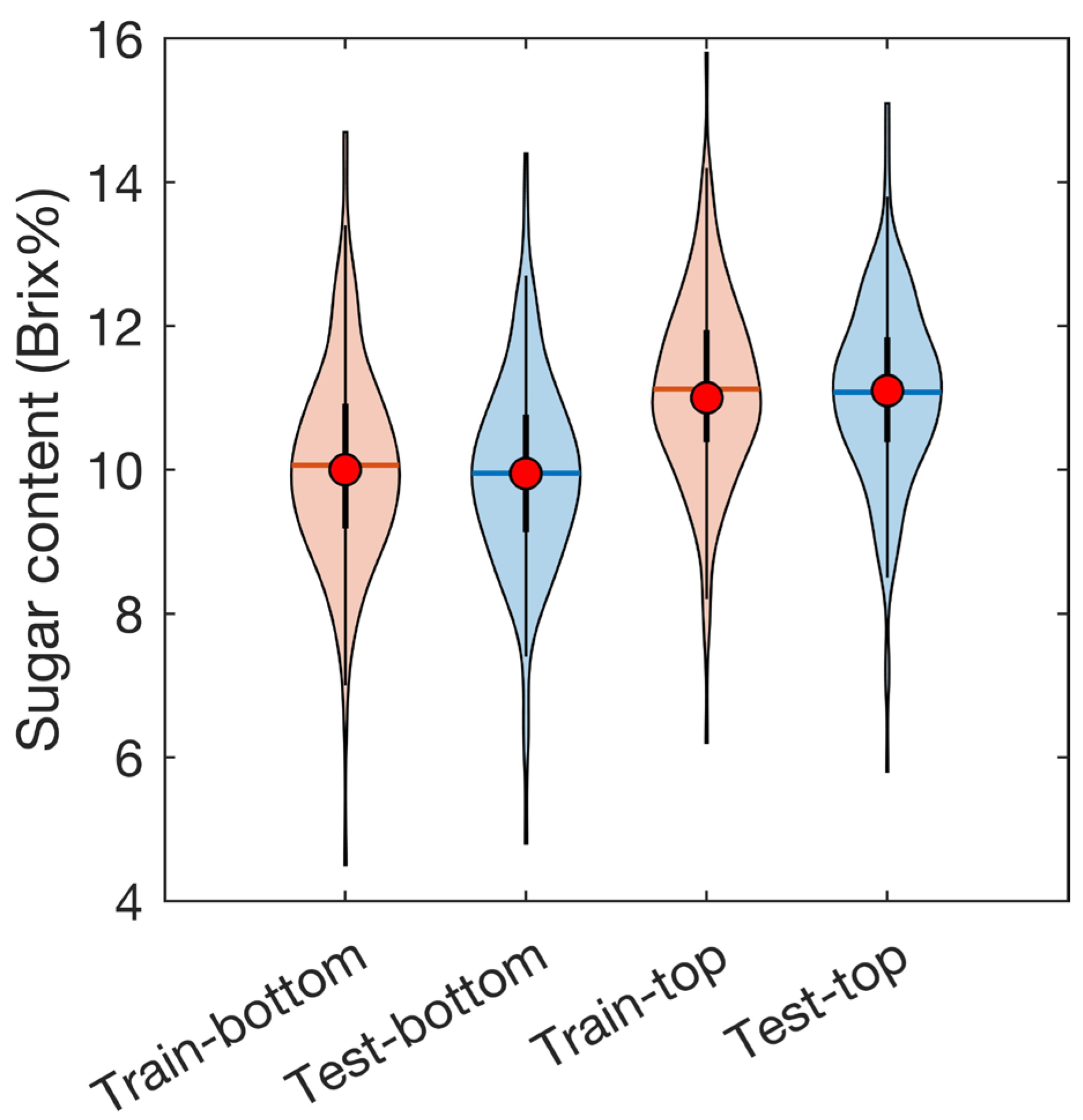
3.2. PLSR Model
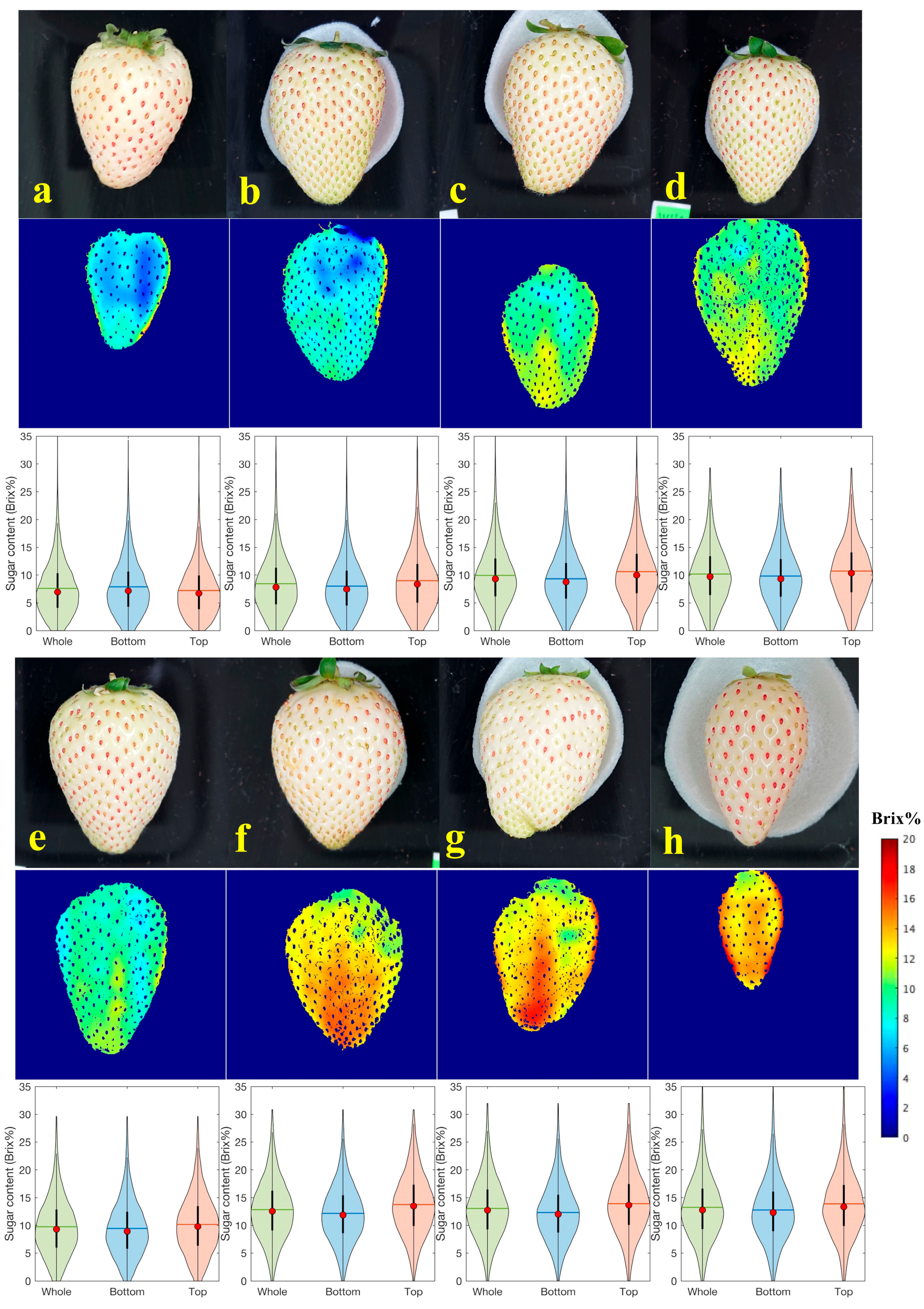
3.3. Visualization of the Sugar Content Distribution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva, F.L.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Muñoz, C.; Hoffmann, T.; Escobar, N.M.; Ludemann, F.; Botella, M.A.; Valpuesta, V.; Schwab, W. The strawberry fruit Fra a allergen functions in flavonoid biosynthesis. Mol. Plant 2010, 3, 113–124. [Google Scholar] [CrossRef]
- Salvatierra, A.; Pimentel, P.; Moya-León, M.A.; Herrera, R. Increased accumulation of anthocyanins in Fragaria chiloensis fruits by transient suppression of FcMYB1 gene. Phytochemistry 2013, 90, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jiang, L.; Chen, Q.; Li, Y.; Zhang, Y.; Luo, Y.; Zhang, Y.; Sun, B.; Wang, X.; Tang, H. Comparative Transcriptome Profiling Analysis of Red- and White-Fleshed Strawberry (Fragaria × ananassa) Provides New Insight into the Regulation of the Anthocyanin Pathway. Plant Cell Physiol. 2018, 5, 1844–1859. [Google Scholar] [CrossRef]
- Tsurumi, R.; Nakanishi, T.; Ishihara, Y.; Ohashi, T.; Kojima, N.; Saitou, Y.; Kobayashi, Y.; Hatakeyama, A.; Iimura, K.; Handa, T. Breeding of a New Strawberry Cultivar with White Fruits ‘Tochigi i W1 go’. Bull. Tochigi Prefect. Agric. Exp. Stn. 2020, 81, 67–82. [Google Scholar]
- Shrestha, B.P.; Nagata, M.; Cao, Q. Study on Image Processing for Quality Estimation of Strawberries (Part 1). Shokubutsu Kojo Gakkaishi 2001, 13, 115–122. [Google Scholar] [CrossRef]
- ElMasry, G.; Wang, N.; ElSayed, A.; Ngadi, M. Hyperspectral imaging for nondestructive determination of some quality attributes for strawberry. J. Food Eng. 2007, 81, 98–107. [Google Scholar] [CrossRef]
- Sturm, K.; Koron, D.; Stampar, F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003, 83, 417–422. [Google Scholar] [CrossRef]
