Occurrence of Campylobacter in Faeces, Livers and Carcasses of Wild Boars Hunted in Tuscany (Italy) and Evaluation of MALDI-TOF MS for the Identification of Campylobacter Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. Sample Preparation and Campylobacter Enrichment
2.4. Campylobacter Isolation and Pre-Identification
2.5. Molecular Identification
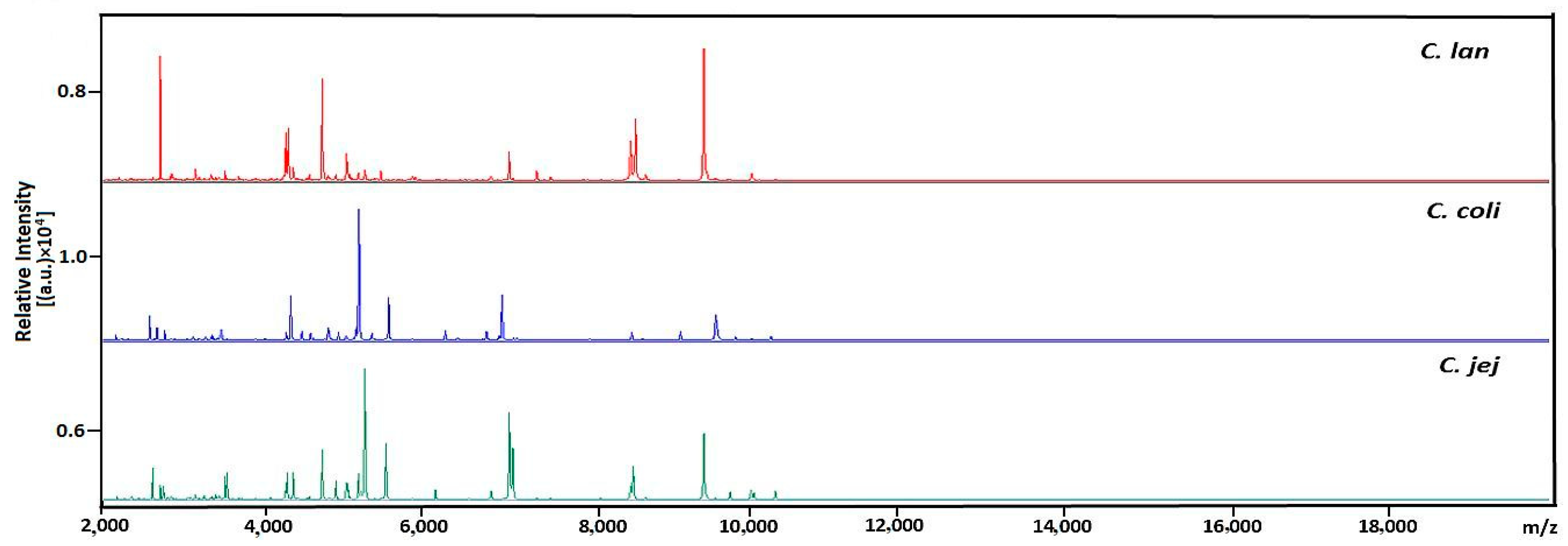
2.6. MALDI-TOF MS Identification
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC); Laughlin, M.E.; Chatham-Stephens, K.; Geissler, A.L. Campylobacteriosis. In CDC Yellow Book 2020: Health Information for International Travel; Brunette, G.W., Nemhauser, J.B., Eds.; Oxford University Press: New York, NY, USA, 2019. Available online: http://www.cdc.gov/campylobacter/additional.html (accessed on 2 December 2022).
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound. Emerg. Dis. 2016, 65, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef]
- Iacolina, L.; Pertoldi, C.; Amills, M.; Kusza, S.; Megens, H.J.; Bâlteanu, V.A.; Bakan, J.; Cubrik-Curik, V.; Oja, R.; Saarma, U.; et al. Hotspots of recent hybridization between pigs and wild boars in Europe. Sci. Rep. 2018, 8, 17372. [Google Scholar] [CrossRef]
- Jori, F.; Massei, G.; Licoppe, A.; Ruiz-Fons, F.; Linden, A.; Václavek, P.; Chenais, E.; Rosell, C. Management of wild boar populations in the European Union before and during the ASF crisis. In Understanding and combatting African Swine Fever: A European perspective; Iacolina, L., Penrith, M.L., Bellini, S., Chenais, E., Jori, F., Montoya, M., Ståhl, K., Gavier-Widén, D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 263–271. [Google Scholar]
- Tuscany Regional Council. Information note on the implementation of regional policies n. 40. Implementation of the Regional Law 10/2016 “Objective Law for the management of Ungulates in Tuscany”. 2018. Available online: http://www.consiglio.regione.toscana.it/upload/COCCOINA/documenti/nota%20informativa%2040_pub.pdf (accessed on 2 December 2022). In Italian.
