Abstract
The Republic of Seychelles is located in Western-Central Indian Ocean, and marine capture fisheries play a key role in the country’s economic and social life in terms of food security, employment, and cultural identity. The Seychellois are among the highest per capita fish-consuming people in the world, with a high reliance on fish for protein. However, the diet is in transition, moving towards a Western-style diet lower in fish and higher in animal meat and easily available, highly processed foods. The aim of this study was to examine and evaluate the protein content and quality of a wide range of marine species exploited by the Seychelles industrial and artisanal fisheries, as well as to further to assess the contribution of these species to the daily intake recommended by the World Health Organization (WHO). A total of 230 individuals from 33 marine species, including 3 crustaceans, 1 shark, and 29 teleost fish, were collected from the Seychelles waters during 2014–2016. All analyzed species had a high content of high-quality protein, with all indispensable amino acids above the reference value pattern for adults and children. As seafood comprises almost 50% of the consumed animal protein in the Seychelles, it is of particular importance as a source of essential amino acids and associated nutrients, and as such every effort to sustain the consumption of regional seafood should be encouraged.
1. Introduction
The significance of global food and nutrition security is anchored in the United Nations Sustainable Development Goals (SDGs) SDG2 “Zero Hunger” and SDG 3 “Good Health and Well-Being” [1]. It is strongly encouraged that an increased food production should come from well-managed ocean resources. Land-based resources are limited, and agricultural food production is one of the major greenhouse gas (GHG) emitters [2,3].
Seafood plays an important role in food and nutrition security, particularly in low- and middle-income countries [4]. The nutritional recommendations to eat fish are based on their lipid content and fatty acid composition [5], although seafood is also an important source of vitamins and minerals [6,7] and high-quality proteins [8,9] that are important for human health and disease prevention. Seafood is also recognized as a rich source of taurine [10], considered to have a positive impact on cardiovascular diseases [11,12]. Seafood may also be a source of toxic heavy metals such as mercury, arsenic, lead, and cadmium [6,13,14], as well as persistent organic pollutants such as dioxins and dioxin-like polychlorinated biphenyls (PCBs) [15,16,17,18]. However, public agencies in Europe have reviewed available evidence through 2021 and concluded that the possible adverse effects of mercury, dioxin, and dioxin-like PCB exposure are offset by the benefits of seafood consumption on cardio-metabolic diseases in general [16,17] and that seafood consumption during pregnancy is likely to benefit the neurocognitive development of children [15]. Additionally, a relatively recent review conducted by Hibbeln et al. [19] concluded with moderate and consistent evidence that seafood consumption during pregnancy and childhood had beneficial associations with neurocognitive outcomes. Since 1986, an ongoing research project in the Seychelles (Seychelles Child Development Study, SCDS; https://www.urmc.rochester.edu/labs/seychelles.aspx, accessed on 1 January 2023) has been examining associations between maternal methylmercury exposure and neurodevelopment in children [20,21,22].
The Republic of Seychelles, one of the 38 United Nations member states of the Small Island Developing States’ group, is located in Western-Central Indian Ocean. It includes a land surface of only 459 km2 divided into 115 tropical islands scattered within an Exclusive Economic Zone (EEZ) of 1.3 million square kilometers [23]. The majority of the population resides on three islands of a large submerged mid-oceanic shelf called the Mahé Plateau. Marine capture fisheries play a key role in the country’s economic and social life. In addition to the industrial tuna fisheries being a major pillar of the economy, artisanal fisheries are continuously of great importance to the local population in terms of food security, employment, and cultural identity. Fish is seen not only as a staple food but also as a delicacy in the local Creole cuisine, and the Seychellois are among the highest per capita fish-consuming people in the world, with a high reliance on fish for protein—consuming about 59 kg per year measured as live weight [23], which is equivalent to 48% of the animal protein consumed [24]. Pregnant women and mothers have been reported to consume as many as 12 meals consisting of fish per week [25]. However, the diet in the Seychelles, as elsewhere, is in transition, moving towards a Western-style diet lower in fish and higher in animal meat and easily available, highly processed foods [26]. This has contributed to the increase in the prevalence of obesity (BMI ≥ 30 kg/m2) between 1998 and 2004 from 4 to 15% in men and from 23 to 34% in women [27], and it highlights the importance of fish in the diet.