- Darbellay, C.; Carlen, C.; Azodanlou, R.; Villettaz, J. Measurement of the organoleptic quality of strawberries. Acta Hortic. 2002, 567, 819–822. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W.; Nicolaï, B. NIR Spectroscopy Applications for Internal and External Quality Analysis of Citrus Fruit—A Review. Food Bioprocess Technol. 2011, 5, 425–444. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; De la Haba, M.J.; Benítez-López, M.; Fernández-Novales, J.; Garrido-Varo, A.; Pérez-Marín, D. Non-destructive characterization and quality control of intact strawberries based on NIR spectral data. J. Food Eng. 2012, 110, 102–108. [Google Scholar] [CrossRef]
- Włodarska, K.; Szulc, J.; Khmelinskii, I.; Sikorska, E. Non-destructive determination of strawberry fruit and juice quality parameters using ultraviolet. visible, and near-infrared spectroscopy. J. Sci. Food Agric. 2019, 99, 5953–5961. [Google Scholar] [CrossRef]
- Pathmanaban, P.; Gnanavel, B.; Anandan, S.S. Recent application of imaging techniques for fruit quality assessment. Trends Food Sci. Technol. 2019, 94, 32–42. [Google Scholar] [CrossRef]
- Mo, C.; Kim, M.S.; Kim, G.; Lim, J.; Delwiche, S.R.; Chao, K.; Lee, H.; Cho, B.-K. Spatial assessment of soluble solid contents on apple slices using hyperspectral imaging. Biosyst. Eng. 2017, 159, 10–21. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Inagaki, T.; Yang, H.; Tsuchikawa, S. Noncontact evaluation of soluble solids content in apples by near-infrared hyperspectral imaging. J. Food Eng. 2019, 224, 53–61. [Google Scholar] [CrossRef]
