Effect of Huanglongbing on the Volatile Organic Compound Profile of Fruit Juice and Peel Oil in ‘Ray Ruby’ Grapefruit
Abstract
1. Introduction
2. Materials and Methods
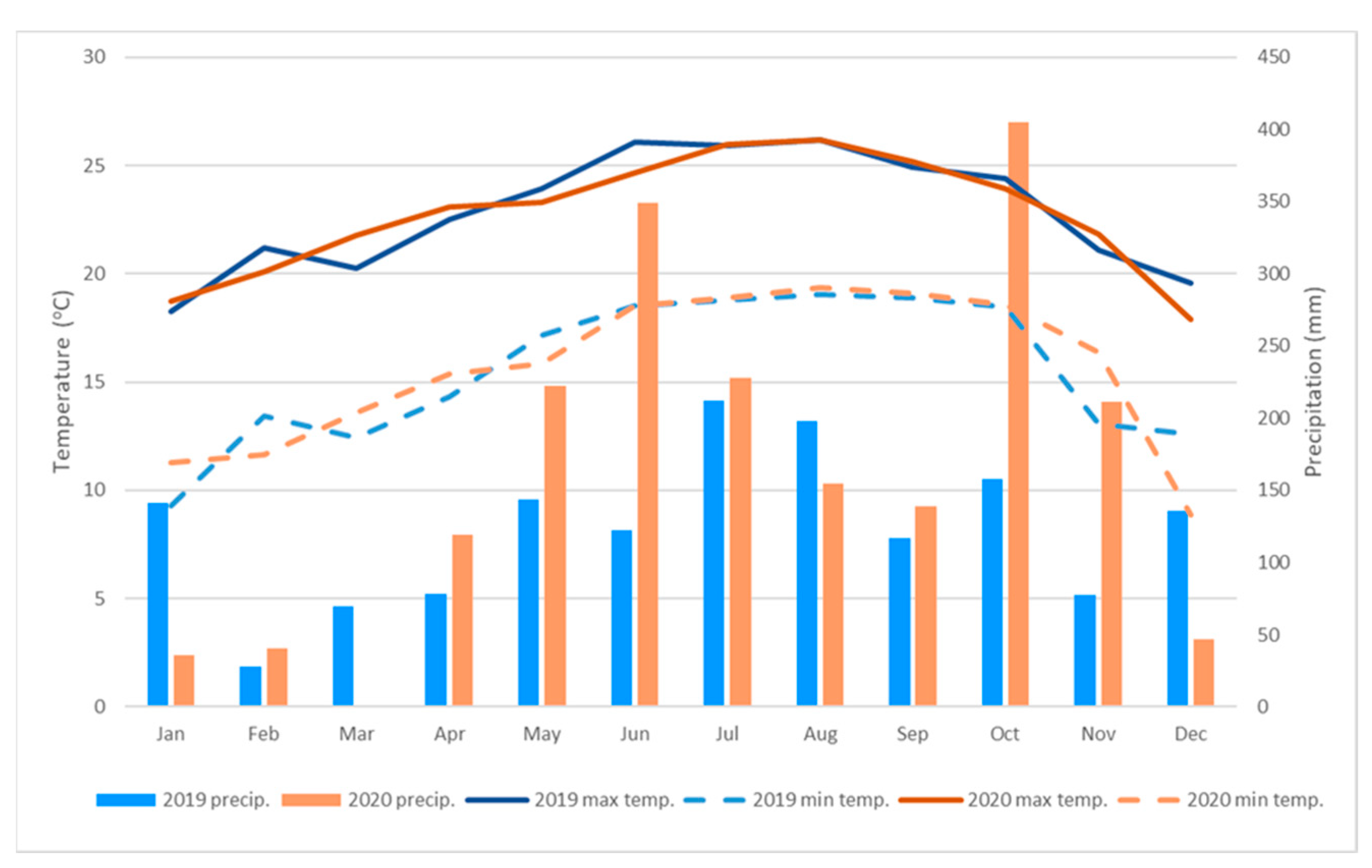
2.1. Site Location, Plant Material, and Cultural Practices
2.2. ‘Candidatus Liberibacter asiaticus’ Detection
2.3. Fruit Harvest and Measurements of Fruit Color, Size, Soluble Solids Contents, and Acidity
2.4. Peel Oil Extraction and Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.5. Juice Extraction and GC-MS Analysis
2.6. Identification and Quantification of VOCs
2.7. Statistical Analyses
3. Results and Discussion
3.1. Huanglongbing Effect on Fruit Size, Color, SSC, and TA
3.2. Huanglongbing Effects on the Volatile Profile of “Ray Ruby” Grapefruit Peel Oil
3.3. Huanglongbing Effects on the Volatile Profile of ‘Ray Ruby’ Grapefruit Juice
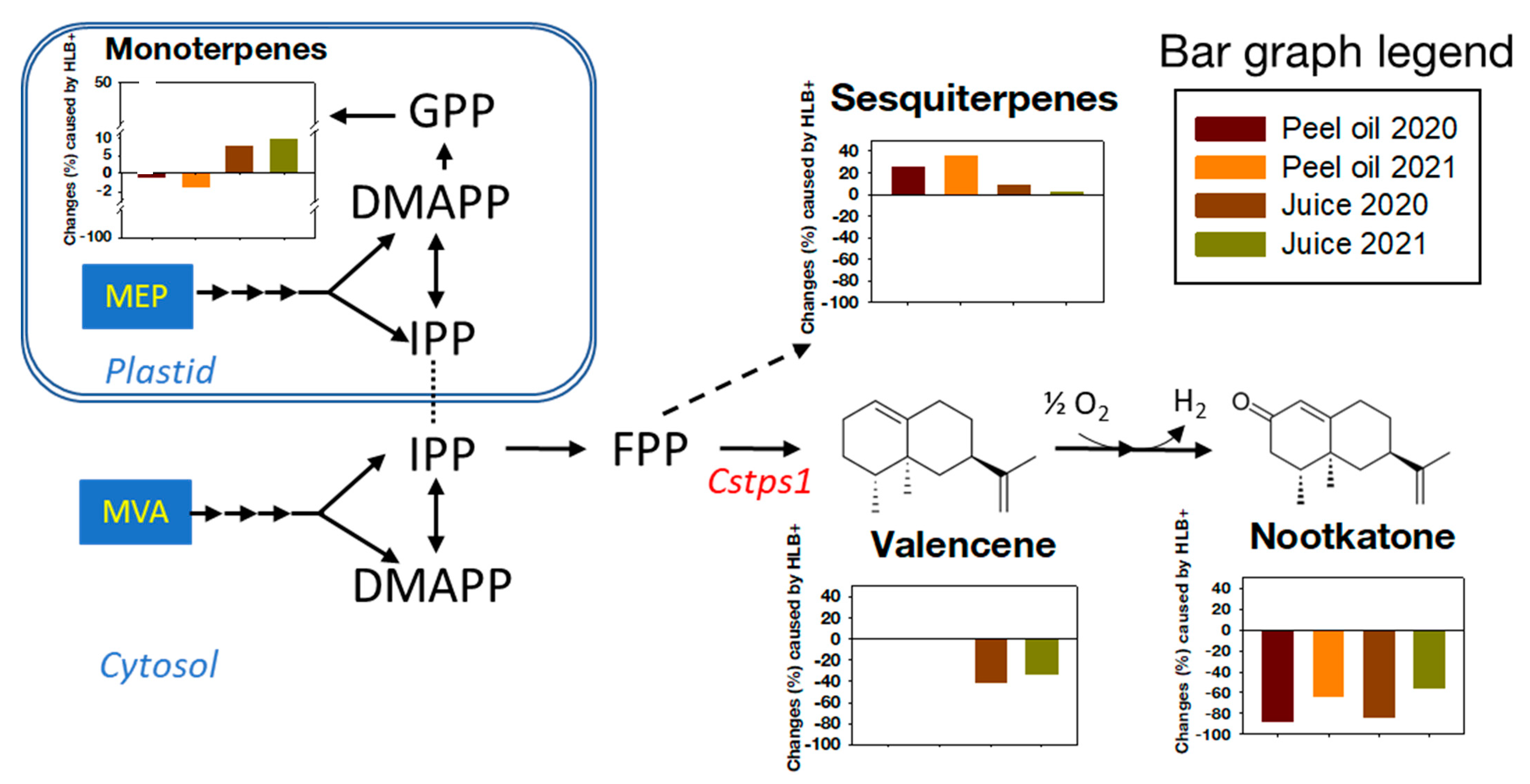
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA-NASS. Florida Citrus Statistics 2020–2021. Available online: http://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/cit/2013-14/cit0514.pdf (accessed on 28 June 2022).
