Millet Fermented by Different Combinations of Yeasts and Lactobacilli: Effects on Phenolic Composition, Starch, Mineral Content and Prebiotic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Millet Samples and Fermentation Processes
2.3. Microorganism Growth Conditions and Enumeration
2.4. Phenolic Compound Extraction
2.5. Peptide Extraction
2.6. HPLC-DAD and HPLC-DAD-MS/MS Analyses
2.7. Quantitation of Phenolic Compounds by HPLC-DAD
2.8. Determination of Resistant, Digestible and Total Starch
2.9. Mineral Content
2.10. In Vitro Evaluation of the Prebiotic Activity
2.11. Statistical Analysis
3. Results
3.1. Unfermented Millet Samples: Phenolic and Starch Composition
3.2. Microbiological Composition and Chemical Evaluation of Fermented Millet
3.3. Evaluation of Bacterial Growth Stimulation on FPM2 and FPM3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet grains: Nutritional quality, processing, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Mahajan, P.; Bera, M.; Panesar, P.S.; Chauhan, A. Millet starch: A review. Int. J. Biol. Macromol. 2021, 180, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Mirzababaee, S.M.; Ozmen, D.; Hesarinejad, M.A.; Toker, O.S.; Yeganehzad, S. A study on the structural, physicochemical, rheological and thermal properties of high hydrostatic pressurized pearl millet starch. Int. J. Biol. Macrom. 2022, 223, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, L.; Hou, D.; Liaqat, H.; Shen, Q. Millet: A review of its nutritional and functional changes during processing. Food Res. Int. 2021, 142, 110197. [Google Scholar] [CrossRef]
- Aisoni, J.E.; Yusha, U.M.; Orole, O.O. Processing effects on physicochemical and proximate composition of finger millet (Eleusine coracana). GJBS 2018, 8, 14–20. [Google Scholar] [CrossRef]
- Urooj, A. Impact of household processing methods on the nutritional characteristics of pearl millet (Pennisetum typhoideum): A review. MOJFPT 2017, 4, 28–32. [Google Scholar]
- Gabaza, M.; Shumoy, H.; Louwagie, L.; Muchuweti, M.; Vandamme, P.; Du Laing, G.; Raes, K. Traditional fermentation and cooking of finger millet: Implications on mineral binders and subsequent bioaccessibility. J. Food Compos. Anal. 2018, 68, 87–94. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Mikušová, L.; Magala, M.; Kohajdová, Z.; Karovicová, J. Nonwheat Cereal-Fermented-Derived Products. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 417–432. [Google Scholar]