- Pu, Y.-Y.; Sun, D.-W.; Riccioli, C.; Buccheri, M.; Grassi, M.; Cattaneo, T.M.P.; Gowen, A. Calibration Transfer from Micro NIR Spectrometer to Hyperspectral Imaging: A Case Study on Predicting Soluble Solids Content of Bananito Fruit (Musa acuminata). Food Anal. Methods 2018, 11, 1021–1033. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Feng, X.; Wu, F.; Zhou, R.; Jin, J.; Xu, K.; Yu, X.; He, Y. SSC and pH for sweet assessment and maturity classification of harvested cherry fruit based on NIR hyperspectral imaging technology. Postharvest Biol. Technol. 2018, 143, 112–118. [Google Scholar] [CrossRef]
- Tsuta, M.; Sugiyama, J.; Sagara, Y. Near-infrared imaging spectroscopy based on sugar absorption band for melons. J. Agric. Food Chem. 2001, 50, 48–52. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, D.; Liu, L.; Wang, Z. How to predict the sugariness and hardness of melons: A near-infrared hyperspectral imaging method. Food Chem. 2017, 218, 413–421. [Google Scholar] [CrossRef]
- Zhu, H.; Chu, B.; Fan, Y.; Tao, X.; Yin, W.; He, Y. Hyperspectral Imaging for Predicting the Internal Quality of Kiwifruits Based on Variable Selection Algorithms and Chemometric Models. Sci. Rep. 2017, 7, 7845. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, C.T.; Liu, F.; Kong, W.W.; He, Y.; Lou, B.G. Hyperspectral imaging analysis for ripeness evaluation of strawberry with support vector machine. J. Food Eng. 2016, 179, 11–18. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Zubik, M.; Mazurek, W.; Sosnowska, B. Detection of fungal infections in strawberry fruit by VNIR/SWIR hyperspectral imaging. Postharvest Biol. Technol. 2018, 139, 115–126. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, K.; Peng, J.; Xing, M.; Pan, L.; Tu, K. Identification of bruise and fungi contamination in strawberries using hyperspectral imaging technology and multivariate analysis. Food Anal. Methods 2018, 11, 1518–1527. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, K.; Xiao, H.; Tu, S.; Sun, K.; Sun, Y.; Pan, L.; Tu, K. Near-Infrared Hyperspectral Imaging Rapidly Detects the Decay of Postharvest Strawberry Based on Water-Soluble Sugar Analysis. Food Anal. Methods 2019, 12, 936–946. [Google Scholar] [CrossRef]