- Phuyal, D.; Nogueira, T.A.R.; Jani, A.D.; Kadyampakeni, D.M.; Morgan, K.T.; Ferrarezi, R.S. ‘Ray Ruby’ Grapefruit Affected by Huanglongbing I. Planting Density and Soil Nutrient Management. HortScience 2020, 55, 1411–1419. [Google Scholar] [CrossRef]
- Baldwin, E.; Bai, J.; Plotto, A.; Ritenour, M. Citrus fruit quality assessment: Producer and consumer perspectives. Stewart Postharvest Rev. 2014, 10, 1. [Google Scholar]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of Huanglongbing or Greening Disease on Orange Juice Quality, a Review. Front. Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.; Plotto, A.; Bai, J.; Manthey, J.; Zhao, W.; Raithore, S.; Irey, M. Effect of abscission zone formation on orange (Citrus sinensis) fruit/juice quality for trees affected by Huanglongbing (HLB). J. Agric. Food Chem. 2018, 66, 2877–2890. [Google Scholar] [CrossRef]
- Zhao, W.; Baldwin, E.A.; Bai, J.; Plotto, A.; Irey, M. Comparative analysis of the transcriptomes of the calyx abscission zone of sweet orange insights into the huanglongbing-associated fruit abscission. Hortic. Res. 2019, 6, 71. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Manthey, J.; McCollum, G.; Bai, J.; Irey, M.; Cameron, R.; Luzio, G. Effect of Liberibacter infection (Huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef]
- Dagulo, L.; Danyluk, M.D.; Spann, T.M.; Valim, M.F.; Goodrich-Schneider, R.; Sims, C.; Rouseff, R. Chemical characterization of orange juice from trees infected with citrus greening (Huanglongbing). J. Food Sci. 2010, 75, C199–C207. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Raithore, S.; Manthey, J.A.; Baldwin, E.A.; Bai, J.; Zhao, W.; Glória, M.B.A.; Plotto, A. Active taste compounds in juice from oranges symptomatic for Huanglongbing (HLB) citrus greening disease. LWT 2018, 91, 518–525. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.; Stover, E.; Driggers, R.; Hearn, J. Volatile profile comparison of USDA sweet orange-like hybrids versus ‘Hamlin’ and ‘Ambersweet’. HortScience 2014, 49, 1262–1267. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; McCollum, G.; Plotto, A.; Manthey, J.A.; Widmer, W.W.; Luzio, G.; Cameron, R. Changes in volatile and non-volatile flavor chemicals of “Valencia” orange juice over the harvest seasons. Foods 2016, 5, 4. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile constituents of redblush grapefruit (Citrus paradisi) and Pummelo (Citrus grandis) peel essential oils from kenya. J. Agric. Food Chem. 2005, 53, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Rouseff, R.L. Characterization of aroma-impact compounds in cold-pressed grapefruit oil using time–intensity GC–olfactometry and GC–MS. Flavour Fragr. J. 2001, 16, 457–463. [Google Scholar] [CrossRef]
- Bun Ng, T.; Bekhit, A.E.A.; Fang, E.F.; Li, X.; Lu, Q.; Guo, H.; Wong, J.H. Grapefruit (Citrus paradisi) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press, Inc.: London, UK, 2016; pp. 463–470. [Google Scholar]
- Murase, T.; Misawa, K.; Haramizu, S.; Minegishi, Y.; Hase, T. Nootkatone, a characteristic constituent of grapefruit, stimulates energy metabolism and prevents diet-induced obesity by activating AMPK. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, 266–275. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Xu, M.; Li, T.; Fan, K.; Yan, T.; Xiao, F.; Bi, K.; Jia, Y. Nootkatone, a neuroprotective agent from Alpiniae Oxyphyllae Fructus, improves cognitive impairment in lipopolysaccharide-induced mouse model of Alzheimer’s disease. Int. Immunopharmacol. 2018, 62, 77–85. [Google Scholar] [CrossRef]
- Xu, B.M.; Baker, G.L.; Sarnoski, P.J.; Goodrich-Schneider, R.M. A comparison of the volatile components of cold pressed Hamlin and Valencia (Citrus sinensis (L.) Osbeck) orange oils affected by Huanglongbing. J. Food Qual. 2017, 2017, 6793986. [Google Scholar] [CrossRef]
- Xu, B.M.; Sims, C.A.; Etxeberria, E.; Schneider, R.M.G. Physicochemical and sensory properties of cold pressed oils from florida hamlin and valencia oranges affected by huanglongbing. J. Food Sci. 2017, 82, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Kiefl, J.; Kohlenberg, B.; Hartmann, A.; Obst, K.; Paetz, S.; Krammer, G.; Trautzsch, S. Investigation on key molecules of Huanglongbing (HLB)-induced orange juice off-flavor. J. Agric. Food Chem. 2017, 66, 2370–2377. [Google Scholar] [CrossRef]
- Sun, X.; Yang, H.; Zhao, W.; Bourcier, E.; Baldwin, E.A.; Plotto, A.; Irey, M.; Bai, J. Huanglongbing and foliar spray programs affect the chemical profile of “Valencia” orange peel oil. Front. Plant Sci. 2021, 12, 611449. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Qureshi, J.A.; Wright, A.L.; Ritenour, M.A.; Macan, N.P.F. Citrus production under screen as a strategy to protect grapefruit trees from Huanglongbing disease. Front. Plant Sci. 2019, 10, 1598. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Bai, J.; Abe, K.; Kurooka, H. Effect of harvest maturity, perforation ratio of polyethylene package and storage temperature on quality of hassaku (Citrus hassaku) fruits. J. Jpn. Soc. Cold Preserv. Food 1990, 16, 97–107. [Google Scholar] [CrossRef]
- Nawaz, R.; Abbasi, N.A.; Khan, M.R.; Ali, I.; Hasan, S.Z.U.; Hayat, A. Color development in ‘Feutrell’s Early’ (Citrus reticulata Blanco) affects peel composition and juice biochemical properties. Int. J. Fruit Sci. 2020, 20, 871–890. [Google Scholar] [CrossRef]
- Uysal, B.; Sozmen, F.; Aktas, O.; Oksal, B.S.; Kose, E.O. Essential oil composition and antibacterial activity of the grapefruit (Citrus Paradisi L.) peel essential oils obtained by solvent-free microwave extraction: Comparison with hydrodistillation. Int. J. Food Sci. Technol. 2011, 46, 1455–1461. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2022. [CrossRef]
- Florida Senate. The 2019 Florida Statutes. Chapter 601: Florida Citrus Code. 2019. Available online: http://www.leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0600-0699/0601/0601.html (accessed on 16 November 2022).
- Bassanezi, R.B.; Montesino, L.H.; Stuchi, E.S. Effects of huanglongbing on fruit quality of sweet orange cultivars in Brazil. Eur. J. Plant Pathol. 2009, 125, 565–572. [Google Scholar] [CrossRef]
- Lado, J.; Cronje, P.; Alquézar, B.; Page, A.; Manzi, M.; Gómez-Cadenas, A.; Stead, A.D.; Zacarías, L.; Rodrigo, M.J. Fruit shading enhances peel color, carotenes accumulation and chromoplast differentiation in red grapefruit. Physiol. Plant. 2015, 154, 469–484. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.; Liao, H.-L.; Zhao, W.; Kostenyuk, I.; Burns, J.; Irey, M. Extraction of DNA from Orange Juice, and Detection of Bacterium Candidatus Liberibacter asiaticus by Real-Time PCR. J. Agric. Food Chem. 2013, 61, 9339–9346. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xie, B.J.; Zhang, Y.; Zhang, Y.; Fan, G.; Yao, X.L.; Pan, S.Y. Characterization of aroma active compounds in fruit juice and peel oil of Jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cacho, P.R.; Rouseff, R.L. Fresh squeezed orange juice odor: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 681–695. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a natural base chemical in sustainable chemistry: A critical review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Kaliaperumal, K.; Zhong, B. Variations of the chemical composition of Citrus sinensis Osbeck cv. Newhall fruit in relation to the symptom severity of Huanglongbing. J. Food Compos. Anal. 2022, 105, 104269. [Google Scholar] [CrossRef]