Adequate protein intake is essential for tissue maintenance and growth, with amino acids being important as building blocks of proteins and as intermediates in various metabolic pathways. The nutritional quality of a protein is dependent on the content of indispensable, also called essential, amino acids, i.e., amino acids that are not synthesized in our body to meet the human requirements. The World Health Organization (WHO) recommends a daily dietary intake of protein of 830 mg protein/kg body weight for healthy adults; an additional 1, 9, and 31 g protein/day for pregnant women in the first, second, and third trimester, respectively; and 910 mg/kg body weight for children, in addition to specific recommendations for each of the indispensable amino acids [28,29].
The protein content of marine capture fisheries can significantly vary between species and even within species depending on habitat, region, and season [30]. Access to local and up-to-date food composition data is therefore essential for dietary counselling, clinical nutrition, and improvements in nutrition security and the development of effective food- and nutrition-related policies [31]. To our understanding, the protein contents and quality of different marine capture species from the Seychelles have not been investigated, nor have any data been published.
The objectives of this work were to examine the amino acid composition and to evaluate the protein content and protein quality of a wide range of marine species exploited by Seychelles industrial and artisanal fisheries, as well as further to assess the contribution of these species to the daily intake of proteins and essential amino acids recommended by the WHO.
2. Materials and Methods
2.1. Sample Collection and Preparation
A total of 230 individuals from 33 marine species, including 3 crustaceans, 1 shark, and 29 teleost fish, were collected from Seychelles waters during 2014–2016 (Table 1). Nearshore species were caught on the Mahé Plateau, where most of the artisanal fishing grounds are located [32], and offshore species were caught around the Mahé Plateau within the exclusive economic zone (EEZ) (Figure 1). After their capture, all organisms were measured (cephalothorax length (CL) for crustaceans, lower jaw-fork length (LJFL) for swordfish, and fork length (FL) and total length (TL) for other species) and weighted, and a piece of the edible part was collected from the tail for crustaceans and dorsal muscle for other species before being immediately stored at −80 °C. Samples were then freeze-dried for 72 h and ground to powder before amino acid analyses.

Table 1.
Marine species collected from the Seychelles waters, with associated details. Length (presented as mean ± SD) refers to the mean carapace length for crustaceans, the mean lower jaw-fork length for swordfish, and the mean fork length for other teleost fish and for sharks. N = number of individuals.
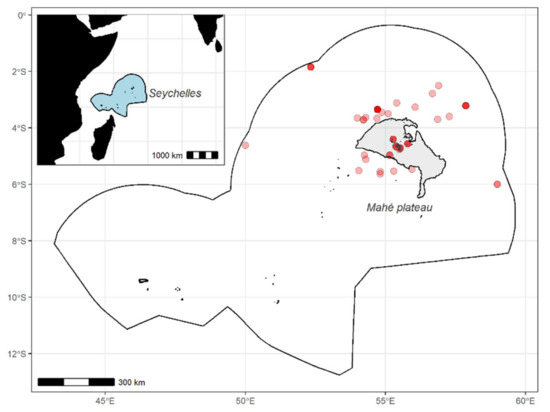
Figure 1.
Fishing locations of the 33 marine species on and around the Mahé Plateau, the Seychelles (Western Indian Ocean).
2.2. Amino Acid Composition and Protein Content
Amino acid composition was analyzed by dissolving approximately 40 mg of dried samples in 0.7 mL of distilled H2O and 0.5 mL of 20 mM norleucine (internal standard), which was then hydrolyzed as previously described [33,34]. Following hydrolysis, 100 µL aliquots of the hydrolysates were evaporated under nitrogen gas until complete dryness and re-dissolved to a suitable concentration in a lithium citrate buffer at pH 2.2. All amino acids were chromatographically analyzed using an ion exchange column followed by ninhydrin post column derivatization on a Biochrom 30 amino acid analyzer (Biochrom Co., Cambridge, UK). Amino acid residues were identified using the A9906 physiological amino acid standard (Sigma Chemical Co., St. Louis, MO, USA), as described previously [35]. The concentrations of 20 amino acids (histidine, his; isoleucine, ile; leucine, leu; lysine, lys; methionine, met; phenylalaline, phe; threonine, thr; valine, val; alanine, ala; b-alanine; b-ala; arginine, arg; asparagine, asn; aspartic acid, asp; cysteine, cys; glutamine, gln; glutamic acid, glu; glycine, gly; hydroxyproline, hyp; proline, pro; serine, ser; tyrosine, tyr; taurine, tau) were converted from dry weight to wet weight by using a mean moisture percentage of 72–81%, depending on species, and expressed in mg per 100 g of raw edible portion (noted mg/100 g). Tryptophan is denatured during acid hydrolysis, while glutamine and asparagine deaminate during acid hydrolysis and were therefore included in the category of glutamate and aspartic acid.