- Terefe, N.S. Food Fermentation. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Sharma, R.; Sharma, S. Anti-nutrient & bioactive profile, in vitro nutrient digestibility, techno-functionality, molecular and structural interactions of foxtail millet (Setaria italica L.) as influenced by biological processing techniques. Food Chem. 2022, 368, 130815. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and potentially probiotic yeasts—Characteristics and food application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef]
- Gabaza, M.; Joossens, M.; Cnockaert, M.; Muchuweti, M.; Raes, K.; Vandamme, P. Lactococci dominate the bacterial communities of fermented maize, sorghum and millet slurries in Zimbabwe. Int. J. Food Microbiol. 2019, 289, 77–87. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar]
- Majid, A.; Priyadarshini, P.C.G. Millet derived bioactive peptides: A review on their functional properties and health benefits. Crit. Rev. Food Sci. Nutr. 2020, 60, 3342–3351. [Google Scholar] [CrossRef] [PubMed]
- Purewal, S.; Sandhu, K.; Salar, R.K.; Kaur, P. Fermented pearl millet: A product with enhanced bioactive compounds and DNA damage protection activity. J. Food Meas. Charact. 2019, 13, 1479–1488. [Google Scholar] [CrossRef]
- Balli, D.; Bellumori, M.; Pucci, M.G.; Longo, V.; Paoli, P.; Melani, F.; Mulinacci, N.; Innocenti, M. Does Fermentation Really Increase the Phenolic Content in Cereals? A Study on Millet. Foods 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Gabaza, M.; Shumoy, H.; Muchuweti, M.; Vandamme, P.; Raes, K. Effect of Fermentation and Cooking on Soluble and Bound Phenolic Profiles of Finger Millet Sour Porridge. J. Agric. Food Chem. 2016, 64, 7615–7621. [Google Scholar] [CrossRef]
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Sodi, C.; Granchi, L. Influence of different leavening agents on technological and nutritional characteristics of whole grain breads obtained from ancient and modern flour varieties. Eur. Food Res. Technol. 2021, 247, 1701–1710. [Google Scholar] [CrossRef]
- Galli, V.; Mazzoli, L.; Luti, S.; Venturi, M.; Guerrini, S.; Paoli, P.; Vincenzini, M.; Granchi, L.; Pazzagli, L. Effect of selected strains of lactobacilli on the antioxidant and anti-inflammatory properties of sourdough. Int. J. Food Microbiol. 2018, 286, 55–65. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Pini, N.; Guerrini, S.; Granchi, L.; Vincenzini, M. Liquid and firm sourdough fermentation: Microbial robustness and interactions during consecutive backsloppings. LWT 2019, 105, 9–15. [Google Scholar] [CrossRef]