- Weng, S.; Yu, S.; Guo, B.; Tang, P.; Liang, D. Non-Destructive Detection of Strawberry Quality Using Multi-Features of Hyperspectral Imaging and Multivariate Methods. Sensors 2020, 11, 3074. [Google Scholar] [CrossRef]
- Weng, S.Z.; Yu, S.; Dong, R.L.; Pan, F.F.; Liang, D. Nondestructive detection of storage time of strawberries using visible near-infrared hyperspectral imaging. Int. J. Food Prop. 2020, 23, 269–281. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K.; Lawson, P. Short-wavelength near-infrared spectra of sucrose, glucose, and fructose with respect to sugar concentration and temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Hanhineva, K.; Beleggia, R.; Dai, N.; Rogachev, I.; Nikiforova, V.J.; Fernie, A.R.; Aharoni, A. Reconfiguration of the Achene and Receptacle Metabolic Networks during Strawberry Fruit Development. Plant Physiol. 2008, 148, 730–750. [Google Scholar] [CrossRef]
- Kobori, H.; Gorretta, N.; Rabatel, G.; Bellon-Maurel, V.; Chaix, G.; Roger, J.-M.; Tsuchikawa, S. Applicability of Vis-NIR hyperspectral imaging for monitoring wood moisture content (MC). Holzforschung 2012, 67, 307–314. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Li, H.-D.; Xu, Q.-S.; Liang, Y.-Z. libPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- The MathWorks, Inc. MATLAB, Version 2021a. 2021. Available online: https://jp.mathworks.com/ (accessed on 16 January 2023).
- Bechtold, B. Violin Plots for Matlab, Github Project. 2016. Available online: https://github.com/bastibe/Violinplot-Matlab (accessed on 16 January 2023).
