Effect of Proofing on the Rheology and Moisture Distribution of Corn Starch-Hydroxypropylmethylcellulose Gluten-Free Dough
Abstract
:1. Introduction
2. Materials and methods
2.1. Materials
2.2. Dough Preparation
2.3. Proofing Curves Determined by Rheofermentometer
2.4. Soluble Carbohydrate Composition
2.5. Water Morphology Determined by DSC
2.6. Water Mobility Analysis
2.7. Dynamic Rheological Characterization
2.7.1. Dynamic Strain Sweep
2.7.2. Dynamic Frequency Sweep
2.7.3. Temperature Sweep
2.8. Creep and Recovery Behavior
2.9. Statistical Analysis
3. Results and Discussion
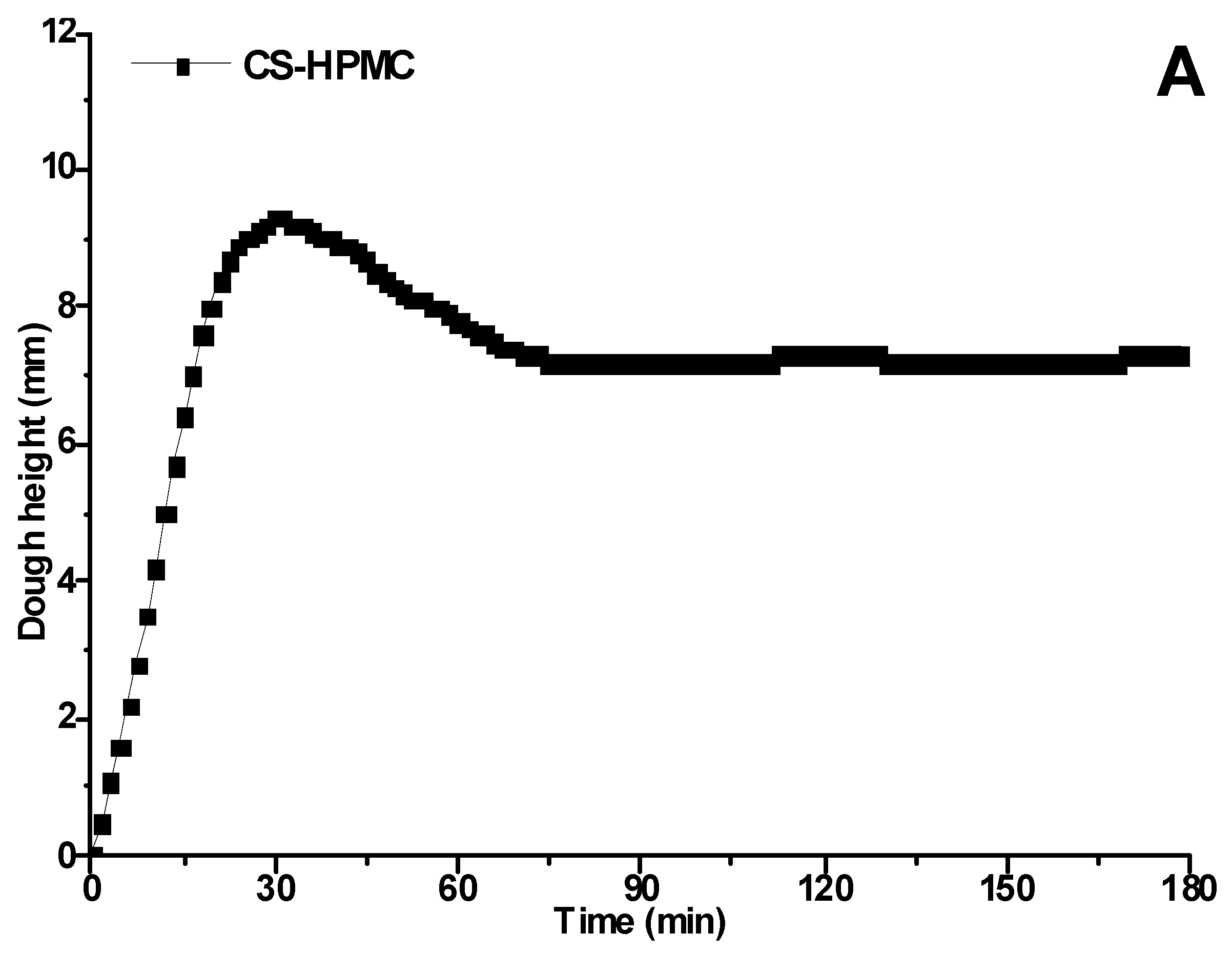
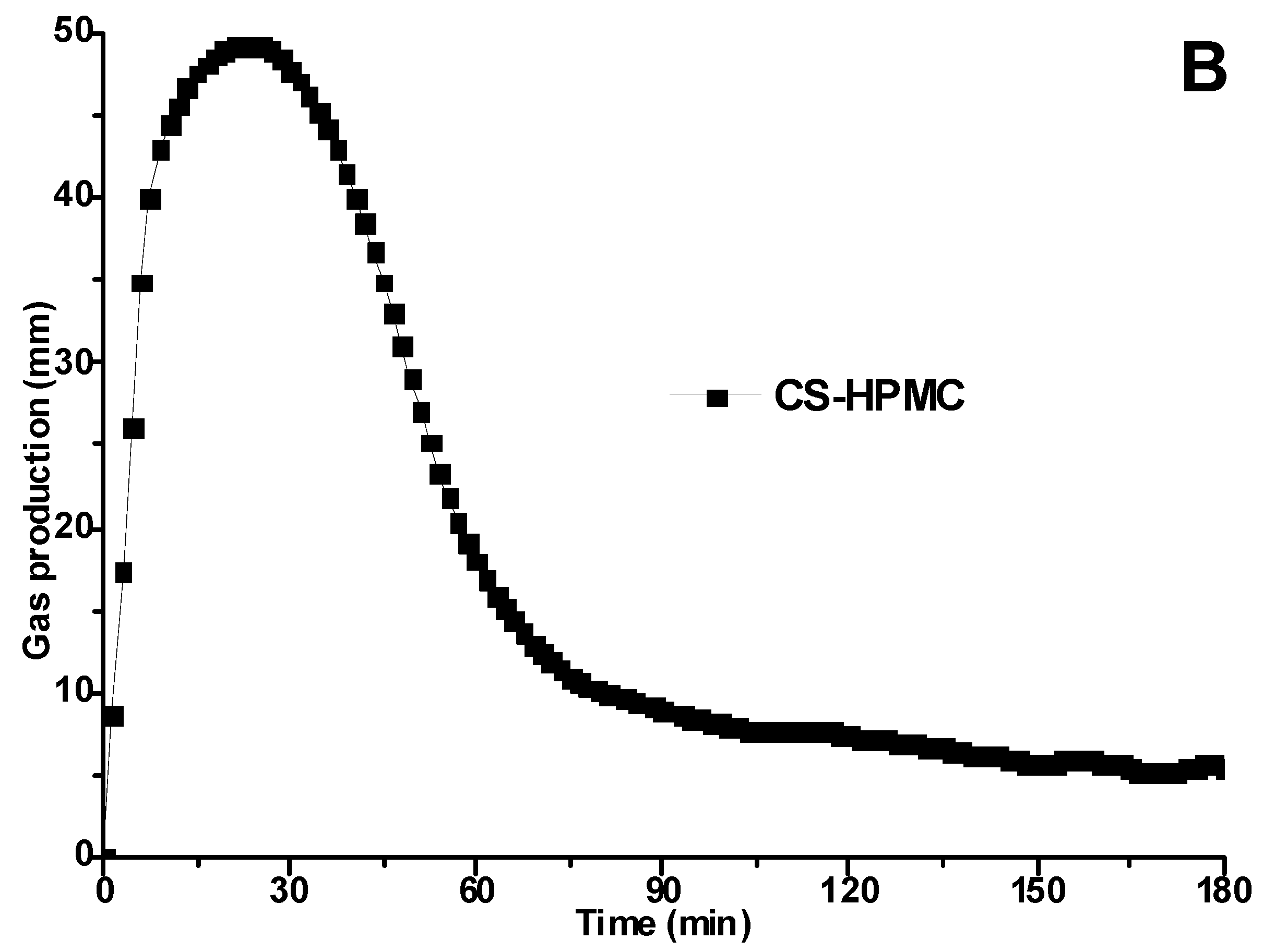
3.1. Proofing Curves Determined by Rheofermentometer
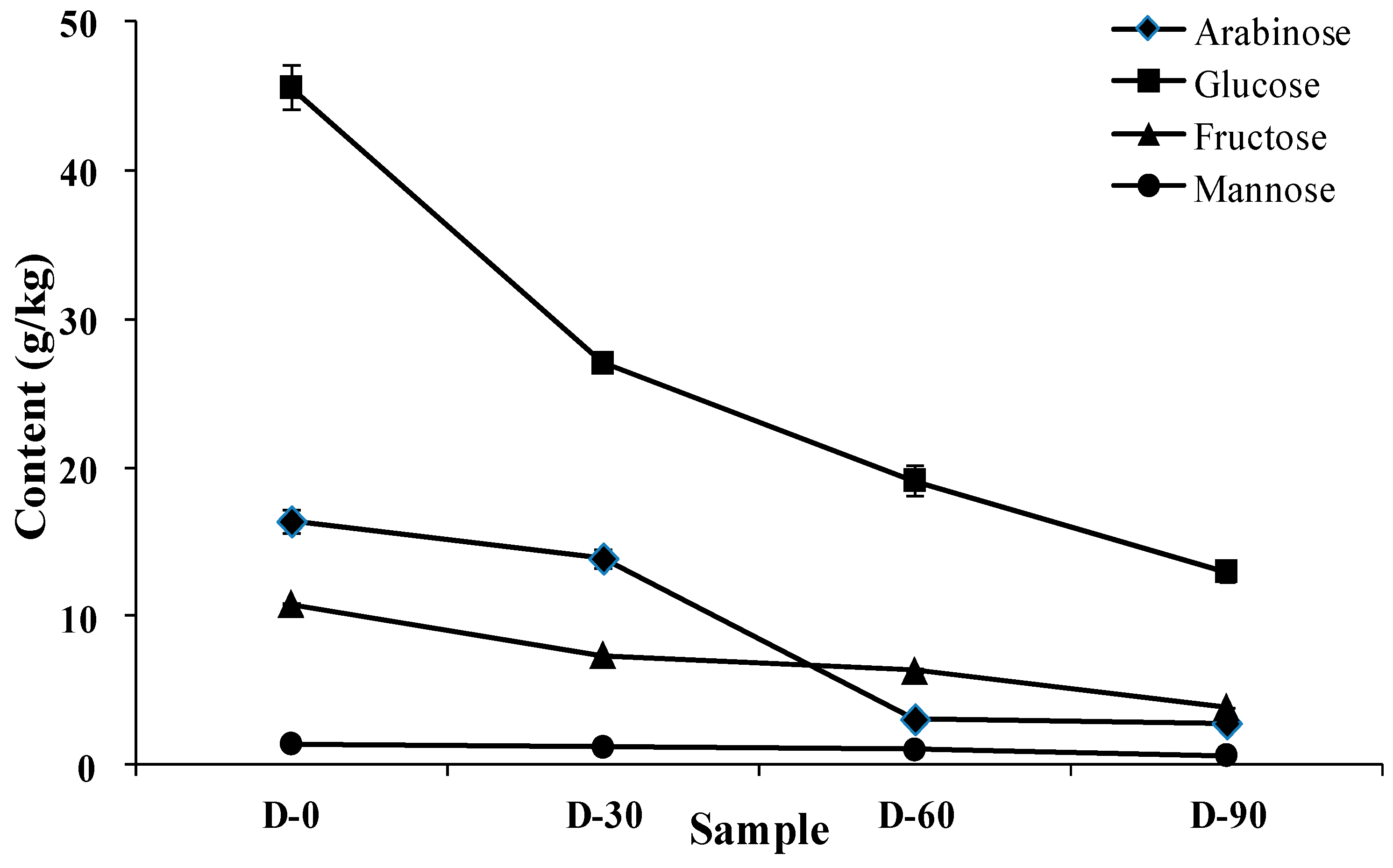