- Shaw, P.E.; Wilson III, C.W. Importance of nootkatone to the aroma of grapefruit oil and the flavor of grapefruit juice. J. Agric. Food Chem. 1981, 29, 677–679. [Google Scholar] [CrossRef]
- Ortuño, A.; Garcia-Puig, D.; Fuster, M.; Perez, M.; Sabater, F.; Porras, I.; Garcia-Lidon, A.; Del Rio, J. Flavanone and nootkatone levels in different varieties of grapefruit and pummelo. J. Agric. Food Chem. 1995, 43, 1–5. [Google Scholar] [CrossRef]
- Plotto, A.; Bai, J.; Baldwin, E. Fruits. In Springer Handbook of Odor; Buettner, A., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 170–190. [Google Scholar]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef]
- Miyazaki, T.; Plotto, A.; Baldwin, E.A.; Reyes-De-Corcuera, J.I.; Gmitter, F.G., Jr. Aroma characterization of tangerine hybrids by gas-chromatography–olfactometry and sensory evaluation. J. Sci. Food Agric. 2012, 92, 727–735. [Google Scholar] [CrossRef]
- Jella, P.; Rouseff, R.; Goodner, K.; Widmer, W. Determination of key flavor components in methylene chloride extracts from processed grapefruit juice. J. Agric. Food Chem. 1998, 46, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.R.; Jayaprakasha, G.; Patil, B.S. Identification of volatile profiles of Rio Red grapefruit at various developmental to maturity stages. J. Essent. Oil Res. 2017, 30, 77–83. [Google Scholar] [CrossRef]
- Hayes, J.D.; Pulford, D.J. The glut athione S-transferase supergene family: Regulation of GST and the contribution of the lsoenzymes to cancer chemoprotection and drug resistance part I. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–520. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Ayoubi, A.S.; Burke, Y.D. Antitumorigenic effects of limonene and perillyl alcohol against pancreatic and breast cancer. Diet. Phytochem. Cancer Prev. Treat. 1996, 401, 131–136. [Google Scholar]
- Gould, M.N. Cancer chemoprevention and therapy by monoterpenes. Environ. Health Perspect. 1997, 105, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Gmitter Jr, F.G.; Bai, J.; Baldwin, E.; Biotteau, A.; Leclair, C.; McCollum, T.G.; Plotto, A. Effect of fruit maturity on volatiles and sensory descriptors of four mandarin hybrids. J. Food Sci. 2020, 85, 1548–1564. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Baldwin, E.; Hearn, J.; Driggers, R.; Stover, E. Volatile and nonvolatile flavor chemical evaluation of USDA orange–Mandarin hybrids for comparison to sweet orange and Mandarin fruit. J. Am. Soc. Hortic. Sci. 2016, 141, 339–350. [Google Scholar] [CrossRef]
- Hung, W.-L.; Wang, Y. Metabolite profiling of Candidatus Liberibacter infection in Hamlin sweet oranges. J. Agric. Food Chem. 2018, 66, 3983–3991. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, M.; Jia, H.; Yu, Y.; Plotto, A.; Baldwin, E.A.; Bai, J.; Wang, N.; Gmitter, F.G., Jr. Deficiency of valencene in mandarin hybrids is associated with a deletion in the promoter region of the valencene synthase gene. BMC Plant Biol. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Fraatz, M.A.; Berger, R.G.; Zorn, H. Nootkatone—A biotechnological challenge. Appl. Microbiol. Biotechnol. 2009, 83, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Plotto, A.; Baldwin, E.A.; Bai, J.; Huang, M.; Yu, Y.; Dhaliwal, H.S.; Gmitter, F.G. Proteomic and metabolomic analyses provide insight into production of volatile and non-volatile flavor components in mandarin hybrid fruit. BMC Plant Biol. 2015, 15, 76. [Google Scholar] [CrossRef]
- Sharon-Asa, L.; Shalit, M.; Frydman, A.; Bar, E.; Holland, D.; Or, E.; Lavi, U.; Lewinsohn, E.; Eyal, Y. Citrus fruit flavor and aroma biosynthesis: Isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound Valencene. Plant J. 2003, 36, 664–674. [Google Scholar] [CrossRef]
- Cha, Y.; Li, W.; Wu, T.; You, X.; Chen, H.; Zhu, C.; Zhuo, M.; Chen, B.; Li, S. Probing the synergistic ratio of P450/CPR to improve (+)-Nootkatone production in Saccharomyces cerevisiae. J. Agric. Food Chem. 2022, 70, 815–825. [Google Scholar] [CrossRef]
- Ouyang, X.; Cha, Y.; Li, W.; Zhu, C.; Zhu, M.; Li, S.; Zhuo, M.; Huang, S.; Li, J. Stepwise engineering of Saccharomyces cerevisiae to produce (+)-Valencene and its related sesquiterpenes. RSC Adv. 2019, 9, 30171–30181. [Google Scholar] [CrossRef]
- Bai, J.; Jordán, M.J.; Li, J. Metabolism of fruit volatile organic compounds. Front. Plant Sci. 2022, 13, 873515. [Google Scholar] [CrossRef]
- Killiny, N.; Jones, S.E.; Nehela, Y.; Hijaz, F.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. All roads lead to Rome: Towards understanding different avenues of tolerance to huanglongbing in citrus cultivars. Plant Physiol. Biochem. 2018, 129, 1–10. [Google Scholar] [CrossRef]
- Hijaz, F.; El-Shesheny, I.; Killiny, N. Herbivory by the insect d iaphorina citri induces greater change in citrus plant volatile profile than does infection by the bacterium, Candidatus Liberibacter asiaticus. Plant Signal. Behav. 2013, 8, e25677. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Possible role of plant volatiles in tolerance against huanglongbing in citrus. Plant Signal. Behav. 2016, 11, e1138193. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, T.; Poole, G.; McCollum, T.; Hall, D.; Hartung, J.; Bai, J.; Luo, W.; Posny, D.; Duan, Y.-P.; Taylor, E. Canine olfactory detection of a vectored phytobacterial pathogen, Liberibacter asiaticus, and integration with disease control. Proc. Natl. Acad. Sci. USA 2020, 117, 3492–3501. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Bidirectional Interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of chitosan-essential oil coatings on safety and quality of fresh blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef]
- Suh, J.H.; Guha, A.; Wang, Z.; Li, S.-Y.; Killiny, N.; Vincent, C.; Wang, Y. Metabolomic analysis elucidates how shade conditions ameliorate the deleterious effects of greening (Huanglongbing) disease in citrus. Plant J. 2021, 108, 1798–1814. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Sun, J.; Yuan, X.; Deng, W.; Zhong, B.; Chun, J. Characterization of volatile organic compounds of healthy and Huanglongbing-infected navel orange and pomelo leaves by HS-GC-IMS. Molecules 2020, 25, 4119. [Google Scholar] [CrossRef]

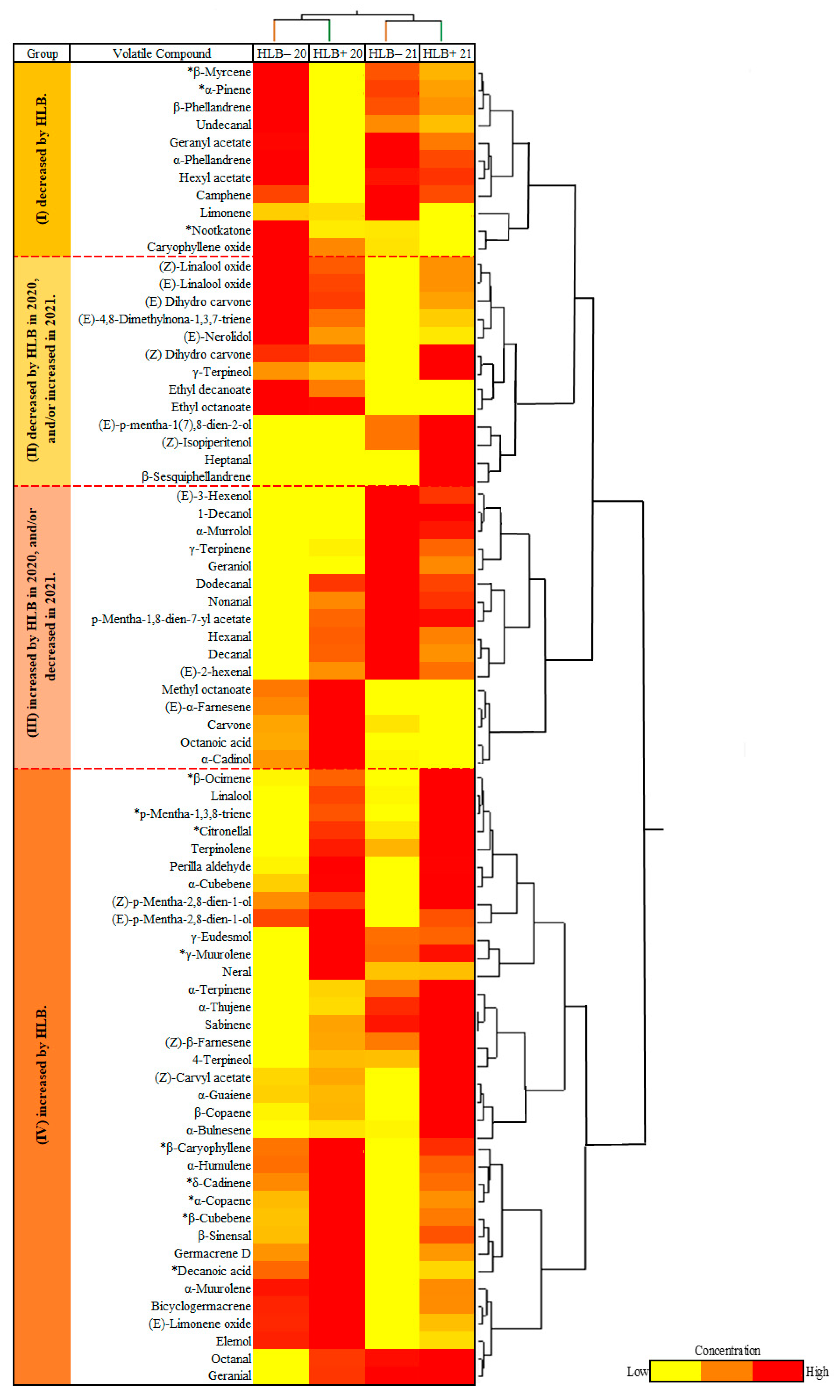
 and HLB+ 20
and HLB+ 20  ) and 2021 (HLB− 21
) and 2021 (HLB− 21  and HLB+ 21
and HLB+ 21  ) harvests. VOCs corresponding to the numbers are listed in Table 2.