- Balli, D.; Bellumori, M.; Orlandini, S.; Cecchi, L.; Mani, E.; Pieraccini, G.; Mulinacci, N.; Innocenti, M. Optimized hydrolytic methods by response surface methodology to accurately estimate the phenols in cereal by HPLC-DAD: The case of millet. Food Chem. 2020, 303, 125393. [Google Scholar] [CrossRef]
- Wiśniewski, J.; Zougman, A.; Nagaraj, N. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Dani, F.R.; Pieraccini, G. Chapter Four—Proteomics of arthropod soluble olfactory proteins. Methods Enzymol. 2020, 642, 81–102. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Sloane, N.; Draga, A.; Lazewska, I. Measurement of Total Dietary Fiber Using AOAC Method 2009.01 (AACC International Approved Method 32-45.01): Evaluation and Updates. Cereal Chem. 2013, 90, 396–414. [Google Scholar]
- Bellumori, M.; Silva, N.A.C.; Vilca, L.; Andrenelli, L.; Cecchi, L.; Innocenti, M.; Balli, D.; Mulinacci, N. A Study on the Biodiversity of Pigmented Andean Potatoes: Nutritional Profile and Phenolic Composition. Molecules 2020, 10, 3169. [Google Scholar] [CrossRef]
- Aloisio, I.; Prodam, F.; Giglione, E.; Bozzi Cionci, N.; Solito, A.; Bellone, S.; Baffoni, L.; Mogna, L.; Pane, M.; Bona, G.; et al. Three-month feeding integration with bifidobacterium strains prevents gastrointestinal symptoms in healthy newborns. Front. Nutr. 2018, 5, 39. [Google Scholar] [CrossRef]
- Biavati, B.; Mattarelli, P. The Family Bifidobacteriaceae prokaryotes: A Handbook on the Biology of Bacteria, 3rd ed.; Springer: New York, NY, USA, 2006; Volume 3, pp. 322–382. [Google Scholar]
- Rada, V.; Petr, J. A new selective medium for the isolation of glucose non-fermenting bifidobacteria from hen caeca. J. Microbiol. Methods 2000, 43, 127–132. [Google Scholar] [CrossRef]
- Kaimal, A.M.; Mujumdar, A.S.; Thorat, B.N. Resistant starch from millets: Recent developments and applications in food industries. Trends Food Sci. Technol. 2021, 111, 563–580. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Siroha, A.K. Relationships between physiochemical, thermal, rheological and in vitro digestibility properties of starches from pearl millet cultivars. LWT-Food Sci. Technol. 2017, 83, 213–224. [Google Scholar]
- Jayawardana, S.A.S.; Samarasekera, J.K.R.R.; Hettiarachchi, G.H.C.M.; Gooneratne, J.; Mazumdar, S.D.; Banerjee, R. Dietary fibers, starch fractions and nutritional composition of finger millet varieties cultivated in Sri Lanka. J. Food Compos. Anal. 2019, 82, 103249. [Google Scholar] [CrossRef]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Sharma, B.; Gujral, H.S. Influence of nutritional and antinutritional components on dough rheology and in vitro protein & starch digestibility of minor millets. Food Chem. 2019, 299, 125115. [Google Scholar] [CrossRef] [PubMed]