- Geisser, H.; Reyer, H.U. Efficacy of hunting, feeding, and fencing to reduce crop damage by wild boars. J. Wildl. Manag. 2004, 68, 939–946. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; London, L.; Skrzypczak, T.; Kantala, T.; Laamanen, I.; Biström, M.; Maunula, L.; Gadd, T. Foodborne zoonoses common in hunted wild boars. Ecohealth 2020, 17, 512–522. [Google Scholar] [CrossRef]
- Wahlström, H.; Tysen, E.; Olsson Engvall, E.; Brändstrom, B.; Eriksson, E.; Mörner, T.; Vågsholm, I. Survey of Campylobacter species, VTEC 0157 and Salmonella species in Swedish wildlife. Vet. Rec. 2003, 153, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wacheck, S.; Fredriksson-Ahomaa, M.; König, M.; Stolle, A.; Stephan, R. Wild boars as an important reservoir for foodborne pathogens. Foodborne Pathog. Dis. 2010, 7, 307–312. [Google Scholar] [CrossRef]
- Díaz-Sánchez, S.; Sánchez, S.; Herrera-León, S.; Porrero, C.; Blanco, J.; Dahbi, G.; Vidal, D. Prevalence of Shiga toxin-producing Escherichia coli, Salmonella spp. and Campylobacter spp. in large game animals intended for consumption: Relationship with management practices and livestock influence. Vet. Microbiol. 2013, 163, 274–281. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, N.; Ugarte-Ruiz, M.; Porrero, M.C.; Zamora, L.; Mentaberre, G.; Serrano, E.; Domínguez, L. Campylobacter shared between free-ranging cattle and sympatric wild ungulates in a natural environment (NE Spain). Ecohealth 2014, 11, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, A.; Paniagua, J.; Torralbo, A.; Arenas-Montes, A.; Borge, C.; García-Bocanegra, I. Campylobacter infection in wild artiodactyl species from southern Spain: Occurrence, risk factors and antimicrobial susceptibility. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, L.; Scotti, E.; Gallo, M.; Nanni Costa, L.; Dall’Olio, S. Authentication of “mono-breed” pork products: Identification of a coat colour gene marker in Cinta Senese pigs useful to this purpose. Livest. Sci. 2016, 184, 71–77. [Google Scholar] [CrossRef]
- On, S.L.W. Isolation, identification and subtyping of Campylobacter. Where to from here? J. Microbiol. Methods 2013, 95, 3–7. [Google Scholar] [CrossRef]
- Ricke, S.C.; Feye, K.M.; Chaney, W.E.; Shi, Z.; Pavlidis, H.; Yang, Y. Developments in rapid detection methods for the detection of foodborne Campylobacter in the United States. Front. Microbiol. 2019, 9, 3280. [Google Scholar] [CrossRef]
- Eberle, K.N.; Kiess, A.S. Phenotypic and genotypic methods for typing Campylobacter jejuni and Campylobacter coli in poultry. Poult. Sci. 2012, 91, 255–264. [Google Scholar] [CrossRef]
- Rychert, J. Benefits and limitation of MALDI-TOF Mass Spectrometry for the identification of microorganisms. J. Infectology. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Pavlovic, M.; Huber, I.; Konrad, R.; Busch, U. Application of MALDI-TOF MS for the identification of food borne bacteria. Open Microbiol. J. 2013, 7, 135–141. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Hamada, M.; Al-Dubaib, M.; Alyamani, E.; Moussa, I.M.; AlRowaidhan, A.; Hemeg, H.A. Application of MALDI-TOF MS fingerprinting as a quick tool for identification and clustering of foodborne pathogens isolated from food products. New Microbiol. 2017, 40, 269–278. [Google Scholar]
- Sulaiman, I.M.; Hsieh, Y.H.; Simpson, S. Species identification of Campylobacter jejuni and Campylobacter coli isolates from raw poultry products by MALDI–TOF MS and rRNA sequence analysis. J. AOAC Int. 2020, 103, 197–204. [Google Scholar] [CrossRef]
- Lévesque, S.; Lemay, F.; Bekal, S.; Frost, E.H.; Michaud, S. First reported case of Campylobacter lanienae enteritis in a human. JMM Case Rep. 2016, 3, e005045. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Royuela, C.; Gomariz, R.P.; Luis Tellería, J. Age determination of European wild boar. Wildl. Soc. Bull. 1989, 17, 326–329. [Google Scholar]
- Tuscany Regional Council, Implementing Regulation 5 September 2017, n. 48/R of Tuscany Regional Law 12 January 1994 n. 3 DPGR 48/R/2017. 2017. Available online: http://raccoltanormativa.consiglio.regione.toscana.it/articolo?urndoc=urn:nir:regione.toscana:regolamento.giunta:2017-09-05;48/R (accessed on 2 December 2022). (In Italian).