Protein content (g/100 g) was determined as the sum of the individual amino acid residues (the molecular weight of each amino acid after the subtraction of the molecular weight of H2O), as recommended by the FAO [36], using norleucine as internal standard.
2.3. Statistical Analyses
Statistical analyses were performed using IBM SPSS statistics 27. All samples were measured in duplicate, and the number of individuals analyzed from each marine species is presented in Table 1.
3. Results
3.1. Protein Content
The protein content, calculated as the sum of amino acids minus the molecular weight of water, was relatively constant among all fish species (Figure 2), varying between 13 and 17 g/100 g. Crustaceans had a lower protein content of approximately 11–12 g/100 g. The total amount of essential amino acids (EAAs) constituted half of the protein content for all species (Figure 2).
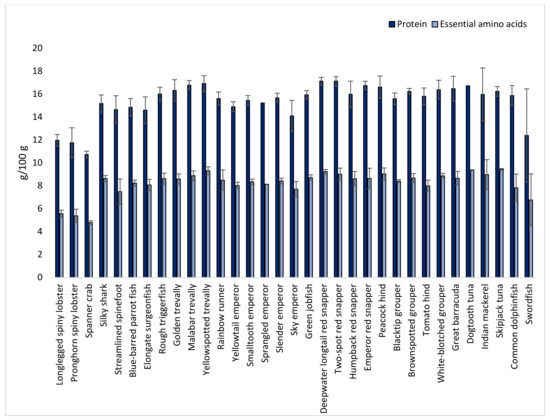
Figure 2.
Protein and sum of essential amino acid (threonine, valine, methionine, isoleucine, leucine, phenylalanine, lysine, and histidine; tryptophan is denatured during acid hydrolysis and is thus not included) content (g/100 g) in species caught in the Seychelles waters.
3.2. Distribution of Essential Amino Acids
The distribution of EAAs was similar for all fish species (Figure 3), with leucine and the commonly limiting amino acid lysine being the most abundant amino acids (1500–1700 mg/100 g and 1800–2000 mg/100 g, respectively). These two amino acids were also the most abundant in crustaceans, although their contributions were slightly lower than in fish. The concentrations of histidine were highly variable among the 33 studied species (from 250 to 1350 mg/100 g), with the highest relative content being measured in tunas and mackerels. The contents of threonine (776–1093 mg/100 g), valine (867–1091 mg/100 g), methionine (450–697 mg/100 g), isoleucine (799–1071 mg/100 g), and phenylalanine (650–908 mg/100 g) were higher in the fish species compared with the crustaceans (on average 569, 646, 402, 623, and 578 mg/100 g, respectively).
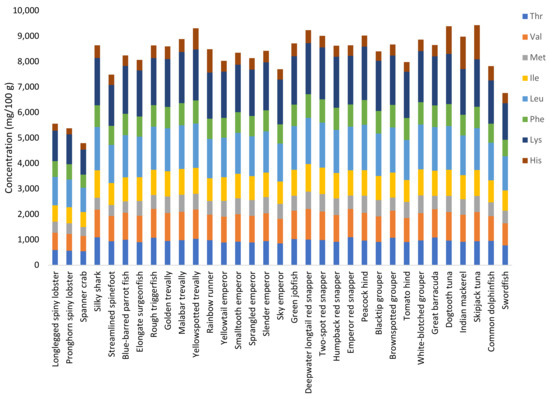
Figure 3.
Distribution of essential amino acids (mg/100 g) in species caught in the Seychelles waters. Thr, threonine; Val, valine; Met, methionine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Lys, lysine; His, histidine (tryptophan is denatured during acid hydrolysis and is thus not included).
3.3. Taurine Concentration
The concentration of taurine considerably varied within and among the studied species (Figure 4). Skipjack tuna and common dolphinfish were lowest in taurine (<20 mg/100 g), while humpback red snapper and peacock hind were highest in taurine (440 mg/100 g).
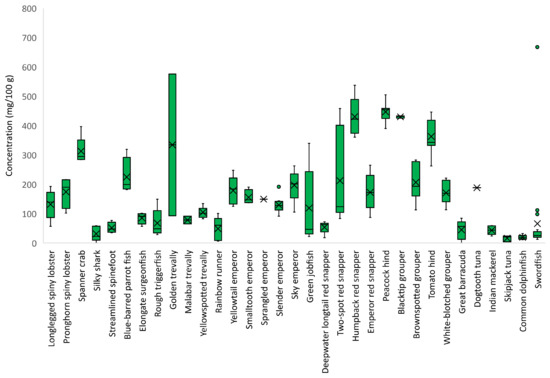
Figure 4.