- Ikegaya, A.; Toyoizumi, T.; Ohba, S.; Nakajima, T.; Kawata, T.; Ito, S.; Arai, E. Effects of distribution of sugars and organic acids on the taste of strawberries. Food Sci. Nutr. 2019, 7, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Amodio, M.L.; Ceglie, F.; Chaudhry, M.M.A.; Piazzolla, F.; Colelli, G. Potential of NIR spectroscopy for predicting internal quality and discriminating among strawberry fruits from different production systems. Postharvest Biol. Technol. 2017, 125, 112–121. [Google Scholar] [CrossRef]
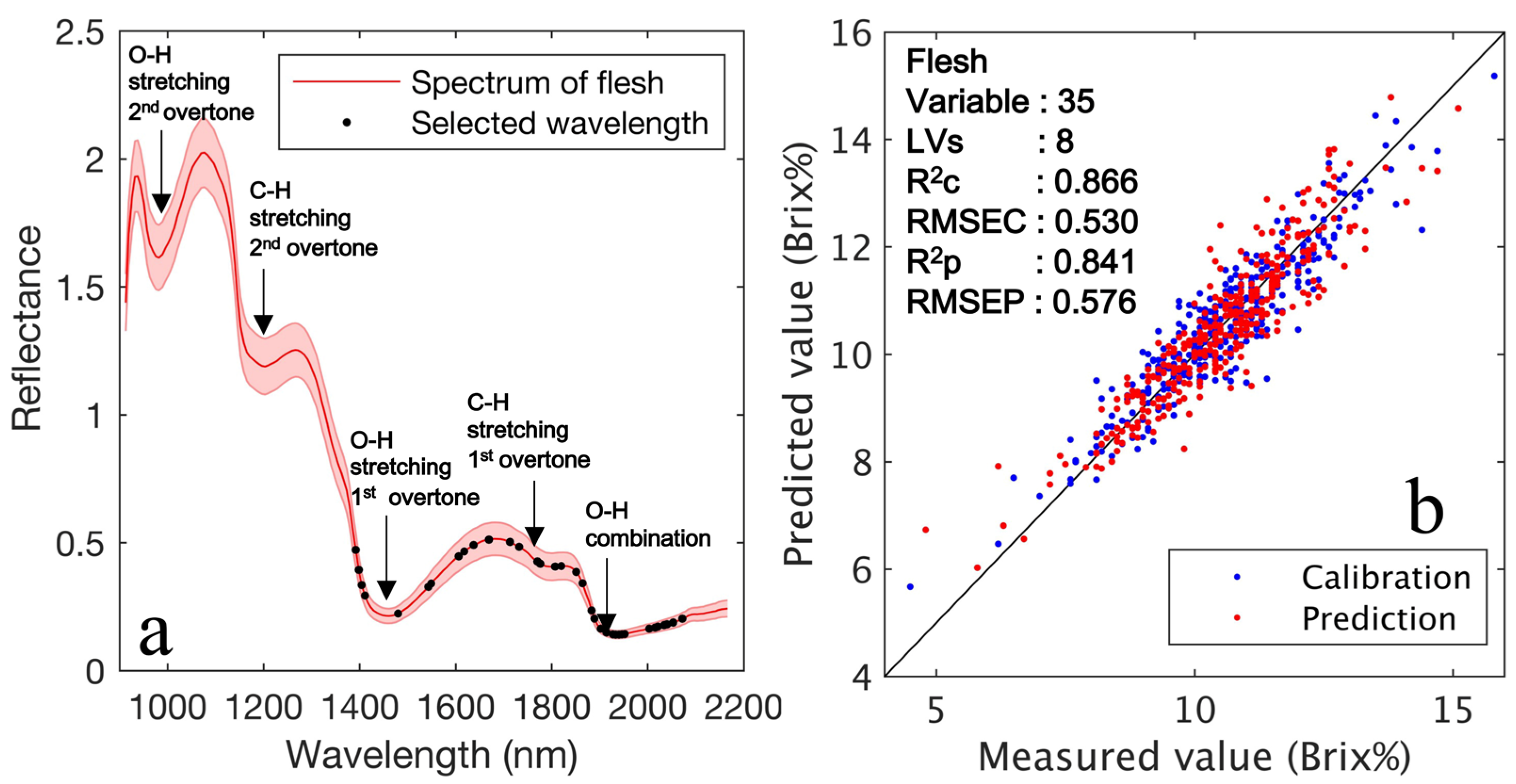
| Pretreat Method | Variable and LVs Selection | ROI | Variable | LVs | RMSECV (Brix%) | RMSEC (Brix%) | RMSEP (Brix%) | R2C | R2P |
|---|---|---|---|---|---|---|---|---|---|
| SNV + 2nd derivative | CARS + CV | Achene | 49 | 4 | 1.095 | 1.029 | 1.043 | 0.494 | 0.477 |
| 2nd derivative | CARS + CV | Achene | 75 | 5 | 1.068 | 0.997 | 1.038 | 0.525 | 0.482 |
| Raw | CARS + CV | Achene | 100 | 11 | 0.906 | 0.825 | 0.904 | 0.674 | 0.607 |
| SNV | CV | Achene | 200 | 16 | 0.798 | 0.630 | 0.805 | 0.810 | 0.688 |
| SNV | CARS + CV | Achene | 31 | 9 | 0.776 | 0.727 | 0.799 | 0.747 | 0.693 |
| SNV + 2nd derivative | CV | Achene | 185 | 10 | 0.841 | 0.735 | 0.793 | 0.742 | 0.697 |
| 2nd derivative | CV | Achene | 185 | 19 | 0.773 | 0.614 | 0.767 | 0.820 | 0.717 |
| 2nd derivative | CARS + CV | Flesh | 145 | 4 | 0.739 | 0.703 | 0.742 | 0.764 | 0.735 |
| 2nd derivative | CARS + CV | Fruit | 157 | 4 | 0.731 | 0.694 | 0.728 | 0.770 | 0.745 |
| Raw | CV | Achene | 200 | 19 | 0.722 | 0.567 | 0.716 | 0.846 | 0.753 |
| SNV | CARS + CV | Flesh | 37 | 8 | 0.572 | 0.537 | 0.714 | 0.862 | 0.755 |
| SNV + 2nd derivative | CARS + CV | Fruit | 88 | 4 | 0.680 | 0.649 | 0.692 | 0.799 | 0.769 |
| SNV + 2nd derivative | CARS + CV | Flesh | 34 | 5 | 0.691 | 0.645 | 0.683 | 0.801 | 0.775 |