- Amadou, I.; Gounga, M.E.; Shi, Y.H.; Le, G.W. Fermentation and heat-moisture treatment induced changes on the physicochemical properties of foxtail millet (Setaria italica) flour. Food Bioprod. Process. 2014, 92, 38–45. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef]
- Azeez, S.O.; Chinma, C.E.; Bassey, S.O.; Eze, U.R.; Makinde, A.F.; Sakariyah, A.A.; Okubanjo, S.S.; Danbaba, N.; Adebo, O.A. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT 2022, 154, 112734. [Google Scholar] [CrossRef]
- Di Gioia, D.; Aloisio, I.; Mazzola, G.; Biavati, B. Bifidobacteria: Their impact on gut microbiota composition and their applications as probiotics in infants. Appl. Microbial. Biotech. 2014, 98, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, R.G.; Morris, L.F.; Harvey, M.L.; Tran, L.T.; Mitchell, H.L.; Playne, M.J. Selection of a Bifidobacterium strain to complement resistant starch in a synbiotic yoghurt. J. Appl. Microbiol. 2001, 90, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.; Giuliani, C.; Rossi, F.; Adessi, A.; Al-Tamimi, A.; Mazzola, G.; Di Gioia, D.; Innocenti, M.; Mulinacci, N. Polysaccharides from by-products of the Wonderful and Laffan pomegranate varieties: New insight into extraction and characterization. Food Chem. 2017, 235, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, G.; Aloisio, I.; Biavati, B.; Di Gioia, D. Development of a synbiotic product for newborns and infants. LWT 2015, 64, 727–734. [Google Scholar]
- Baruzzi, F.; de Candia, S.; Quintieri, L.; Caputo, L.; De Leo, F. Development of a synbiotic beverage enriched with bifidobacteria strains and fortified with whey proteins. Front. Microbiol. 2017, 8, 640. [Google Scholar] [CrossRef]
- Harris, S.; Monteagudo-Mera, A.; Kosik, O.; Charalampopoulos, D.; Shewry, P.; Lovegrove, A. Comparative prebiotic activity of mixtures of cereal grain polysaccharides. AMB Express 2019, 9, 203. [Google Scholar] [CrossRef]
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic microbiology: The role of Bifidobacterium breve as food supplement for the prevention/treatment of paediatric diseases. Nutrients 2018, 10, 1723. [Google Scholar] [CrossRef]
- Simone, M.; Gozzoli, C.; Quartieri, A.; Mazzola, G.; Di Gioia, D.; Amaretti, A.; Raimondi, S.; Rossi, M. The probiotic Bifidobacterium breve B632 inhibited the growth of Enterobacteriaceae within colicky infant microbiota cultures. BioMed Res. Int. 2014, 2014, 301053. [Google Scholar] [CrossRef] [PubMed]