Taurine concentration (mg/100 g) in species caught in the Seychelles waters. Mean values are represented by an ×, median values are represented by −, the boxes cover the interquartile range, and the whiskers represent the minimum and maximum values without outliers. Outliers are plotted as individual points.
4. Discussion
4.1. Protein Content
The protein content was calculated based on the amount of total amino acids minus the molecular weight of water, as recommended by the FAO [36]. This procedure efficiently hydrolyzes most of the peptide bonds while also reducing some amino acids. Tryptophan is denatured during acid hydrolysis, while glutamine and asparagine deaminate during acid hydrolysis and were therefore included in the category of glutamate and aspartic acid [37]. This may have resulted in a potential underestimation of the actual protein content and a lower protein content compared with that measured with the commonly used Kjeldahl´s method [33]. The protein content was similarly high in all marine species, with the exceptions of spanner crab and lobsters that showed slightly lower protein contents.
4.2. Contribution to Daily Recommended Intake
The Codex nutritional reference values for protein are based on the best available scientific knowledge of the daily amount needed for good health (830 mg protein/kg body weight for adults and 910 mg kg body weight for children). Based on these reference values and considering a portion size of 150 g for adults and 75 g for children, the contributions of one portion of each capture fishery species to the recommended dietary intake (RDI) for a 65 kg adult person, a 65 kg pregnant woman in the third trimester, and a 10-year-old child (average body weight of 30 kg) were estimated (Figure 5). One portion of swordfish and crustaceans (spiny lobster and spanner crab) would cover 30% of the adult, pregnant woman, and child RDIs. All other species would contribute 40–45% of these RDIs.
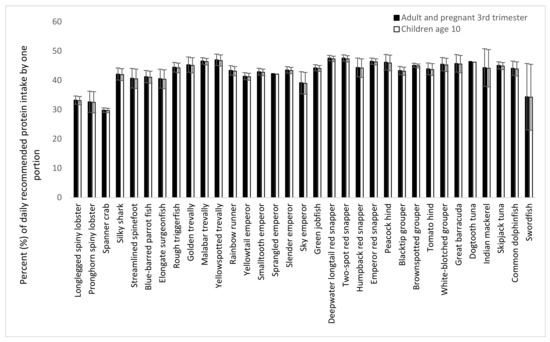
Figure 5.
Percent of recommended dietary intake (RDI) of protein for an adult (65 kg), a pregnant woman in the 3rd trimester (65 kg), or for a 10-year-old child (30 kg), that is covered by one portion of 150 or 75 g, respectively. Recommended dietary intake values from Codex [29] and the WHO [28] are used.
The FAO and WHO have recommended the dietary intake of each of the indispensable amino acids, based on growth and nitrogen balance. The percentage coverage of each of these amino acids for a 65 kg person by a 150 g portion of different species is illustrated in Figure 6. One portion of crustacean from the Palinuridae (spiny lobster) and Raninidae (spanner crab) families covered 50% of phenylalanine; 60–67% of valine, leucine, isoleucine, and histidine; and 90% of threonine, methionine, and lysine. One portion of fish covered approximately 70% of the daily recommended amount of phenylalanine and around or above 100% of the daily recommended amount of the other indispensable amino acids.
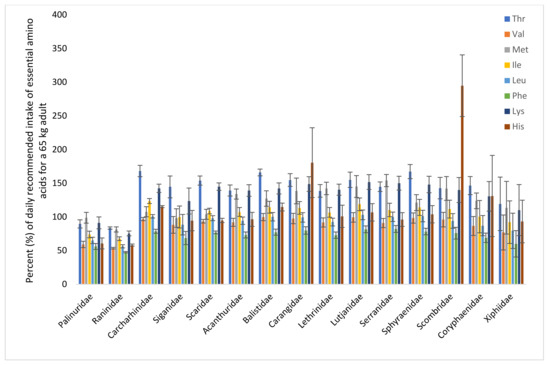
Figure 6.
Percent of recommended dietary intake of the essential amino acids for a 65 kg person by a portion of 150 g. Recommended dietary allowance values from the WHO [28] are used. Thr, threonine; Val, valine; Met, methionine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Lys, lysine; His, histidine; (tryptophan is denatured during acid hydrolysis and is thus not included).