) harvests. VOCs corresponding to the numbers are listed in Table 2.
 and HLB+ 20
and HLB+ 20  ) and 2021 (HLB− 21
) and 2021 (HLB− 21  and HLB+ 21
and HLB+ 21  ) harvests. VOCs corresponding to the numbers are listed in Table 2.
) harvests. VOCs corresponding to the numbers are listed in Table 2.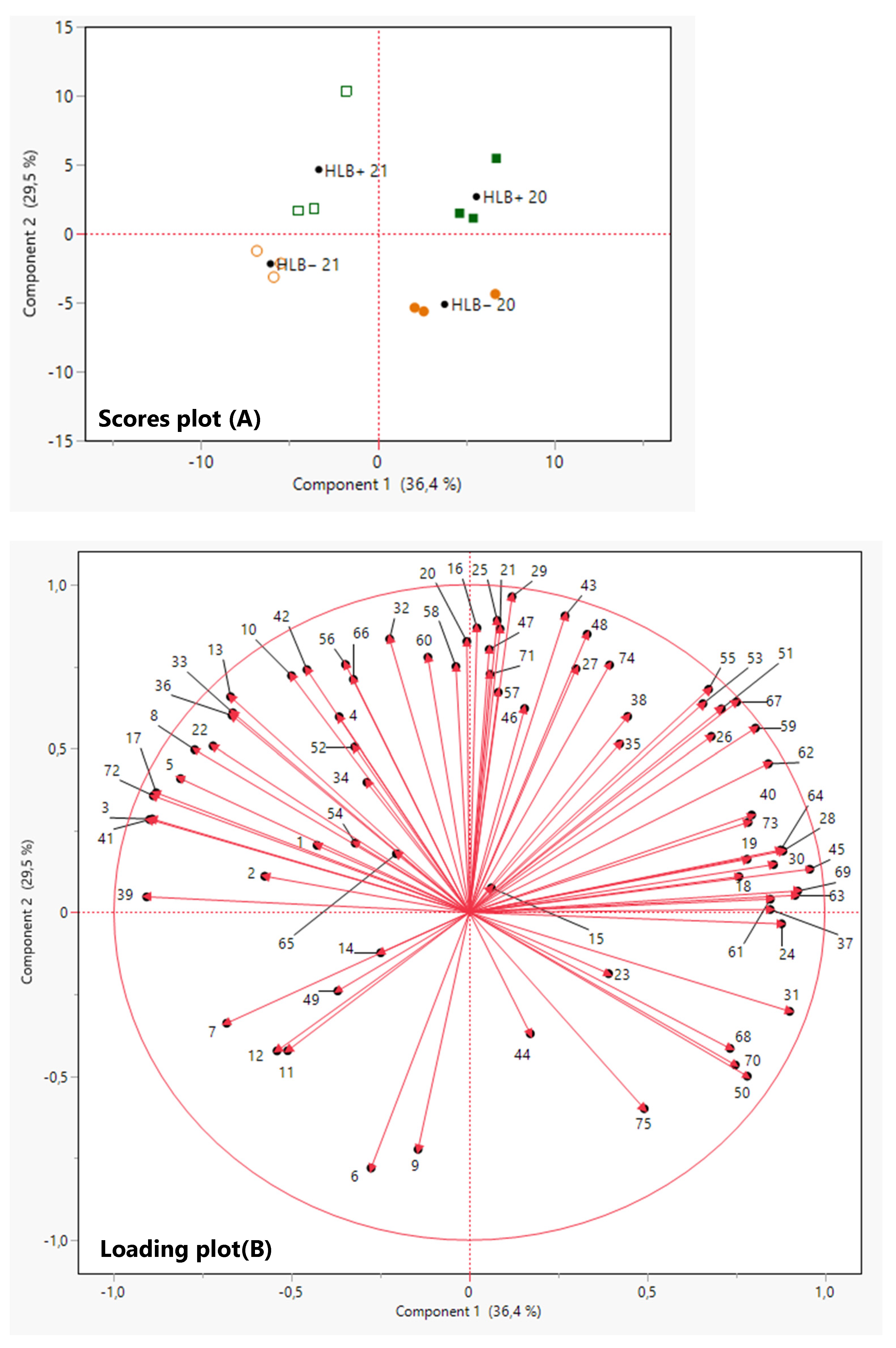
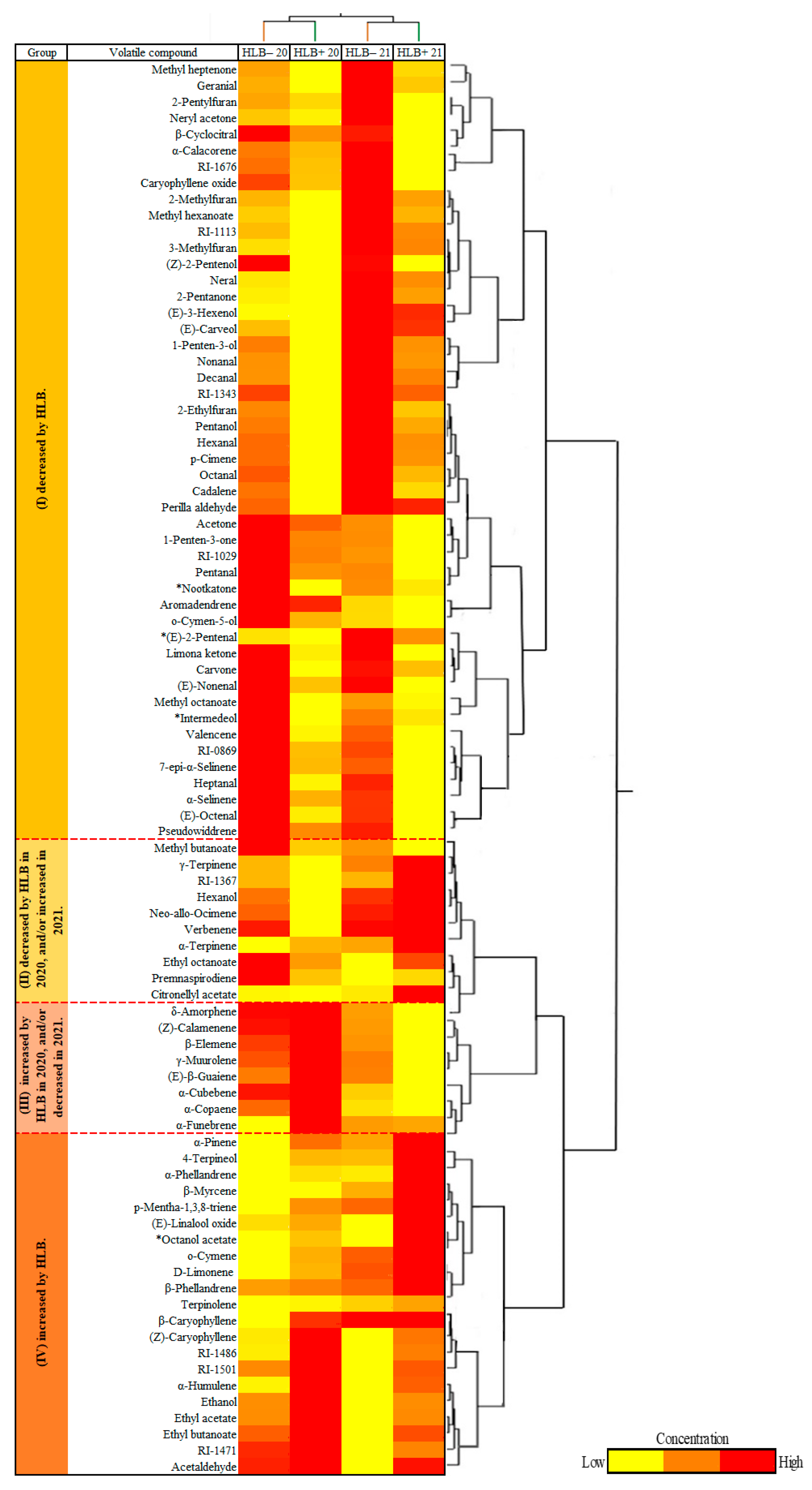
 and HLB+ 20
and HLB+ 20  ) and 2021 (HLB− 21
) and 2021 (HLB− 21  and HLB+ 21
and HLB+ 21  ) harvests. VOCs corresponding to the numbers are listed in Table 3.
) harvests. VOCs corresponding to the numbers are listed in Table 3.
 and HLB+ 20
and HLB+ 20  ) and 2021 (HLB− 21
) and 2021 (HLB− 21  and HLB+ 21
and HLB+ 21  ) harvests. VOCs corresponding to the numbers are listed in Table 3.