- Moore, J.E. An optimized recovery method for thermophilic Campylobacter from liver. BMC Microbiol. 2001, 1, 32. [Google Scholar] [CrossRef]
- ISO 10272-1:201; Microbiology of the food chain—Horizontal method for detection and enumeration of Campylobacter spp.—Part 1: Detection method. International Organization for Standardization ISO Standards: Geneva, Switzerland, 2017.
- Pedonese, F.; Nuvoloni, R.; Turchi, B.; Torracca, B.; Di Giannatale, E.; Marotta, F.; Cerri, D. Prevalence, phenotypic and genetic diversity of Campylobacter in poultry fresh meat and poultry products on retail sale in Tuscany (Italy). Vet. Ital. 2017, 5, 29–37. [Google Scholar] [CrossRef]
- FDA. Chapter 7: Campylobacter, Bacteriological Analytical Manual (BAM). 2001. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-7-campylobacter (accessed on 2 December 2022).
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef]
- Di Giannatale, E.; Garofolo, G.; Alessiani, A.; Di Donato, G.; Candeloro, L.; Vencia, W.; Decastelli, L.; Marotta, F. Tracing back clinical Campylobacter jejuni in the Northwest of Italy and assessing their potential source. Front. Microbiol. 2016, 7, 887. [Google Scholar] [CrossRef]
- Marotta, F.; Garofolo, G.; Di Marcantonio, L.; Di Serafino, G.; Neri, D.; Romantini, R.; Sacchini, L.; Alessiani, A.; Di Donato, G.; Nuvoloni, R.; et al. Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PLoS ONE 2019, 14, e0223804. [Google Scholar] [CrossRef]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, S.A.; Delia, A.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar]
- Tomino, Y.; Andoh, M.; Horiuchi, Y.; Shin, J.; Ai, R.; Nakamura, T.; Toda, M.; Yonemitsu, K.; Takano, A.; Shimoda, H.; et al. Surveillance of Shiga toxin-producing Escherichia coli and Campylobacter spp. in wild Japanese deer (Cervus nippon) and boar (Sus scrofa). J. Vet. Med. Sci. 2020, 82, 1287–1294. [Google Scholar] [CrossRef]
- Hulánková, R.; Bořilová, G.; Plhal, R. Occurrence and characterization of selected bacterial pathogens in the intestinal tract of wild boars hunted in the Czech Republic. In Proceedings of the 12th International Symposium on Wild Boar and Other Suids, Lázně Bělohrad, Czech Republic, 4–7 September 2018; p. 50. Available online: https://wmrg.ldf.mendelu.cz/wcd/w-ldf-wmrg/wbs_2018_cp_final_2ku.pdf#page=51 (accessed on 2 December 2022).