4.3. Protein Quality
In addition to being building blocks for protein synthesis, each amino acid has its own metabolic pathway. The 20 proteogenic amino acids are classified as non-essential or essential. The nine essential amino acids (threonine, valine, methionine, isoleucine, leucine, phenylalanine, lysine, histidine, and tryptophan) cannot be synthesized in the human body from naturally occurring precursors at a rate needed to meet the metabolic requirements.
In this work, protein quality was determined based on the amount of essential amino acids. All analyzed fish species were high in lysine and threonine, which are strictly indispensable [1]. One portion of crustacean meat or fish filet was found to significantly contribute to the daily requirements of the indispensable amino acids, and one portion of fish filet was found to meet the requirements of threonine, methionine, and lysine. However, it is important to mention that all samples were analyzed raw, and several factors may influence amino acid contents during processing and household preparations such as boiling, baking, frying, and smoking [38,39], which may affect the amino acid contribution to the diet. These values thus indicate the amount available in pre-processed food and not the exact actually absorbed amount. The chemical score of amino acids used to assess the amount of limiting amino acids, can be used to determine if a diet meets the required amount of indispensable amino acids. The chemical score equals the ratio between each indispensable amino acid in the food protein and the corresponding amino acid in a reference protein proposed by the FAO/WHO. The protein-digestibility-corrected amino acid score can thereafter be calculated as the amino acid score multiplied by the true digestibility in humans [40]. Proteins of animal source normally have a chemical score of 1.0, while the scores of cereal proteins normally range from 0.4 to 0.6. All species analyzed in this work had a high protein quality, with the contents of all indispensable amino acids above the reference-scoring pattern for adults [40] indicating that the protein quality was superior. As tryptophan was denatured during the acid hydrolysis of the samples, it was not possible to assess whether it is a limiting amino acid.
4.4. Taurine
Accumulating evidence supports the idea that an increased dietary intake of taurine, a naturally occurring sulfonic acid, may be beneficial, as it has been documented to attenuate hypertension, suppress atherosclerosis, and exhibit antioxidative and anti-inflammatory properties [41,42,43]. Fish is recognized as a rich source of taurine [44], and urinary taurine may be used as a marker of the level of fish consumption [45]. In this study, taurine content greatly varied not only between species but also within different specimens of a given species. As a free amino acid, taurine is easily lost in handling and preparation, and its content is often found to significantly vary, even within one fillet [38,46]. A stricter control of all parts of the value chain would be necessary to avoid such variation. The levels of taurine measured in this study were within normal ranges compared to seafood in general, but as this is the first report of the levels of taurine for many of these species, comparison is challenging. The highest content was analyzed in the demersal species, humpback red snapper and tomato hind.
4.5. Regional Food and Nutrition Security
Food traditions are important. Food provides nutrients, and changes in lifestyles include nutrition transitions; the decreasing consumption of local foods is often associated with an increase in the consumption of carbohydrate-dense and highly processed foods. Such foods are normally cheaper and excessive in sugar, fat and additives. The high intake of refined food products has led to a worldwide elevated burden of overweight and obesity [47], and Seychellois are not an exception [26]. Malnutrition, excessive caloric consumption, and coexisting micronutrient deficiencies combined with declining activity levels may imply increases in and the earlier onset of lifestyle diseases, and global food systems may be leading to the poorer health of many [48,49,50].
5. Conclusions
This study provides detailed information on the concentrations of essential and non-essential amino acids and the protein content and quality of a wide range of tropical capture fishery species from the Seychelles (Western Indian Ocean) caught in both nearshore and offshore waters. The species’ contributions to the recommended daily intake values of indispensable amino acids from the WHO were assessed, and implications for regional food and nutrition security was discussed.
The captured fish species analyzed in this work had high contents of high-quality protein, with all indispensable amino acids above the reference value pattern for adults and children. Such species with high protein contents of superior quality are perceived as healthy foods. As fish makes up as much as 48% of the consumed animal protein in the Seychelles, it is of particular importance as a source of essential amino acids and associated nutrients such as fatty acids, taurine, vitamins and minerals. Accordingly, every effort to sustain the consumption of regional fish should be encouraged.