| A. | |||||
|---|---|---|---|---|---|
| Code | Plant Name | Origin | Color | Plant Genera | Species |
| PMN | Pearl millet | Nigeria | yellow/brown | Pennisetum | glaucum |
| FGM | Finger millet | Nigeria | red/brown | Eleusine | coracana |
| FXM | Foxtail millet | Nigeria | yellow | Setaria | italica |
| PMI | Pearl millet | Italy | yellow/brown | Pennisetum | glaucum |
| B. | |||||
| Code | Fermentative Microorganisms | Fermentation Time | Temperature | Drying Method | |
| FPM1 | Saccharomyces boulardii | 72 h | 30 °C | Spray drying | |
| FPM2 | Saccharomyces cerevisiae + Companilactobacillus paralimentarius | 24 h | 28 °C | Oven drying | |
| FPM3 | Hanseniaspora uvarum + Fructilactobacillus sanfranciscens | 24 h | 28 °C | Oven drying | |
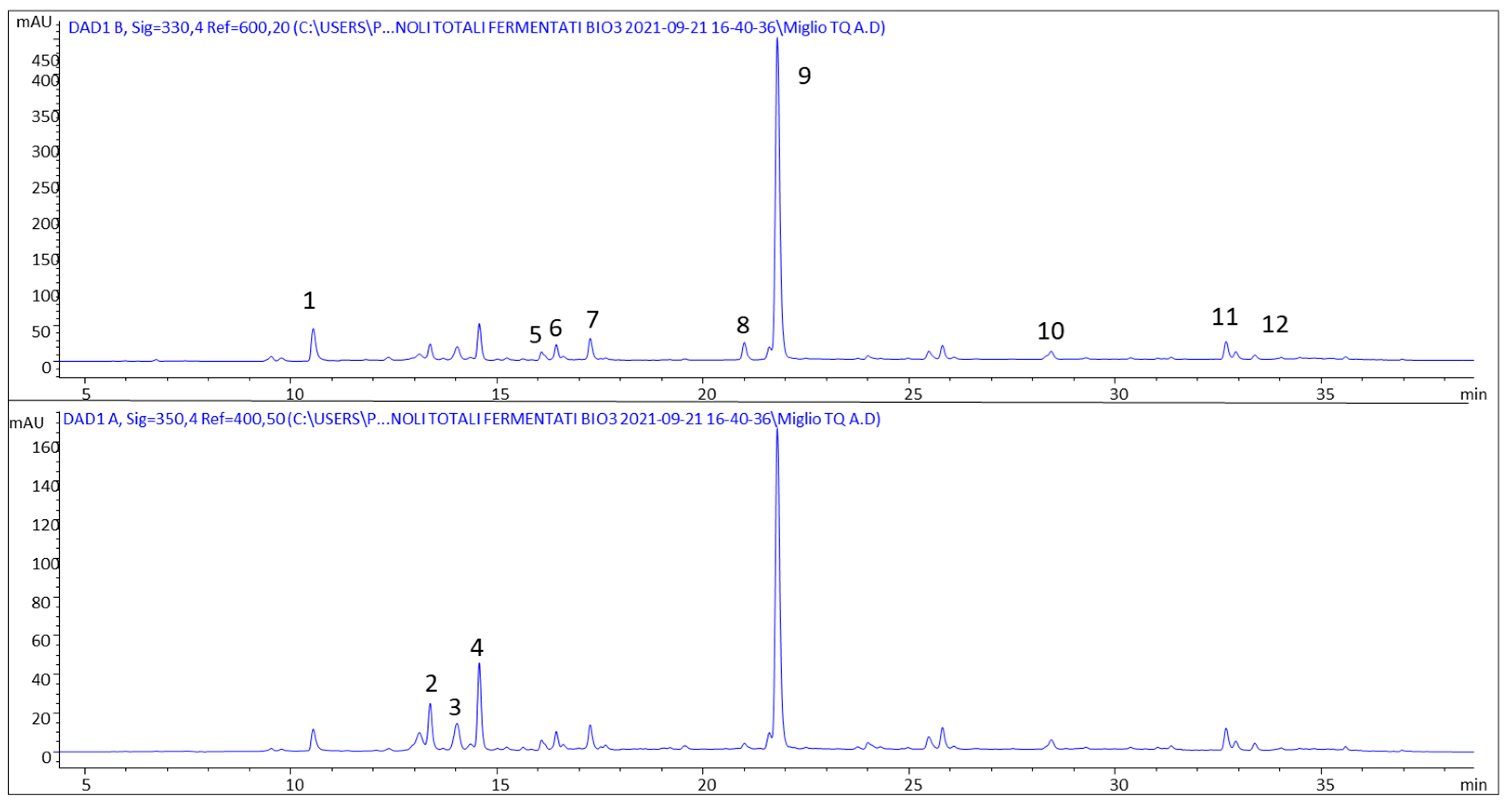
| Analytes | [M − H]− | Identified Compounds |
|---|---|---|
| 1 | 468 | N1,N4-dicaffeoylspermidine |
| 2 | 609 | luteolin-(7-O-glucopyranosyl)-8-C-glucopyranoside |
| 3 | 577 | vitexin-2″-O-rhamnoside |
| 4 | 431 | vitexin |
| 5 | 339 | ferulic acid rhamnoside |
| 6 | 339 | ferulic acid rhamnoside isomer |
| 7 | 193 | isoferulic acid |
| 8 | 177 | methyl hydroxycinnamate |
| 9 | 207 | methyl ferulate |
| (A) | ||||||
|---|---|---|---|---|---|---|
| Phenolic Composition | Starch Content | |||||
| FP mg/g | TPB mg/g | TPA mg/g | RS g/100 g | SDS g/100 g | TS g/100 g | |
| PMN | 0.74 ± 0.02 c | 1.13 ± 0.05 d | 2.26 ± 0.03 b | 11.23 ± 1.72 b | 23.85 ± 2.15 b | 31.34 ± 3.09 b |
| FxM | 0.26 ± 0.04 a | 0.52 ± 0.07 a | 2.15 ± 0.07 a | 9.26 ± 1.12 a | 15.41 ± 1.02 a | 24.68 ± 2.11 a |
| FgM | 0.30 ± 0.03 b | 0.81 ± 0.05 b | 2.32 ± 0.06 c | 24.25 ± 0.76 c | 25.39 ± 1.25 b | 49.65 ± 2.68 c |
| PMI | 0.30 ± 0.01 b | 1.02 ± 0.07 c | 2.24 ± 0.05 b | 11.83 ± 0.65 b | 38.52 ± 0.48 c | 50.35 ± 1.61 c |
| (B) | ||||||
| Phenolic Composition | Starch Content | |||||
| FP mg/g | TPA mg/g | RS g/100 g | SDS g/100 g | TS g/100 g | ||
| FPM1 | 0.12 ± 0.01 a | 1.20 ± 0.02 a | 0.71 ± 0.03 a | 3.91 ± 0.78 a | 4.62 ± 3.82 a | |
| FPM2 | 0.27 ± 0.01 c | 2.74 ± 0.10 c | 9.83 ± 0.24 c | 50.18 ± 0.69 b | 60.00 ± 0.93 b | |
| FPM3 | 0.25 ± 0.01 b | 2.57 ± 0.03 b | 4.03 ± 0.40 b | 55.39 ± 1.40 c | 59.42 ± 1.79 b | |
| Sample | Final pH | TTA | LAB | Yeasts | Total Sugars | Lactic Acid | Acetic Acid | Glycerin | Ethanol |
| (mL) | (CFU/g) | (CFU/g) | (g/L) | (g/L) | (g/L) | (g/L) | (%) | ||