- Atanassova, V.; Apelt, J.; Reich, F.; Klein, G. Microbiological quality of freshly shot game in Germany. Meat. Sci. 2008, 78, 414–419. [Google Scholar] [CrossRef]
- Ercolini, C.; Serracca, L.; Migone, L.; Goria, M.; Ferrari, A. Prevalenza di Campylobacter spp., Yersinia enterocolitica, E. coli 0157: H7 in tessuto muscolare di bovino, suino, equino e cinghiale. Il Prog. Vet. 2007, 10, 453–456. (In Italian) [Google Scholar]
- Stella, S.; Tirloni, E.; Castelli, E.; Colombo, F.; Bernardi, C. Microbiological evaluation of carcasses of wild boar hunted in a hill area of northern Italy. J. Food Prot. 2018, 81, 1519–1525. [Google Scholar] [CrossRef]
- Edwards, D.S.; Milne, L.M.; Morrow, K.; Sheridan, P.; Verlander, N.Q.; Mulla, R.; Richardson, J.F.; Pender, A.; Lilley, M.; Reacher, M. Campylobacteriosis outbreak associated with consumption of undercooked chicken liver pâté in the East of England, September 2011: Identification of a dose–response risk. Epidemiol. Infect. 2014, 142, 352–357. [Google Scholar] [CrossRef] [PubMed]
- von Altrock, A.; Hamedy, A.; Merle, R.; Waldmann, H. Campylobacter spp.—Prevalence on pig livers and antimicrobial susceptibility. Prev. Vet. Med. 2013, 109, 152–157. [Google Scholar] [CrossRef]
- Little, C.L.; Richardson, J.F.; Owen, R.J.; de Pinna, E.; Threlfall, E.J. Campylobacter and Salmonella in raw red meats in the United Kingdom: Prevalence, characterization and antimicrobial resistance pattern, 2003–2005. Food Microbiol. 2008, 25, 538–543. [Google Scholar] [CrossRef]
- Marotta, F.; Di Marcantonio, L.; Janowicz, A.; Pedonese, F.; Di Donato, G.; Ardelean, A.; Nuvoloni, R.; Di Giannatale, E.; Garofolo, G. Genotyping and antibiotic resistance traits in Campylobacter jejuni and coli from pigs and wild boars in Italy. Front. Cell Infect. Microbiol. 2020, 15, 592512. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Marín, M.; López-Olvera, J.R.; Ayats, T.; Fernandez Aguilar, X.; Lavín, S.; Mentaberre, G.; Cerdà-Cuéllar, M. Zoonotic Campylobacter spp. and Salmonella spp. carried by wild boars in a metropolitan area: Occurrence, antimicrobial susceptibility and public health relevance. Sci. Total Environ. 2022, 822, 153444. [Google Scholar] [CrossRef]
- Koene, M.G.Y.; Houvers, D.J.; Djkrstra, J.R.; Dium, B.; Wagenaar, J.A. Simultaneous presence of multiple Campylobacter species in dogs. J. Clin. Microbiol. 2004, 42, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Thépault, A.; Poezevara, T.; Quesne, S.; Rose, V.; Chemaly, M.; Rivoal, K. Prevalence of thermophilic Campylobacter in cattle production at slaughterhouse level in France and link between C. jejuni bovine strains and campylobacteriosis. Front. Microbiol. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Ghaznavi-Rad, E. A report of two clinical coinfections with Campylobacter jejuni and Campylobacter coli in infantile dysentery. Arch. Pediatr. Infect. Dis 2019, 7, e80116. [Google Scholar] [CrossRef]