) harvests. VOCs corresponding to the numbers are listed in Table 3.
| Parameter | 2020 | 2021 | ||||
|---|---|---|---|---|---|---|
| HLB− | HLB+ | p-Value | HLB− | HLB+ | p-Value | |
| Fruit weight (g) | 482.8 a | 230.8 b | <0.001 *** | 500.6 a | 228.8 b | <0.001 *** |
| Fruit length (mm) | 89.3 a | 74.0 b | <0.001 *** | 96.7a | 74.8 b | <0.001 *** |
| Fruit diameter (mm) | 104.8 a | 79.9 b | <0.001 *** | 99.9 a | 81.2 b | <0.001 *** |
| Peel a* | 8.50 a | −7.25 b | <0.001 *** | 8.56 | −0.19 | 0.058 |
| Peel b* | 42.62 | 46.54 | 0.053 | 45.13 | 52.89 | 0.083 |
| Peel a*/b* ratio | 0.199 a | −0.156 b | <0.001 *** | 0.190 a | −0.036 b | <0.001 *** |
| Hue angle (°) | 78.8 b | 98.8 a | <0.001 *** | 79.3 b | 92.3 a | <0.001 *** |
| SSC (%) | 10.77 a | 9.50 b | 0.009 *** | 9.63 a | 8.35 b | 0.008 *** |
| TA (%) | 1.10 a | 1.15 a | 0.408 | 0.89 a | 0.99 a | 0.347 |
| SSC/TA ratio | 9.79 a | 8.28 b | 0.029 * | 10.93 a | 8.61 b | 0.042 * |
| Peak No. | VOC | Calculated RI z | Reference RI y | 2020 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HLB− | HLB+ | p-Value | Difference x | HLB− | HLB+ | p-Value | Difference | ||||
| 1 | Hexanal | 795 | 805 | 0.001 | 0.003 | 0.0124 * | 200.00% | 0.004 | 0.003 | 0.2907 | −25.00% |
| 2 | (E)-2-Hexenal | 852 | 853 | 0.003 | 0.008 | 0.0058 * | 166.70% | 0.014 | 0.009 | 0.3918 | −35.70% |
| 3 | (E)-3-Hexenol | 855 | 855 | 0.000 w | 0 | - v | - | 0.017 | 0.013 | 0.4063 | −23.50% |
| 4 | Heptanal | 902 | 903 | 0 | 0 | - | - | 0 | 0.001 | 0.0091 * | ++ u |
| 5 | α-Thujene | 937 | 932 | 0.002 | 0.003 | 0.3252 | 50.00% | 0.003 | 0.003 | 0.546 | 0.00% |
| 6 | α-Pinene | 949 | 940 | 0.724 | 0.587 | <0.001 * | −18.90% | 0.689 | 0.638 | 0.0120 * | −7.40% |
| 7 | Camphene | 967 | 951 | 0.003 | 0.003 | 0.0585 | 0.00% | 0.004 | 0.003 | 0.1556 | −25.00% |
| 8 | Sabinene | 988 | 976 | 0.2 | 0.256 | 0.0195 * | 28.00% | 0.336 | 0.352 | 0.6949 | 4.80% |
| 9 | β-Myrcene | 997 | 990 | 2051 | 1744 | 0.0004 * | −15.00% | 1,949 | 1836 | 0.0025 * | −5.80% |
| 10 | Octanal | 1007 | 1003 | 0.09 | 0.218 | 0.0119 * | 142.20% | 0.244 | 0.257 | 0.7337 | 5.30% |
| 11 | Hexyl acetate | 1013 | 1006 | 0.002 | 0 | 0.0005 * | −100.00% | 0.002 | 0.002 | 0.3624 | 0.00% |
| 12 | α-Phellandrene | 1019 | 1010 | 0.045 | 0.036 | 0.0024 * | −20.00% | 0.045 | 0.042 | 0.0516 | −6.70% |
| 13 | α-Terpinene | 1031 | 1020 | 0.004 | 0.005 | 0.1954 | 25.00% | 0.008 | 0.011 | 0.1022 | 37.50% |
| 14 | D-Limonene | 1053 | 1030 | 93,782 | 93,709 | 0.7855 | −0.10% | 94,799 | 93,529 | 0.4629 | −1.30% |
| 15 | β-Phellandrene | 1054 | 1031 | 0.181 | 0.178 | 0.5735 | −1.70% | 0.18 | 0.179 | 0.9456 | −0.60% |
| 16 | β-Ocimene | 1054 | 1035 | 0.081 | 0.115 | 0.0072 * | 42.00% | 0.079 | 0.137 | 0.0036 * | 73.40% |
| 17 | γ-Terpinene | 1072 | 1062 | 0.01 | 0.014 | 0.0779 | 40.00% | 0.083 | 0.054 | 0.5522 | −34.90% |
| 18 | (Z)-Linalool oxide | 1086 | 1080 | 0.304 | 0.265 | 0.3216 | −12.80% | 0.195 | 0.242 | 0.1633 | 24.10% |
| 19 | (E)-Linalool oxide | 1101 | 1086 | 0.152 | 0.138 | 0.4642 | −9.20% | 0.101 | 0.123 | 0.2279 | 21.80% |
| 20 | Terpinolene | 1103 | 1088 | 0.013 | 0.019 | 0.1568 | 46.20% | 0.015 | 0.02 | 0.1705 | 33.30% |
| 21 | Linalool | 1106 | 1101 | 0.136 | 0.165 | 0.102 | 21.30% | 0.138 | 0.176 | 0.297 | 27.50% |
| 22 | Nonanal | 1108 | 1102 | 0.035 | 0.047 | 0.0802 | 34.30% | 0.059 | 0.054 | 0.4627 | −8.50% |
| 23 | (E)-4,8-Dimethylnona-1,3,7-triene | 1120 | 1116 | 0.012 | 0.011 | 0.2153 | −8.30% | 0.009 | 0.01 | 0.7871 | 11.10% |
| 24 | Methyl octanoate | 1124 | 1126 | 0.004 | 0.007 | 0.0122 * | 75.00% | 0 | 0 | - | - |
| 25 | p-Mentha-1,3,8-triene | 1127 | 1118 | 0 | 0.001 | 0.0054 * | ++ | 0 | 0.002 | 0.0001 * | ++ |
| 26 | (E)-p-Mentha-2,8-dien-1-ol | 1137 | 1128 | 0.021 | 0.024 | 0.5517 | 14.30% | 0.013 | 0.02 | 0.25 | 53.80% |
| 27 | (Z)-p-Mentha-2,8-dien-1-ol | 1151 | 1138 | 0.013 | 0.015 | 0.2621 | 15.40% | 0.011 | 0.016 | 0.2967 | 45.50% |
| 28 | (E)-Limonene oxide | 1153 | 1139 | 0.013 | 0.014 | 0.6275 | 7.70% | 0.008 | 0.01 | 0.5024 | 25.00% |
| 29 | Citronellal | 1158 | 1152 | 0.038 | 0.068 | 0.0268 * | 78.90% | 0.042 | 0.076 | 0.0309 * | 81.00% |
| 30 | Octanoic acid | 1160 | 1180 | 0.017 | 0.051 | 0.0050 * | 200.00% | 0 | 0 | - | - |
| 31 | Ethyl octanoate | 1194 | 1195 | 0.011 | 0.01 | 0.8238 | −9.10% | 0 | 0 | - | - |
| 32 | 4-Terpinenol | 1197 | 1182 | 0.025 | 0.03 | 0.2734 | 20.00% | 0.029 | 0.043 | 0.1799 | 48.30% |
| 33 | (E)-p-mentha-1(7),8-dien-2-ol | 1206 | 1204 | 0 | 0 | - | - | 0.005 | 0.009 | 0.2007 | 80.00% |
| 34 | Decanal | 1208 | 1205 | 0.351 | 0.391 | 0.2134 | 11.40% | 0.414 | 0.379 | 0.4399 | −8.50% |
| 35 | (Z) Dihydro carvone | 1214 | 1210 | 0.008 | 0.008 | 0.7397 | 0.00% | 0.005 | 0.009 | 0.1795 | 80.00% |
| 36 | (Z)-Isopiperitenol | 1220 | 1228 | 0 | 0 | - | - | 0.01 | 0.018 | 0.1877 | 80.00% |
| 37 | (E) Dihydro carvone | 1223 | 1230 | 0.008 | 0.007 | 0.5321 | −12.50% | 0.004 | 0.005 | 0.1548 | 25.00% |
| 38 | Neral | 1248 | 1240 | 0.031 | 0.061 | 0.067 | 96.80% | 0.038 | 0.039 | 0.937 | 2.60% |
| 39 | Geraniol | 1261 | 1250 | 0 | 0 | - | - | 0.011 | 0.005 | 0.0033 * | −54.50% |
| 40 | Carvone | 1263 | 1242 | 0.021 | 0.029 | 0.0214 * | 38.10% | 0.017 | 0.016 | 0.7048 | −5.90% |
| 41 | 1-Decanol | 1275 | 1270 | 0 | 0 | - | - | 0.006 | 0.006 | 0.665 | 0.00% |