| FPM1 | 4.31 ± 0.16 c | 26.10 ± 1.40 c | (1.20 ± 0.32) × 108 a | (3.12 ± 0.51) × 108 b | 2.16 ± 0.49 b | 1.97 ± 0.23 c | 0.26 ± 0.02 b | 0.36 ± 0.14 ab | 0.17 ± 0.06 a |
| FPM2 | 3.93 ± 0.13 b | 13.76 ± 1.02 a | (3.70 ± 0.42) × 109 c | (5.34 ± 1.50) × 108 c | 1.54 ± 0.33 ab | 0.23 ± 0.03 a | 0.14 ± 0.02 a | 0.17 ± 0.02 a | 0.09 ± 0.01 a |
| FPM3 | 3.59 ± 0.10 a | 16.94 ± 1.05 b | (1.95 ± 0.18) × 109 b | (4.25 ± 0.35) × 107 a | 1.27 ± 0.31 a | 0.49 ± 0.09 b | 0.22 ± 0.06 b | 0.46 ± 0.10 b | 0.36 ± 0.09 b |
| Mass | emPAI | Description | Identification | Fermented Samples | ||
|---|---|---|---|---|---|---|
| 168,430 | 0.02 | A0A1D8KW99_CENAM | DNA-directed RNA polymera se subunit beta | FPM2 | ||
| 113,891 | 0.03 | A0A0B5ACT4_CENAM | NBS-LRR-like protein | FPM1 | FPM2 | FPM3 |
| 95,958 | 0.03 | A0A4Y1NYR9_CENAM | Calmodulin-binding transcription activator 4 | FPM3 | ||
| 82,491 | 0.04 | A0A024BLE7_CENAM | Photosystem I P700 chlorophyll a apoprotein A2 | FPM3 | ||
| 80,220 | 0.04 | E5FQ64_CENAM | Heat-shock protein 90 | FPM3 | ||
| 72,966 | 0.04 | A4ZYQ0_CENAM | Chloroplast heat-shock protein 70 | FPM1 | FPM2 | FPM3 |
| 69,627 | 0.05 | A0A2R3STY0_CENAM | Putative kinase-like protein TMKL1 | FPM3 | ||
| 56,674 | 0.06 | B5TSR3_CENAM | DELLA protein | FPM2 | ||
| 53,877 | 0.06 | A0A024BLC0_CENAM | ATP synthase subunit beta | FPM3 | ||
| 53,466 | 0.06 | A0A068EUE1_CENAM | Glutathione reductase | FPM3 | ||
| 52,531 | 0.06 | A0A1B0RMG0_CENAM | Purple acid phospatase | FPM2 | ||
| 48,657 | 0.22 | A0A172DYZ9_CENAM | Calreticulin | FPM2 | FPM3 | |
| 47,705 | 0.07 | M1PSE1_CENAM | Ribulose bisphosphate carboxylase large chain | FPM2 | ||
| 46,281 | 0.07 | Q8LKI5_CENAM | Opaque-2-like protein | FPM1 | ||
| 45,994 | 0.07 | B5AKW1_CENAM | eIF-4A | FPM1 | ||
| 43,045 | 0.16 | I3RJV4_CENAM | DELLA protein | FPM1 | FPM3 | |
| 42,984 | 0.08 | I3RJV7_CENAM | DELLA protein | FPM1 | FPM2 | |
| 42,983 | 0.16 | I3RJW9_CENAM | DELLA protein | FPM2 | ||
| 42,870 | 0.08 | A0A076Q103_CENAM | Calcium-dependent protein kinase | FPM3 | ||
| 40,915 | 0.17 | Q94IL8_CENAM | Alcohol dehydrogenase | FPM2 | FPM3 | |
| 39,548 | 0.08 | A0A024BKG2_CENAM | Silicon transport protein | FPM2 | FPM3 | |
| 39,210 | 0.08 | A0A0S1MNE3_CENAM | CaFPM1eoyl CoA O-methyltransferase | FPM1 | ||
| 38,910 | 0.08 | A0A024BKF6_CENAM | Photosystem II protein D1 | FPM1 | ||
| 36,942 | 0.09 | A0A089N0T7_CENAM | Tubulin beta chain | FPM2 | ||
| 36,874 | 0.19 | Q8LRN0_CENAM | Glyoxalase II | FPM2 | FPM3 | |
| 35,920 | 0.09 | A0A4Y1NY14_CENAM | Dehydration-responsive element-binding protein 2 A | FPM3 | ||