- Thépault, A.; Rose, V.; Queguiner, M.; Chemaly, M.; Rivoal, K. Dogs and Cats: Reservoirs for highly diverse Campylobacter jejuni and a potential source of human exposure. Animals. 2020, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.T.; Schiaffino, F.; Huynh, S.; Olortegui, M.P.; Peñataro Yori, P.; Garcia Bardales, P.F.; Pinedo Vasquez, T.; Curico Huansi, G.E.; Manzanares Villanueva, K.; Shapiama Lopez, W.V.; et al. Shotgun metagenomics of fecal samples from children in Peru reveals frequent complex co-infections with multiple Campylobacter species. PLoS Negl. Trop. Dis. 2022, 4, e0010815. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Surewaard, B.G.J.; Wong, C.H.Y. Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat. Immunol. 2018, 19, 1100–1111. [Google Scholar] [CrossRef]
- Green, M.S.; Schwartz, N.; Peer, V. Sex differences in campylobacteriosis incidence rates at different ages—A seven country, multi-year, meta-analysis. A potential mechanism for the infection. BMC Infect. Dis. 2020, 20, 625. [Google Scholar] [CrossRef]
- Han, Z.; Pielsticker, C.; Gerzova, L.; Rychlik, I.; Rautenschlein, S. The influence of age on Campylobacter jejuni infection in chicken. Dev. Comp. Immunol. 2016, 62, 58–71. [Google Scholar] [CrossRef]
- Connerton, P.L.; Richards, P.J.; Lafontaine, G.L.; O’Kane, P.M.; Ghaffar, N.; Cummings, N.J.; Smith, D.L.; Fish, N.M.; Connerton, I.F. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Fang, S.; He, M.; Huang, X.; Hui Yang, H.; Yang, B.; Chen, C.; Huang, L. Age-based dynamic changes of phylogenetic composition and interaction networks of health pig gut microbiome feeding in a uniformed condition. BMC Vet. Res. 2019, 15, 172. [Google Scholar] [CrossRef]
- Bessède, E.; Solecki, O.; Sifre, E.; Labadi, L.; Megraud, F. Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Clin. Microbiol. Infect. 2011, 17, 1735–1739. [Google Scholar] [CrossRef]
- Lawton, S.J.; Weis, A.M.; Byrne, B.A.; Fritz, H.; Taff, C.C.; Townsend, A.K.; Weimer, B.C.; Mete, A.; Wheeler, S.; Boyce, W.M. Comparative analysis of Campylobacter isolates from wild birds and chickens using MALDI-TOF MS, biochemical testing, and DNA sequencing. J. Vet. Diagn. 2018, 30, 354–361. [Google Scholar] [CrossRef]
- Cuénod, A.; Foucault, F.; Pflüger, V.; Egli, A. Factors associated with MALDI-TOF mass spectral quality of species identification in clinical routine diagnostics. Front. Cell Infect. Microbiol. 2021, 11, 646648. [Google Scholar] [CrossRef]
- Belkum, A.; Welker, M.; Pincus, D.; Charrier, J.P.; Girard, V. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry in clinical microbiology: What are the current issues? Ann. Lab. Med. 2017, 37, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Ziino, G.; Marotta, S.M.; Giarratana, F.; Giuffrida, A.; Panebianco, F. Reliability evaluation of MALDI-TOF MS associated with SARAMIS software in rapid identification of thermophilic Campylobacter isolated from food. Food Anal. Methods 2019, 12, 1128–1132. [Google Scholar] [CrossRef]
- Kérouanton, A.; Chidaine, B.; Rose, V.; Samson, V.; Denis, M. Direct detection of Campylobacter from feces of organic and conventional pigs highlighted the presence of Campylobacter lanienae. In Proceedings of the Epidemiology and Control of Hazards in Pork Production Chain, Safepork: One Health Approach Under a Concept of Farm to Fork, Porto, Portugal, 7–10 September 2015; pp. 53–57. [Google Scholar]
- Fornefett, J.; Busch, A.; Döpping, S.; Hotzel, H.; Rimek, D. Bacterial gastroenteritis caused by the putative zoonotic pathogen Campylobacter lanienae: First reported case in Germany. Access Microbiol. 2021, 25, 000199. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.M.; Burnens, A.; Linton, D.; Lawson, A.J.; Stanley, J. Campylobacter lanienae sp. nov., a new species isolated from workers in an abattoir. Int. J. Syst. Evol. Microbiol. 2000, 50, 865–872. [Google Scholar] [CrossRef]