| 42 | Geranial | 1277 | 1271 | 0.03 | 0.055 | 0.0178 * | 83.30% | 0.062 | 0.062 | 0.9822 | 0.00% |
| 43 | Perilla aldehyde | 1295 | 1272 | 0.016 | 0.031 | 0.0004 * | 93.80% | 0.015 | 0.031 | 0.2256 | 106.70% |
| 44 | Undecanal | 1307 | 1305 | 0.018 | 0.01 | 0.1639 | −44.40% | 0.014 | 0.012 | 0.2492 | −14.30% |
| 45 | Decanoic acid | 1351 | 1370 | 0.007 | 0.011 | 0.0411 * | 57.10% | 0 | 0.002 | 0.0235 * | ++ |
| 46 | γ-Terpineol | 1359 | 1200 | 0.019 | 0.018 | 0.7496 | −5.30% | 0.017 | 0.021 | 0.3144 | 23.50% |
| 47 | (Z)-Carvyl acetate | 1362 | 1360 | 0.017 | 0.018 | 0.4091 | 5.90% | 0.016 | 0.024 | 0.1349 | 50.00% |
| 48 | α-Cubebene | 1369 | 1361 | 0.007 | 0.009 | 0.0546 | 28.60% | 0.006 | 0.009 | 0.0313 * | 50.00% |
| 49 | Geranyl acetate | 1377 | 1380 | 0.083 | 0.062 | 0.0141 * | −25.30% | 0.084 | 0.074 | 0.4158 | −11.90% |
| 50 | Ethyl decanoate | 1387 | 1391 | 0.002 | 0.001 | 0.0190 * | −50.00% | 0 | 0 | - | - |
| 51 | α-Copaene | 1402 | 1380 | 0.151 | 0.197 | 0.0175 * | 30.50% | 0.134 | 0.162 | 0.0181 * | 20.90% |
| 52 | Dodecanal | 1407 | 1405 | 0.02 | 0.024 | 0.0671 | 20.00% | 0.025 | 0.024 | 0.6882 | −4.00% |
| 53 | β-Cubebene | 1413 | 1426 | 0.136 | 0.172 | 0.0149 * | 26.50% | 0.124 | 0.149 | 0.0124 * | 20.20% |
| 54 | p-Mentha-1,8-dien-7-yl acetate | 1442 | 1436 | 0.004 | 0.004 | 0.757 | 0.00% | 0.005 | 0.005 | 0.9257 | 0.00% |
| 55 | β-Caryophyllene | 1454 | 1420 | 0.412 | 0.501 | 0.0297 * | 21.60% | 0.304 | 0.464 | 0.0098 * | 52.60% |
| 56 | (Z)-β-Farnesene | 1457 | 1443 | 0.012 | 0.017 | 0.2619 | 41.70% | 0.019 | 0.025 | 0.4055 | 31.60% |
| 57 | β-Copaene | 1460 | 1430 | 0.005 | 0.006 | 0.7414 | 20.00% | 0.005 | 0.007 | 0.0813 | 40.00% |
| 58 | α-Guaiene | 1462 | 1440 | 0.005 | 0.006 | 0.7193 | 20.00% | 0.005 | 0.007 | 0.0080 * | 40.00% |
| 59 | α-Humulene | 1489 | 1455 | 0.064 | 0.076 | 0.1021 | 18.80% | 0.047 | 0.066 | 0.0221 * | 40.40% |
| 60 | γ-Muurolene | 1502 | 1477 | 0 | 0.01 | <0.0001 * | ++ | 0.006 | 0.009 | 0.0066 * | 50.00% |
| 61 | (E)-α-Farnesene | 1508 | 1506 | 0.011 | 0.023 | 0.0657 | 109.10% | 0 | 0 | - | - |
| 62 | Germacrene D | 1515 | 1482/1519 | 0.08 | 0.103 | 0.08 | 28.80% | 0.063 | 0.079 | 0.0299 * | 25.40% |
| 63 | α-Muurolene | 1525 | 1500 | 0.027 | 0.028 | 0.741 | 3.70% | 0.013 | 0.02 | 0.0011 * | 53.80% |
| 64 | Bicyclogermacrene | 1531 | 1502 | 0.024 | 0.026 | 0.7317 | 8.30% | 0.014 | 0.02 | 0.0298 * | 42.90% |
| 65 | α-Bulnesene | 1535 | 1510 | 0.003 | 0.004 | 0.06 | 33.30% | 0.003 | 0.009 | 0.3503 | 200.00% |
| 66 | β-Sesquiphellandrene | 1540 | 1521 | 0 | 0 | - | - | 0 | 0.003 | 0.0096 * | ++ |
| 67 | δ-Cadinene | 1547 | 1523 | 0.177 | 0.211 | 0.0179 * | 19.20% | 0.148 | 0.184 | 0.0455 * | 24.30% |
| 68 | (E)-Nerolidol | 1570 | 1535 | 0.028 | 0.017 | 0.0191 * | −39.30% | 0.009 | 0.01 | 0.3918 | 11.10% |
| 69 | Elemol | 1577 | 1551 | 0.041 | 0.045 | 0.5934 | 9.80% | 0.022 | 0.025 | 0.6186 | 13.60% |
| 70 | Caryophyllene oxide | 1630 | 1580 | 0.024 | 0.017 | 0.1633 | −29.20% | 0.012 | 0.011 | 0.1373 | −8.30% |
| 71 | γ-Eudesmol | 1675 | 1622 | 0 | 0.008 | 0.0138 * | ++ | 0.005 | 0.005 | 0.5735 | 0.00% |
| 72 | α-Murrolol | 1686 | 1642 | 0 | 0 | - | - | 0.012 | 0.011 | 0.981 | −8.30% |
| 73 | α-Cadinol | 1695 | 1653 | 0.009 | 0.012 | 0.1668 | 33.30% | 0.007 | 0.007 | 0.8972 | 0.00% |
| 74 | β -Sinensal | 1719 | 1706 | 0.02 | 0.026 | 0.2684 | 30.00% | 0.017 | 0.023 | 0.4113 | 35.30% |
| 75 | Nootkatone | 1843 | 1820 | 0.127 | 0.016 | 0.0264 * | −87.40% | 0.019 | 0.007 | 0.0059 * | −63.20% |
| Chemical class | |||||||||||
| Monoterpene hydrocarbons | 97.11 | 96.68 | 0.1437 | −0.40% | 98.2 | 96.81 | 0.4154 | −1.40% | |||
| Sesquiterpene hydrocarbons | 1.11 | 1.39 | 0.0194 * | 25.20% | 0.89 | 1.21 | 0.0155 * | 36.00% | |||
| Aliphatic aldehydes | 0.52 | 0.7 | 0.0149* | 34.60% | 0.78 | 0.74 | 0.9413 | −5.10% | |||
| Terpene aldehydes | 0.13 | 0.24 | 0.0816 | 84.60% | 0.17 | 0.23 | 0.2666 | 35.30% | |||
| Total aldehydes | 0.65 | 0.94 | 0.0096 * | 44.60% | 0.95 | 0.97 | 0.8814 | 2.10% | |||
| Terpene oxides | 0.49 | 0.44 | 0.3522 | −10.20% | 0.32 | 0.39 | 0.1933 | 21.90% | |||
| Alcohols | 0.29 | 0.33 | 0.1597 | 13.80% | 0.31 | 0.38 | 0.3717 | 22.60% | |||
| Ketones | 0.16 | 0.06 | 0.0437 * | −62.50% | 0.04 | 0.04 | 0.3921 | 0.00% | |||
| Esters | 0.12 | 0.1 | 0.0377 * | −16.70% | 0.11 | 0.1 | 0.8718 | −9.10% | |||
| Peak No. | VOC | Calculated RI z | Reference RI y | 2020 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HLB– | HLB+ | p-Value | Difference x | HLB– | HLB+ | p-Value | Difference | ||||
| 1 | Acetaldehyde | 447 | 380 | 1.37 | 1.49 | 0.6811 | 8.8% | 0.61 | 1.41 | 0.2879 | 131.1% |
| 2 | Ethanol | 475 | 450 | 5.05 | 9.94 | 0.1798 | 96.8% | 1.03 | 5.06 | 0.0570 | 391.3% |
| 3 | Acetone | 500 | 500 | 1.67 | 1.48 | 0.745 | −11.4% | 1.38 | 1.15 | 0.2660 | −16.7% |
| 4 | 2-Methylfuran | 595 | 605 | 0.66 | 0.54 | 0.1301 | −18.2% | 0.96 | 0.70 | 0.2193 | −27.1% |
| 5 | Ethyl acetate | 600 | 610 | 3.05 | 5.62 | 0.1847 | 84.3% | 0.94 | 3.11 | 0.0819 | 230.9% |
| 6 | 3-Methylfuran | 606 | 620 | 0.91 | 0.88 | 0.5746 | −3.3% | 1.08 | 0.98 | 0.6796 | −9.3% |
| 7 | 1-Penten-3-ol | 674 | 680 | 0.15 | 0.09 | 0.1767 | −40.0% | 0.21 | 0.14 | 0.0505 | −33.3% |