| 35,714 | 0.09 | W5QKC5_CENAM | Polygalacturonase inhibitor protein 1 | FPM1 | ||
| 32,918 | 0.1 | Q5NKR7_CENAM | Uncharacterized protein 311G2.2 | FPM3 | ||
| 31,501 | 0.1 | A0A823A7Z2_CENAM | Aquaporin noduline-26-like intrinsic protein 4-1 | FPM1 | ||
| 31,405 | 0.11 | A0A823A730_CENAM | Aquaporin noduline-26-like intrinsic protein 4-1 | FPM2 | ||
| 30,780 | 0.11 | A0A089MYF7_CENAM | Actin-7-like protein | FPM2 | FPM3 | |
| 30,562 | 0.11 | A0A024BKN6_CENAM | Ribosomal protein L2 | FPM2 | ||
| 30,326 | 0.11 | A0A823A8Q5_CENAM | Aquaporin plasma membrane intrinsic protein 2-1 | FPM1 | ||
| 30,061 | 0.11 | Q8HNK2_CENAM | Cytochrome c oxidase subunit 3 | FPM3 | ||
| 29,498 | 0.11 | A0A823ADZ2_CENAM | Aquaporin noduline-26-like intrinsic protein 1-1 | FPM1 | ||
| 29,134 | 0.24 | A6N4D4_CENAM | 27 kDA pennisetin | FPM2 | FPM3 | |
| 27,452 | 0.12 | A4ZYP9_CENAM | L-ascorbate peroxidase | FPM1 | ||
| 27,387 | 0.12 | Q5MJ19_CENAM | RING zinc-finger protein | FPM1 | ||
| 27,247 | 0.12 | G9M131_CENAM | Phytochrome B | FPM1 | FPM3 | |
| 26,887 | 0.12 | CAPSD_MSVSE | Capsid protein | FPM3 | ||
| 26,336 | 0.13 | A0A7T8J1V5_CENAM | PWWP domain family-like protein | FPM3 | ||
| 25,718 | 0.13 | A0A1B1SJY6_CENAM | TIFPM21 | FPM1 | ||
| 25,675 | 0.13 | G9M0H0_CENAM | GIGANTEA | FPM2 | FPM3 | |
| 25,440 | 0.13 | G9M1I7_CENAM | Phytochrome C | FPM2 | ||
| 25,133 | 0.13 | A0A822ZYW4_CENAM | Aquaporin noduline-26 intrinsic protein 3-4 | FPM3 | ||
| 24,652 | 0.14 | B3SU24_CENAM | Elongation factor 1 subunit alpha | FPM2 | FPM3 | |
| 24,520 | 0.14 | Q6R2L1_CENAM | Photosystem I A apoprotein | FPM2 | ||
| 22,459 | 0.15 | A6N4D3_CENAM | 21 kDa pennisetin | FPM1 | ||
| 20,863 | 0.16 | Q9M6M2_CENAM | NB-ARC domain-containing protein | FPM2 | ||
| 18,632 | 0.18 | Q06HR0_CENAM | ATP-dependent Clp protease ATP-binding subunit ClpX1 | FPM1 | ||
| 16,414 | 0.21 | W5QKC6_CENAM | Polygalacturonase inhibitor protein 2 | FPM3 | ||
| 15,149 | 233.85 | A4ZYP8_CENAM | Superoxide dismutase | FPM2 | ||
| 13,526 | 0.25 | A0A024BLF0_CENAM | Ribosomal protein L14 | FPM3 | ||
| 12,402 | 0.62 | D8V069_CENAM | Chitinase | FPM2 | FPM3 | |
| 11,282 | 1.21 | A0A0K1DBU0_CENAM | Glutathione S-transferase | FPM3 | ||
| 10,899 | 0.32 | MP_MSVK | Movement protein | FPM1 | ||
| 10,720 | 0.32 | D7F3V4_CENAM | 30S ribosomial protein S19, chloroplastic | FPM2 | ||
| 9186 | 0.38 | Q32ZI6_CENAM | Truncated vacuolar ATPase subunit C isoform | FPM1 | FPM3 | |
| 5058 | 0.71 | G9M2M4_CENAM | Uncharacterized protein | FPM3 | ||