Author Contributions
Conceptualization, I.-J.J., N.B. and E.O.E.; methodology, I.-J.J., N.B. and R.G.; software, I.-J.J. and N.B.; validation, I.-J.J., E.O.E., N.B. and R.G.; writing—original draft preparation, I.-J.J.; writing—review and editing, E.O.E., I.-J.J., N.B. and R.G.; funding acquisition, I.-J.J., E.O.E., N.B. and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
The present work is a contribution to the SEYFISH project (“Nutrients and Contaminants in Seychelles fisheries resources”) with the financial support of the French Research Institute for Sustainable Development (IRD) and the European Fisheries Partnership Agreement (EU-FPA), as well as UiT—The Arctic University of Norway, project SECURE, Cristin grant ID 2061344. The publication charges for this article were funded by the NTNU Norwegian University of Science and Technology.
Data Availability Statement
The data are available on request.
Acknowledgments
The authors would like to thank all fishermen and crews who assisted with sampling. A special thank goes to the SFA staff (in alphabetic order: Clara Belmont, Dora Lesperance, Kettyna Gabriel, Maria Rose, Natifa Pillay, Rodney Melanie, Rona Arrisol, and Stephanie Hollanda) for their help in processing the samples and to Emmanuel Chassot (IOTC) for assisting with data management. The authors are also grateful to chief engineer Guro K. Edvinsen and senior engineer Hanne K. Mæhre for analytical contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development; UN: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The environmental cost of animal source foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Caldeira, K.; Chopin, T.; Gaines, S.; Haugan, P.; Hemer, M.; Howard, J.; Konar, M.; Krause-Jensen, D.; Lindstad, E. The ocean as a solution to climate change: Five Opportunities for Action. Available online: https://www.wri.org/events/2019/10/ocean-solution-climate-change-5-opportunities-action (accessed on 8 May 2021).
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022; 266p. [Google Scholar]
- Thomsen, S.T.; Assuncao, R.; Afonso, C.; Boué, G.; Cardoso, C.; Cubadda, F.; Garre, A.; Kruisselbrink, J.W.; Mantovani, A.; Pitter, J.G.; et al. Human health risk—benefit assessment of fish andother seafood: A scoping review. Food Sci. Nutr. 2022, 62, 7479–7502. [Google Scholar] [CrossRef]
- Bodin, N.; Lesperance, D.; Albert, R.; Hollanda, S.; Michaud, P.; Degroote, M.; Churlaud, C.; Bustamante, P. Trace elements in oceanic pelagic communitites in the western Indian Ocean. Chemosphere 2017, 174, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.P.W.; Maire, E.; Bodin, N.; Hempson, T.N.; Graham, N.A.J.; Wilson, S.K.; MacNeil, M.A.; Hicks, C.C. Climate-induced increases in micronutrient availability for coral reef fisheries. One Earth 2022, 5, 98–108. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.J.; Walquist, M.; Liaset, B.; Elvevoll, E.O.; Eilertsen, K.E. Dietary intake of cod and scallop reduces atherosclerotic burden in female apolipoprotein E-deficient mice fed a Western-type high fat diet for 13 weeks. Nutr. Metab. 2016, 13, 8. [Google Scholar] [CrossRef]
- Spitze, A.R.; Wong, D.L.; Rogers, Q.R.; Fascetti, A.J. Taurine concentrations in animal feed ingredients; cooking influences taurine content. J. Anim. Physiol. Anim. Nutr. 2003, 87, 251–262. [Google Scholar] [CrossRef]
- Elvevoll, E.O.; Eilertsen, K.E.; Brox, J.; Dragnes, B.T.; Falkenberg, P.; Olsen, J.O.; Kirkhus, B.; Lamglait, A.; Osterud, B. Seafood diets: Hypolipidemic and antiatherogenic effects of taurine and n-3 fatty acids. Atherosclerosis 2008, 200, 396–402. [Google Scholar] [CrossRef]
- Yamori, Y.; Liu, L.; Ikeda, K.; Miura, A.; Mizushima, S.; Miki, T.; Nara, Y.; Disease, W.H.-C.; Alimentary Comprarison Study, G. Distribution of twenty-four hour urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: Results from the WHO-CARDIAC study. Hypertens. Res. 2001, 24, 453–457. [Google Scholar] [CrossRef]