| 8 | 1-Penten-3-one | 678 | 685 | 0.61 | 0.43 | 0.0676 | −29.5% | 0.42 | 0.26 | 0.0378 * | −38.1% |
| 9 | 2-Pentanone | 689 | 695 | 0.17 | 0.13 | 0.3553 | −23.5% | 0.75 | 0.37 | 0.1375 | −50.7% |
| 10 | Pentanal | 692 | 700 | 3.71 | 1.97 | 0.1968 | −46.9% | 2.11 | 0.66 | 0.0084 * | −68.7% |
| 11 | 2-Ethylfuran | 696 | 705 | 0.40 | 0.21 | 0.1229 | −47.5% | 0.60 | 0.30 | 0.1005 | −50.0% |
| 12 | Methyl butanoate | 715 | 720 | 0.28 | 0.18 | 0.2831 | −35.7% | 0.20 | 0.15 | 0.1980 | −25.0% |
| 13 | (E)-2-Pentenal | 754 | 750 | 0.16 | 0.00 v | <0.0001 * | −100.0% | 0.15 | 0.00 | <0.0001 * | −100.0% |
| 14 | Pentanol | 761 | 765 | 0.24 | 0.11 | 0.053 | −54.2% | 0.36 | 0.20 | 0.0640 | −44.4% |
| 15 | (Z)-2-Pentenol | 765 | 770 | 0.15 | 0.09 | 0.0172 * | −40.0% | 0.61 | 0.32 | 0.0981 | −47.5% |
| 16 | Ethyl butanoate | 796 | 800 | 1.80 | 2.39 | 0.5098 | 32.8% | 0.79 | 1.90 | 0.1482 | 140.5% |
| 17 | Hexanal | 801 | 805 | 41.45 | 15.93 | 0.0479 * | −61.6% | 59.57 | 35.27 | 0.1921 | −40.8% |
| 18 | (E)-3-Hexenol | 857 | 855 | 1.77 | 1.69 | 0.7959 | −4.5% | 5.88 | 5.14 | 0.6759 | −12.6% |
| 19 | Hexanol | 867 | 865 | 0.94 | 0.65 | 0.2656 | −30.9% | 1.07 | 1.18 | 0.6968 | 10.3% |
| 20 | RI-0869 w | 869 | - | 3.22 | 2.76 | 0.4301 | −14.3% | 3.04 | 2.60 | 0.2134 | −14.5% |
| 21 | Heptanal | 905 | 903 | 6.89 | 2.33 | 0.0198 * | −66.2% | 6.18 | 2.11 | 0.0050 * | −65.9% |
| 22 | Methyl hexanoate | 922 | 925 | 1.15 | 0.69 | 0.1551 | −40.0% | 2.98 | 1.38 | 0.1128 | −53.7% |
| 23 | α-Pinene | 954 | 940 | 2.97 | 3.53 | 0.7586 | 18.9% | 3.32 | 3.96 | 0.6181 | 19.3% |
| 24 | Methyl heptenone | 986 | 986 | 56.75 | 51.22 | 0.3349 | −9.7% | 66.09 | 53.49 | 0.3871 | −19.1% |
| 25 | β-Myrcene | 993 | 990 | 33.95 | 34.12 | 0.9944 | 0.5% | 39.68 | 51.72 | 0.4990 | 30.3% |
| 26 | 2-Pentylfuran | 996 | 992 | 0.53 | 0.43 | 0.6037 | −18.9% | 0.85 | 0.35 | 0.0245 * | −58.8% |
| 27 | Octanal | 1006 | 1003 | 2.62 | 0.98 | 0.0016 * | −62.6% | 3.45 | 1.67 | 0.0629 | −51.6% |
| 28 | Verbenene | 1021 | 956 | 0.68 | 0.53 | 0.6212 | −22.1% | 0.69 | 0.70 | 0.9706 | 1.4% |
| 29 | α-Phellandrene | 1023 | 1010 | 0.55 | 0.61 | 0.8729 | 10.9% | 0.59 | 1.07 | 0.2119 | 81.4% |
| 30 | RI-1029 | 1029 | - | 0.24 | 0.17 | 0.2728 | −29.2% | 0.16 | 0.09 | 0.2245 | −43.8% |
| 31 | α-Terpinene | 1033 | 1020 | 0.67 | 0.86 | 0.6049 | 28.4% | 0.90 | 1.30 | 0.3318 | 44.4% |
| 32 | o-Cymene | 1040 | 1023 | 3.39 | 3.84 | 0.6628 | 13.3% | 4.28 | 4.79 | 0.4519 | 11.9% |
| 33 | D-Limonene | 1048 | 1030 | 404.14 | 437.84 | 0.8006 | 8.3% | 480.82 | 517.63 | 0.6770 | 7.7% |
| 34 | β-Phellandrene | 1052 | 1031 | 3.22 | 3.59 | 0.8804 | 11.5% | 3.96 | 5.35 | 0.4181 | 35.1% |
| 35 | p-Cimene | 1057 | 1036 | 0.83 | 0.53 | 0.0172 * | −36.1% | 1.04 | 0.75 | 0.0360 * | −27.9% |
| 36 | (E)-Octenal | 1062 | 1060 | 0.83 | 0.30 | 0.0023 * | −63.9% | 0.71 | 0.26 | 0.0122 * | −63.4% |
| 37 | γ-Terpinene | 1071 | 1062 | 0.58 | 0.52 | 0.8246 | −10.3% | 0.62 | 0.73 | 0.5986 | 17.7% |
| 38 | (E)-Linalool oxide | 1084 | 1086 | 0.86 | 0.94 | 0.849 | 9.3% | 0.81 | 1.19 | 0.1313 | 46.9% |
| 39 | Terpinolene | 1101 | 1088 | 1.87 | 2.02 | 0.8953 | 8.0% | 2.53 | 3.14 | 0.4702 | 24.1% |
| 40 | Nonanal | 1105 | 1102 | 4.64 | 3.64 | 0.1575 | −21.6% | 5.93 | 4.59 | 0.0027 * | −22.6% |
| 41 | RI-1113 | 1113 | - | 0.31 | 0.25 | 0.4404 | −19.4% | 0.48 | 0.36 | 0.2803 | −25.0% |
| 42 | Methyl octanoate | 1117 | 1126 | 0.71 | 0.33 | 0.0459 * | −53.5% | 0.49 | 0.35 | 0.2712 | −28.6% |
| 43 | o-Cymen-5-ol | 1125 | - | 0.71 | 0.50 | 0.4011 | −29.6% | 0.46 | 0.42 | 0.6126 | −8.7% |
| 44 | p-Mentha-1,3,8-triene | 1128 | 1118 | 0.15 | 0.17 | 0.5552 | 13.3% | 0.18 | 0.20 | 0.5945 | 11.1% |
| 45 | Neo-allo-Ocimene | 1145 | 1131 | 0.08 | 0.05 | 0.3697 | −37.5% | 0.09 | 0.09 | 0.7875 | 0.0% |
| 46 | Limona ketone | 1147 | 1144 | 0.27 | 0.19 | 0.3673 | −29.6% | 0.27 | 0.18 | 0.0527 | −33.3% |
| 47 | (E)-Nonenal | 1162 | 1162 | 0.32 | 0.16 | 0.1569 | −50.0% | 0.32 | 0.11 | 0.0027 * | −65.6% |
| 48 | Ethyl octanoate | 1187 | 1195 | 2.29 | 1.47 | 0.658 | −35.8% | 0.92 | 1.90 | 0.3276 | 106.5% |
| 49 | Octanol acetate | 1201 | 1211 | 0.00 | 0.10 | 0.0283 * | ++ u | 0.00 | 0.41 | 0.0195 * | ++ |
| 50 | 4-Terpineol | 1203 | 1182 | 0.21 | 0.25 | 0.8277 | 19.0% | 0.25 | 0.35 | 0.4555 | 40.0% |
| 51 | Decanal | 1206 | 1205 | 0.43 | 0.24 | 0.1113 | −44.2% | 0.69 | 0.46 | 0.1254 | −33.3% |
| 52 | (E)-Carveol | 1235 | 1217 | 0.33 | 0.21 | 0.4553 | −36.4% | 0.68 | 0.58 | 0.8259 | −14.7% |
| 53 | β-Cyclocitral | 1244 | 1223 | 1.05 | 0.84 | 0.4535 | −20.0% | 1.00 | 0.68 | 0.0719 | −32.0% |
| 54 | Neral | 1247 | 1240 | 0.40 | 0.37 | 0.7952 | −7.5% | 0.64 | 0.49 | 0.0114 * | −23.4% |
| 55 | Carvone | 1266 | 1242 | 1.20 | 0.77 | 0.5385 | −35.8% | 1.16 | 0.88 | 0.2813 | −24.1% |
| 56 | Geranial | 1274 | 1271 | 0.85 | 0.69 | 0.2611 | −18.8% | 1.18 | 0.80 | 0.0153 * | −32.2% |
| 57 | Perilla aldehyde | 1306 | 1272 | 0.10 | 0.06 | 0.5129 | −40.0% | 0.12 | 0.11 | 0.8090 | −8.3% |
| 58 | RI-1343 | 1343 | - | 0.39 | 0.17 | 0.3146 | −56.4% | 0.46 | 0.35 | 0.4882 | −23.9% |
| 59 | Citronellyl acetate | 1348 | 1355 | 0.29 | 0.28 | 0.9678 | −3.4% | 0.30 | 0.55 | 0.2422 | 83.3% |
| 60 | RI-1367 | 1367 | - | 0.33 | 0.26 | 0.797 | −21.2% | 0.33 | 0.50 | 0.4228 | 51.5% |