| SNV | CARS + CV | Fruit | 60 | 9 | 0.630 | 0.579 | 0.632 | 0.839 | 0.808 |
| Raw | CARS + CV | Fruit | 26 | 9 | 0.600 | 0.566 | 0.633 | 0.847 | 0.808 |
| Raw | CARS + CV | Flesh | 35 | 8 | 0.558 | 0.530 | 0.576 | 0.866 | 0.841 |
| SNV + 2nd derivative | CV | Fruit | 185 | 15 | 0.506 | 0.436 | 0.555 | 0.909 | 0.852 |
| 2nd derivative | CV | Flesh | 185 | 20 | 0.537 | 0.442 | 0.553 | 0.907 | 0.853 |
| 2nd derivative | CV | Fruit | 185 | 20 | 0.516 | 0.433 | 0.541 | 0.910 | 0.859 |
| SNV | CV | Fruit | 200 | 15 | 0.533 | 0.459 | 0.527 | 0.899 | 0.866 |
| SNV | CV | Flesh | 200 | 15 | 0.573 | 0.476 | 0.523 | 0.891 | 0.869 |
| Raw | CV | Flesh | 200 | 17 | 0.528 | 0.450 | 0.520 | 0.903 | 0.870 |
| SNV + 2nd derivative | CV | Flesh | 185 | 16 | 0.517 | 0.438 | 0.512 | 0.908 | 0.874 |
| Raw | CV | Fruit | 200 | 19 | 0.511 | 0.413 | 0.500 | 0.918 | 0.880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seki, H.; Ma, T.; Murakami, H.; Tsuchikawa, S.; Inagaki, T. Visualization of Sugar Content Distribution of White Strawberry by Near-Infrared Hyperspectral Imaging. Foods 2023, 12, 931. https://doi.org/10.3390/foods12050931
Seki H, Ma T, Murakami H, Tsuchikawa S, Inagaki T. Visualization of Sugar Content Distribution of White Strawberry by Near-Infrared Hyperspectral Imaging. Foods. 2023; 12(5):931. https://doi.org/10.3390/foods12050931
Chicago/Turabian StyleSeki, Hayato, Te Ma, Haruko Murakami, Satoru Tsuchikawa, and Tetsuya Inagaki. 2023. "Visualization of Sugar Content Distribution of White Strawberry by Near-Infrared Hyperspectral Imaging" Foods 12, no. 5: 931. https://doi.org/10.3390/foods12050931
APA StyleSeki, H., Ma, T., Murakami, H., Tsuchikawa, S., & Inagaki, T. (2023). Visualization of Sugar Content Distribution of White Strawberry by Near-Infrared Hyperspectral Imaging. Foods, 12(5), 931. https://doi.org/10.3390/foods12050931