- Sabino, M.A.; Bodin, N.; Govinden, R.; Arrisol, R.; Churlaud, C.; Pethybridge, H.; Bustamante, P. The role of tropical small-scale fisheries in trace element delivery for a Small Island Developing State community, the Seychelles. Mar. Pollut. Bull. 2022, 181, 113870. [Google Scholar] [CrossRef]
- Sardenne, F.; Bodin, N.; Médieu, A.; Antha, M.; Arrisol, R.; Le Grand, F.; Bideau, A.; Munaron, J.-M.; Le Loc’h, F.; Chassot, E. Benefit-risk associated with the consumption of fish bycatch from tropical tuna fisheries. Environ. Pollut. 2020, 267, 115614. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Health Benefits of Seafood (Fish and Shellfish) Consumption in Relation to Health Risks Associated with Exposure to Methylmercury; EFSA: Parma, Italy, 2014. [Google Scholar]
- FAO; WHO. Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption; FAO: Rome, Italy, 2011. [Google Scholar]
- VKM. Benefit and Risk Assessment of Fish in the Norwegian Diet. Scientific Opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food and Environment; VKM: Oslo, Norway, 2022. [Google Scholar]
- Munschy, C.; Vigneau, E.; Bely, N.; Héas-Moisan, K.; Olivier, N.; Pollono, C.; Hollanda, S.; Bodin, N. Legacy and emerging organic contaminants: Levels and profiles in top predator fish from the western Indian Ocean in relation to their trophic ecology. Environ. Res. 2020, 188, 109761. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Spiller, P.; Brenna, J.T.; Golding, J.; Holub, B.J.; Harris, W.S.; Kris-Etherton, P.; Lands, B.; Connor, S.L.; Myers, G.; et al. Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: Two systematic reviews. Prostaglandins Leukot Essent Fat. Acids 2019, 151, 14–36. [Google Scholar] [CrossRef]
- Davidson, P.W.; van Wijngaarden, E.; Shamlaye, C.; Strain, J.J.; Myers, G.J. Putting findings from the Seychelles Child Development Study into perspective: The importance of a historical special issue of the Seychelles Medical and Dental Journal. Neurotoxicology 2020, 76, 111–113. [Google Scholar] [CrossRef]
- Strain, J.J.; Love, T.M.; Yeates, A.J.; Weller, D.; Mulhern, M.S.; McSorley, E.M.; Thurston, S.W.; Watson, G.E.; Mruzek, D.; Broberg, K.; et al. Associations of prenatal methylmercury exposure and maternal polyunsaturated fatty acid status with neurodevelopmental outcomes at 7 years of age: Results from the Seychelles Child Development Study Nutrition Cohort 2. Am. J. Clin. Nutr. 2021, 113, 304–313. [Google Scholar] [CrossRef]
- Strain, J.J.; Yeates, A.J.; van Wijngaarden, E.; Thurston, S.W.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Love, T.M.; Smith, T.H.; Yost, K.; et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: Associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am. J. Clin. Nutr. 2015, 101, 530–537. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Country Profiles. The Republic of Seychelles. Available online: http://www.fao.org/fishery/facp/SYC/en (accessed on 20 October 2022).
- Seychelles Fisheries Sector Policy and Strategy. 2019. Available online: http://www.mofa.gov.sc/downloads/Seychelles%20Fisheries%20Sector%20Policy%20.pdf (accessed on 20 October 2022).
- Strain, J.J. Eating fish for two. Nutr. Bull. 2014, 39, 181–186. [Google Scholar] [CrossRef]
- Cardoso, I.; Bovet, P.; Viswanathan, B.; Luke, A.; Marques-Vidal, P. Nutrition transition in a middle-income country: 22-year trends in the Seychelles. Eur. J. Clin. Nutr. 2012, 67, 135–140. [Google Scholar] [CrossRef]
- Bovet, P.; Chiolero, A.; Shamlaye, C.; Paccaud, F. Prevalence of overweight in the Seychelles: 15 year trends and association with socio-economic status. Obes. Rev. 2008, 9, 511–517. [Google Scholar] [CrossRef]
- WHO. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Lewis, J. Codex Nutrient Reference Values; FAO: Rome, Italy, 2019. [Google Scholar]
- Khalili Tilami, S.; Sampels, S. Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. Aquac. 2017, 26, 243–253. [Google Scholar] [CrossRef]
- Greenfield, H.; Southate, D. Food Composition Data: Production, Management, and Use; FAO: Rome, Italy, 2003. [Google Scholar]