| 61 | α-Cubebene | 1381 | 1361 | 3.28 | 3.47 | 0.9063 | 5.8% | 1.96 | 1.59 | 0.5229 | −18.9% |
| 62 | α-Copaene | 1423 | 1380 | 11.31 | 13.93 | 0.6077 | 23.2% | 8.28 | 7.48 | 0.7582 | −9.7% |
| 63 | β-Elemene | 1429 | 1384 | 3.34 | 3.55 | 0.8735 | 6.3% | 3.06 | 2.70 | 0.5631 | −11.8% |
| 64 | (Z)-Caryophyllene | 1463 | 1405 | 2.21 | 2.96 | 0.2626 | 33.9% | 2.14 | 2.58 | 0.1100 | 20.6% |
| 65 | Neryl acetone | 1466 | 1434 | 1.61 | 1.35 | 0.1231 | −16.1% | 2.79 | 1.27 | 0.0002 * | −54.5% |
| 66 | RI-1471 | 1471 | - | 0.79 | 0.81 | 0.9556 | 2.5% | 0.69 | 0.75 | 0.4549 | 8.7% |
| 67 | α-Funebrene | 1476 | 1385 | 0.25 | 0.40 | 0.0358 * | 60.0% | 0.31 | 0.30 | 0.8610 | −3.2% |
| 68 | β-Caryophyllene | 1482 | 1420 | 234.29 | 248.18 | 0.606 | 5.9% | 245.09 | 251.65 | 0.7335 | 2.7% |
| 69 | RI-1486 | 1486 | - | 9.00 | 10.43 | 0.6083 | 15.9% | 7.74 | 9.49 | 0.1092 | 22.6% |
| 70 | Aromadendrene | 1497 | 1440 | 0.57 | 0.51 | 0.7684 | −10.5% | 0.26 | 0.21 | 0.4499 | −19.2% |
| 71 | Premnaspirodiene | 1498 | 1505 | 0.47 | 0.42 | 0.6351 | −10.6% | 0.40 | 0.41 | 0.7714 | 2.5% |
| 72 | RI-1501 | 1501 | - | 2.82 | 3.86 | 0.3824 | 36.9% | 2.74 | 3.31 | 0.1840 | 20.8% |
| 73 | α-Humulene | 1514 | 1455 | 37.54 | 51.31 | 0.461 | 36.7% | 36.83 | 45.93 | 0.2014 | 24.7% |
| 74 | γ-Muurolene | 1520 | 1477 | 1.57 | 1.83 | 0.7173 | 16.6% | 1.44 | 1.02 | 0.2465 | −29.2% |
| 75 | (E)-β-Guaiene | 1532 | 1490 | 0.52 | 0.64 | 0.6221 | 23.1% | 0.51 | 0.38 | 0.4463 | −25.5% |
| 76 | Pseudowiddrene | 1538 | 1523 | 4.86 | 3.74 | 0.4153 | −23.0% | 4.59 | 2.78 | 0.0423 * | −39.4% |
| 77 | Valencene | 1546 | 1492 | 4.58 | 2.69 | 0.2728 | −41.3% | 3.86 | 2.61 | 0.1532 | −32.4% |
| 78 | α-Selinene | 1551 | 1496 | 2.51 | 1.81 | 0.385 | −27.9% | 2.28 | 1.49 | 0.0954 | −34.6% |
| 79 | δ-Amorphene | 1558 | 1512 | 14.79 | 15.08 | 0.9574 | 2.0% | 11.04 | 8.51 | 0.4020 | −22.9% |
| 80 | (Z)-Calamenene | 1566 | 1518 | 1.85 | 1.91 | 0.8863 | 3.2% | 1.46 | 1.17 | 0.2506 | −19.9% |
| 81 | 7-epi-α-Selinene | 1578 | 1520 | 3.15 | 1.98 | 0.2758 | −37.1% | 2.56 | 1.53 | 0.0370 * | −40.2% |
| 82 | α-Calacorene | 1589 | 1542 | 0.31 | 0.26 | 0.7017 | −16.1% | 0.40 | 0.21 | 0.2672 | −47.5% |
| 83 | Caryophyllene oxide | 1598 | 1580 | 2.56 | 2.03 | 0.3732 | −20.7% | 2.85 | 1.77 | 0.0124 * | −37.9% |
| 84 | RI-1676 | 1676 | - | 1.16 | 0.98 | 0.5716 | −15.5% | 1.38 | 0.85 | 0.0106 * | −38.4% |
| 85 | Cadalene | 1694 | 1674 | 0.39 | 0.33 | 0.5114 | −15.4% | 0.44 | 0.35 | 0.2595 | −20.5% |
| 86 | Intermedeol | 1701 | 1675 | 1.95 | 0.50 | 0.0435 * | −74.4% | 1.27 | 0.65 | 0.0118 * | −48.8% |
| 87 | Nootkatone | 1849 | 1820 | 4.30 | 0.71 | 0.0467 * | −83.5% | 2.35 | 1.05 | 0.0166 * | −55.3% |
| Chemical class | |||||||||||
| Monoterpene hydrocarbons | 453.25 | 488.29 | 0.8315 | 7.7% | 538.85 | 591.71 | 0.6357 | 9.8% | |||
| Sesquiterpene hydrocarbons | 327.8 | 354.98 | 0.6445 | 8.3% | 326.91 | 332.9 | 0.8463 | 1.8% | |||
| Terpene alcohols | 3.2 | 1.46 | 0.1034 | −54.4% | 2.66 | 2 | 0.1868 | −24.8% | |||
| Terpene aldehydes | 2.39 | 1.96 | 0.3414 | −18.0% | 2.94 | 2.08 | 0.0023 * | −29.3% | |||
| Terpene ketones | 5.77 | 1.67 | 0.0495 * | −71.1% | 3.79 | 2.11 | 0.0345 * | −44.3% | |||
| Terpene esters | 2.29 | 1.57 | 0.697 | −31.4% | 0.92 | 2.32 | 0.1967 | 152.2% | |||
| Terpene oxide | 3.56 | 3.14 | 0.6047 | −11.8% | 3.83 | 3.17 | 0.2033 | −17.2% | |||
| Total terpene compounds | 798.25 | 853.06 | 0.8096 | 6.9% | 880.59 | 936.75 | 0.6463 | 6.4% | |||
| Aliphatic aldehydes | 62.42 | 27.04 | 0.0334 * | −56.7% | 79.72 | 46.54 | 0.0489 * | −41.6% | |||
| Aliphatic alcohols | 8.31 | 12.57 | 0.2115 | 51.3% | 9.16 | 12.04 | 0.175 | 31.4% | |||
| Aliphatic ketones | 60.81 | 54.61 | 0.3009 | −10.2% | 71.42 | 56.54 | 0.3248 | −20.8% | |||
| Aliphatic esters | 7.28 | 9.48 | 0.4531 | 30.2% | 5.7 | 7.44 | 0.278 | 30.5% | |||
| Furan | 2.5 | 2.06 | 0.2469 | −17.6% | 3.5 | 2.33 | 0.1537 | −33.4% | |||
| Total non-terpene compounds | 141.32 | 105.76 | 0.0498 * | −25.2% | 168.8 | 124.42 | 0.2137 | −26.3% | |||
| Other | 17.93 | 19.42 | 0.6998 | 8.3% | 16.7 | 17.8 | 0.4713 | 6.6% | |||
| Total | 957.5 | 978.25 | 0.9243 | 2.2% | 1066.09 | 1078.97 | 0.8944 | 1.2% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cruz, M.A.; Plotto, A.; Ferrarezi, R.S.; Leite Junior, R.P.; Bai, J. Effect of Huanglongbing on the Volatile Organic Compound Profile of Fruit Juice and Peel Oil in ‘Ray Ruby’ Grapefruit. Foods 2023, 12, 713. https://doi.org/10.3390/foods12040713
da Cruz MA, Plotto A, Ferrarezi RS, Leite Junior RP, Bai J. Effect of Huanglongbing on the Volatile Organic Compound Profile of Fruit Juice and Peel Oil in ‘Ray Ruby’ Grapefruit. Foods. 2023; 12(4):713. https://doi.org/10.3390/foods12040713
Chicago/Turabian Styleda Cruz, Maria Aparecida, Anne Plotto, Rhuanito Soranz Ferrarezi, Rui Pereira Leite Junior, and Jinhe Bai. 2023. "Effect of Huanglongbing on the Volatile Organic Compound Profile of Fruit Juice and Peel Oil in ‘Ray Ruby’ Grapefruit" Foods 12, no. 4: 713. https://doi.org/10.3390/foods12040713
APA Styleda Cruz, M. A., Plotto, A., Ferrarezi, R. S., Leite Junior, R. P., & Bai, J. (2023). Effect of Huanglongbing on the Volatile Organic Compound Profile of Fruit Juice and Peel Oil in ‘Ray Ruby’ Grapefruit. Foods, 12(4), 713. https://doi.org/10.3390/foods12040713