| Primers | Sequence (5′–3′) |
|---|---|
| 16S_F517 | 5′-GCC AGC AGC CGC GGT-3′ |
| 16S_R517 | 5′-AAG GAG GTG ATC CAG-3′ |
| Total | % of 193 * | % of 86 ** | |
|---|---|---|---|
| Male | 49 | 25.39 | 56.98 |
| Female | 37 | 19.17 | 43.02 |
| Adult | 34 | 17.62 | 39.54 |
| Subadult | 11 | 5.70 | 12.79 |
| Young | 41 | 21.24 | 47.67 |
| Animal Id. | Sex | Age Group | Faecal Swab | Liver | Bile | Carcass |
|---|---|---|---|---|---|---|
| Wb3 | F | A | C. lan # | − | − | \ |
| Wb4 | F | Y | C. lan # | * | − | \ |
| Wb18 | F | SA | − | C. lan # | − | \ |
| Wb24 | F | Y | C. coli # | \ | \ | \ |
| Wb27 | M | A | C. lan # | − | \ | \ |
| Wb28 | M | A | C. coli # | − | − | C. coli |
| Wb29 | M | SA | C. lan # | − | \ | \ |
| Wb32 | M | A | C. coli # | − | − | C. coli |
| Wb35 | F | SA | C. coli # | − | − | \ |
| Wb36 | F | A | C. coli # | C. coli | − | \ |
| Wb37 | F | Y | C. jej # | − | − | \ |
| Wb38 | F | A | C. coli # | − | − | \ |
| Wb39 | F | Y | C. coli # | − | − | \ |
| Wb44 | F | A | \ | C. coli # | \ | \ |
| Wb49 | F | Y | − | − | − | C. coli # |
| Wb55 | M | Y | C. coli | − | − | \ |
| Wb57 | M | A | C. lan # | − | − | C. lan # |
| Wb59 | F | A | C. lan | − | − | − |
| Wb67 | F | A | C. lan | C. jej # | − | \ |
| Wb75 | M | SA | C. lan # | − | − | − |
| Wb76 | F | Y | C. coli # | − | − | − |
| Wb77 | M | Y | C. coli # | − | − | − |
| Wb78 | M | Y | C. coli # | − | \ | C. coli # |
| Wb85 | F | A | C. coli # | − | − | \ |
| Wb98 | F | Y | C. coli # | − | − | C. lan # |
| Wb101 | F | A | C. lan # | − | − | − |
| Wb103 | M | Y | C. lan | − | − | C. lan |
| Wb107 | F | A | C. lan # | − | \ | \ |
| Wb108 | F | A | C. lan # | − | − | \ |
| Wb109 | F | A | C. coli # | − | − | \ |
| Wb110 | F | SA | C. lan # | − | − | \ |
| Wb111 | F | A | C. coli # | − | − | \ |
| Wb112 | M | Y | C. coli # | − | − | \ |
| Wb113 | F | A | C. coli # | − | − | \ |
| Wb115 | F | Y | C. lan # | − | − | \ |
| Wb116 | M | Y | C. lan # | − | − | \ |
| Wb118 | M | Y | C. lan | − | − | \ |
| Wb125 | F | A | C. lan # | − | C. lan # | \ |
| Wb126 | F | A | C. lan | − | − | \ |
| Wb128 | M | Y | C. lan # | − | − | \ |
| Wb129 | F | Y | C. lan # | − | − | \ |
| Wb130 | M | A | C. lan # | − | − | \ |
| Wb133 | M | A | C. coli # | − | \ | \ |
| Wb134 | M | A | C. coli # | − | − | \ |
| Wb136 | F | A | C. coli # | − | − | \ |
| Wb137 | F | Y | C. coli # | − | − | \ |
| Wb138 | F | A | C. coli # | − | − | \ |
| Wb139 | F | Y | C. coli # | − | − | \ |
| Wb140 | F | A | C. coli # | − | − | \ |
| Wb141 | F | Y | C. coli # | − | − | \ |
| Wb142 | F | Y | C. coli | − | − | \ |
| Wb143 | M | Y | C. coli | − | − | \ |
| Wb144 | M | Y | C. coli | C. coli | \ | \ |
| Wb145 | M | Y | C. coli # | C. lan # | − | \ |
| Wb146 | M | Y | C. coli # | \ | \ | \ |
| Wb147 | M | A | − | − | − | C. lan |
| Wb148 | M | SA | C. lan # | − | \ | \ |
| Wb151 | F | A | C. lan # | − | − | − |
| Wb152 | M | A | C. jej | − | \ | \ |
| Wb153 | M | A | C. jej | − | \ | \ |
| Wb155 | F | A | * | C. jej | − | \ |
| Wb156 | M | Y | * | C. jej | − | \ |
| Wb157 | F | Y | C. coli | − | − | \ |