- Robinson, J.P.W.; Robinson, J.; Gerry, C.; Govinden, R.; Freshwater, C.; Graham, N.A.J. Diversification insulates fisher catch and revenue in heavily exploited tropical fisheries. Sci. Adv. 2020, 6, eaaz0587. [Google Scholar] [CrossRef]
- Maehre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein Determination-Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Stein, W.H. Chromatographic determination of amino acids by the use of automatic recording equipment. Meth. Enzymol. 1963, 6, 819–831. [Google Scholar] [CrossRef]
- Maehre, H.K.; Hamre, K.; Elvevoll, E.O. Nutrient evaluation of rotifers and zooplankton: Feed for marine fish larvae. Aquacult. Nutr. 2013, 19, 301–311. [Google Scholar] [CrossRef]
- Maclean, W.C.; Harnly, J.M.; Chen, J.; Chevassus-Agnes, S.; Gilani, G.; Livesey, G.; Mathioudakis, B.; Munoz De Chavez, M.; Devasconcellos, M.T.; Warwick, P. Food Energy—Methods of Analysis and Conversion Factors. In Food and Agriculture Organization of the United Nations Technical Workshop Report; FAO: Rome, Italy, 2003. [Google Scholar]
- Pickering, M.; Newton, P. Amino acid hydrolysis: Old problems, new solutions. LC GC Mag. Sep. Sci. 1990, 8, 778–781. [Google Scholar]
- Larsen, R.; Stormo, S.K.; Dragnes, B.T.; Elvevoll, E.O. Losses of taurine, creatine, glycine and alanine from cod (Gadus morhua L.) fillet during processing. J. Food Comp. Anal. 2007, 20, 396–402. [Google Scholar] [CrossRef]
- Larsen, R.; Mierke-Klemeyer, S.; Mæhre, H.; Elvevoll, E.O. Retention of health beneficial components during hot- And cold-smoking of African catfish (Ciarias gariepinus) fillets. Arch. Für Lebensm. 2010, 61, 31–35. [Google Scholar] [CrossRef]
- FAO. Protein Quality Evaluation in Human Diets. Report of a Joint FAO/WHO Expert Consultation; FAO: Rome, Italy, 1991. [Google Scholar]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The role of taurine in mitochnodria health: More than just an antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Ito, T.; Azyma, J. Clinical significance of taurine. Amino Acids 2013, 46, 1–5. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Zhang, T.-T.; Yu, Z.-L.; Wang, C.-C.; Zhao, Y.-C.; Wang, Y.-M.; Xue, C.-H. Taurine alleviates trimethylamine N-oxide-induced atherosclerosis by regulating bile acid metabolism in ApoE–/– mice. J Agric. Food Chem. 2022, 70, 5738–5747. [Google Scholar] [CrossRef] [PubMed]
- Gormley, T.R.; Neumann, T.; Fagan, J.D.; Brunton, N.P. Taurine content of raw and processed fish fillets/portions. Eur. Food Res. Technol. 2007, 225, 837–842. [Google Scholar] [CrossRef]
- Gibson, R.; Lau, C.-H.E.; Loo, R.L.; Ebbels, T.M.D.; Chekmeneva, E.; Dyer, A.R.; Miura, K.; Ueshima, H.; Zhao, L.; Daviglus, M.L.; et al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am. J. Clin. Nutr. 2020, 111, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Dragnes, B.T.; Larsen, R.; Erntsen, M.H.; Mæhre, H.; Elvevoll, E.O. Impact of processing on the taurine content in processed seafood and their corresponding unprocessed raw materials. Int. J. Food Sci. Nutr. 2009, 60, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, F.; Vita, G.; Müller, D.B. Food Security for an Aging and Heavier Population. Sustainability 2018, 10, 3683. [Google Scholar] [CrossRef]
- Nash, K.L.; MacNeil, M.A.; Blanchard, J.L.; Cohen, P.J.; Farmery, A.K.; Graham, N.A.J.; Thorne-Lyman, A.L.; Watsen, R.A.; Hicks, C.C. Trade and foreign fishing mediate global marine nutrient supply. Sustain. Sci. 2022, 119, e2120817119. [Google Scholar] [CrossRef]
- Fanzo, J.; Drewnowski, A.; Blumberg, J.; Miller, G.; Kraemer, K.; Kennedy, E. Nutrients, foods, diets, people: Promoting healthy eating. Curr. Dev. Nutr. 2020, 4, nzaa069. [Google Scholar] [CrossRef]
- Farmery, A.K.; Alexander, K.; Anderson, K.; Blanchard, J.L.; Carter, C.G.; Evans, K.; Fischer, M.; Fleming, A.; Frusher, S.; Fulton, E.A.; et al. Food for all: Designing sustainable and secure future seafood systems. Rev. Fish Biol. Fish 2022, 32, 101–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).