| Samples | Al | Ca | Cu | Fe | K | Mg | Mn | Mo | Na | Ni | S | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMI | 7.64 ± 0.49 a | 156.64 ± 5.82 a | 4.75 ± 0.15 b | 71.71 ± 0.74 a | 2313.08 ± 6.25 c | 996.37 ± 18.47 a | 14.16 ± 0.24 a | 0.46 ± 0.03 b | 18.85 ± 3.04 a | 2.12 ± 0.03 a | 1051.43 ± 20.60 b | 37.22 ± 0.29 a |
| FPM1 | 13.20 ± 0.64 a | 254.12 ± 2.54 b | 4.28 ± 0.17 a | 71.54 ± 1.13 a | 2477.26 ± 14.78 d | 1395.48 ± 13.39 d | 18.48 ± 0.18 c | 0.22 ± 0.08 a | 215.42 ± 4.67 d | 3.54 ± 0.08 d | 733.64 ± 3.31 a | 40.23 ± 0.88 b |
| FPM2 | 20.47 ± 3.52 b | 282.06 ± 5.45 d | 6.67 ± 0.12 d | 110.41 ± 2.76 c | 2093.95 ± 61.92 b | 1138.98 ± 4.99 c | 18.34 ± 0.32 c | 0.30 ± 0.02 a | 63.47 ± 3.72 c | 3.25 ± 0.04 c | 1405.09 ± 30.76 d | 56.24 ± 1.99 d |
| FPM3 | 21.02 ± 6.26 b | 263.26 ± 3.19 c | 5.99 ± 0.14 c | 96.59 ± 1.01 b | 1988.66 ± 12.90 a | 1073.04 ± 14.45 b | 17.39 ± 0.10 b | 0.28 ± 0.07 a | 56.65 ± 0.12 b | 2.98 ± 0.04 b | 1249.59 ± 13.07 c | 51.53 ± 0.95 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balli, D.; Cecchi, L.; Pieraccini, G.; Venturi, M.; Galli, V.; Reggio, M.; Di Gioia, D.; Furlanetto, S.; Orlandini, S.; Innocenti, M.; et al. Millet Fermented by Different Combinations of Yeasts and Lactobacilli: Effects on Phenolic Composition, Starch, Mineral Content and Prebiotic Activity. Foods 2023, 12, 748. https://doi.org/10.3390/foods12040748
Balli D, Cecchi L, Pieraccini G, Venturi M, Galli V, Reggio M, Di Gioia D, Furlanetto S, Orlandini S, Innocenti M, et al. Millet Fermented by Different Combinations of Yeasts and Lactobacilli: Effects on Phenolic Composition, Starch, Mineral Content and Prebiotic Activity. Foods. 2023; 12(4):748. https://doi.org/10.3390/foods12040748
Chicago/Turabian StyleBalli, Diletta, Lorenzo Cecchi, Giuseppe Pieraccini, Manuel Venturi, Viola Galli, Marta Reggio, Diana Di Gioia, Sandra Furlanetto, Serena Orlandini, Marzia Innocenti, and et al. 2023. "Millet Fermented by Different Combinations of Yeasts and Lactobacilli: Effects on Phenolic Composition, Starch, Mineral Content and Prebiotic Activity" Foods 12, no. 4: 748. https://doi.org/10.3390/foods12040748
APA StyleBalli, D., Cecchi, L., Pieraccini, G., Venturi, M., Galli, V., Reggio, M., Di Gioia, D., Furlanetto, S., Orlandini, S., Innocenti, M., & Mulinacci, N. (2023). Millet Fermented by Different Combinations of Yeasts and Lactobacilli: Effects on Phenolic Composition, Starch, Mineral Content and Prebiotic Activity. Foods, 12(4), 748. https://doi.org/10.3390/foods12040748