| Wb159 | M | A | − | − | C. lan | \ |
| Wb162 | M | Y | C. coli | − | − | − |
| Wb164 | M | Y | C. coli | − | − | − |
| Wb171 | M | A | C. coli | − | − | − |
| Wb172 | M | SA | C. coli | − | − | \ |
| Wb173 | F | Y | C. lan | − | − | \ |
| Wb174 | F | SA | C. lan | − | − | \ |
| Wb175 | F | Y | C. lan | * | − | \ |
| Wb176 | F | Y | C. lan | − | − | \ |
| Wb180 | F | SA | C. lan | − | \ | \ |
| Wb182 | M | A | C. hyo | − | − | \ |
| Wb183 | F | A | C. hyo | − | \ | \ |
| Wb186 | M | SA | C. lan # | − | − | \ |
| Wb189 | M | Y | C. lan # | − | − | − |
| Wb191 | F | Y | C. coli # | − | − | − |
| Wb192 | M | Y | C. hyo | − | − | − |
| Wb193 | F | Y | C. lan # | − | \ | C. lan # |
| Wb194 | F | Y | C. coli # | − | − | C. coli # |
| Wb195 | F | Y | C. coli # | − | − | \ |
| Wb197 | M | Y | * | C. lan # | C. coli # | − |
| Wb198 | F | Y | C. hyo | − | − | \ |
| Wb199 | F | Y | C. coli # | − | − | \ |
| Wb200 | M | SA | C. lan # | − | − | − |
| Total | 86 | 86 | 78 | 9 | 3 | 10 |
| Species | No. of Isolates | No. of Isolates with Id Score < 1.700 | No. of Isolates with Id Score 1.700–1.999 | No. of Isolates with Id Score 2.000–2.299 | No. of Isolates with Id Score 2.300–3.000 |
|---|---|---|---|---|---|
| C. coli | 35 | 0 | 2 | 26 | 7 |
| C. jejuni | 2 | 0 | 0 | 2 | 0 |
| C. lanienae | 29 | 4 | 15 | 10 | 0 |
| C. coli n = 35 | C. jejuni n = 2 | C. lanienae n = 29 | |
|---|---|---|---|
| Comparisons | 35 | 2 | 29 |
| NR | 2 | 0 | 19 |
| Correctly identified | 33 | 2 | 10 |
| Not correctly identified | 0 | 0 | 0 |
| Misidentified | 0 | 0 | 0 |
| Sensitivity (95% CI) | 0.942 (0.814–0.984) | 1.00 (0.342–1.00) | 0.344(0.199–0.526) |
| Specificity (95% CI) | 1 (0.890–1.00) | 1 (0.943–1.00) | 1 (0.905–1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziomek, M.; Gondek, M.; Torracca, B.; Marotta, F.; Garofolo, G.; Wieczorek, K.; Michalak, K.; Fratini, F.; Pedonese, F. Occurrence of Campylobacter in Faeces, Livers and Carcasses of Wild Boars Hunted in Tuscany (Italy) and Evaluation of MALDI-TOF MS for the Identification of Campylobacter Species. Foods 2023, 12, 778. https://doi.org/10.3390/foods12040778
Ziomek M, Gondek M, Torracca B, Marotta F, Garofolo G, Wieczorek K, Michalak K, Fratini F, Pedonese F. Occurrence of Campylobacter in Faeces, Livers and Carcasses of Wild Boars Hunted in Tuscany (Italy) and Evaluation of MALDI-TOF MS for the Identification of Campylobacter Species. Foods. 2023; 12(4):778. https://doi.org/10.3390/foods12040778
Chicago/Turabian StyleZiomek, Monika, Michał Gondek, Beatrice Torracca, Francesca Marotta, Giuliano Garofolo, Kinga Wieczorek, Katarzyna Michalak, Filippo Fratini, and Francesca Pedonese. 2023. "Occurrence of Campylobacter in Faeces, Livers and Carcasses of Wild Boars Hunted in Tuscany (Italy) and Evaluation of MALDI-TOF MS for the Identification of Campylobacter Species" Foods 12, no. 4: 778. https://doi.org/10.3390/foods12040778
APA StyleZiomek, M., Gondek, M., Torracca, B., Marotta, F., Garofolo, G., Wieczorek, K., Michalak, K., Fratini, F., & Pedonese, F. (2023). Occurrence of Campylobacter in Faeces, Livers and Carcasses of Wild Boars Hunted in Tuscany (Italy) and Evaluation of MALDI-TOF MS for the Identification of Campylobacter Species. Foods, 12(4), 778. https://doi.org/10.3390/foods12040778