3.2. Changes of the Soluble Carbohydrate Composition
3.3. Water Morphology
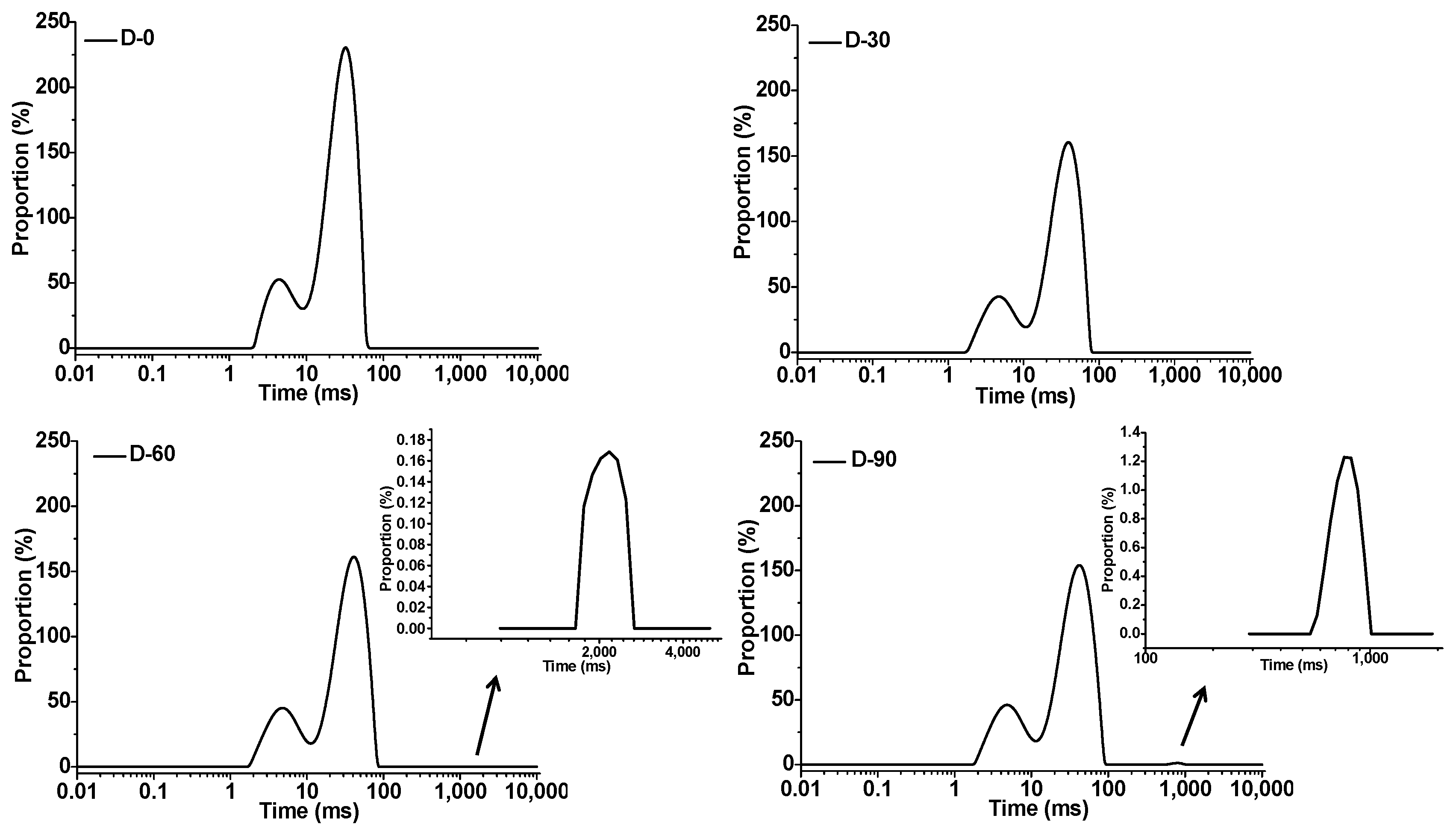
3.4. Water Mobility
3.5. Dynamic Rheological Characterization
3.5.1. Dynamic Strain Sweep
3.5.2. Dynamic Frequency Sweep
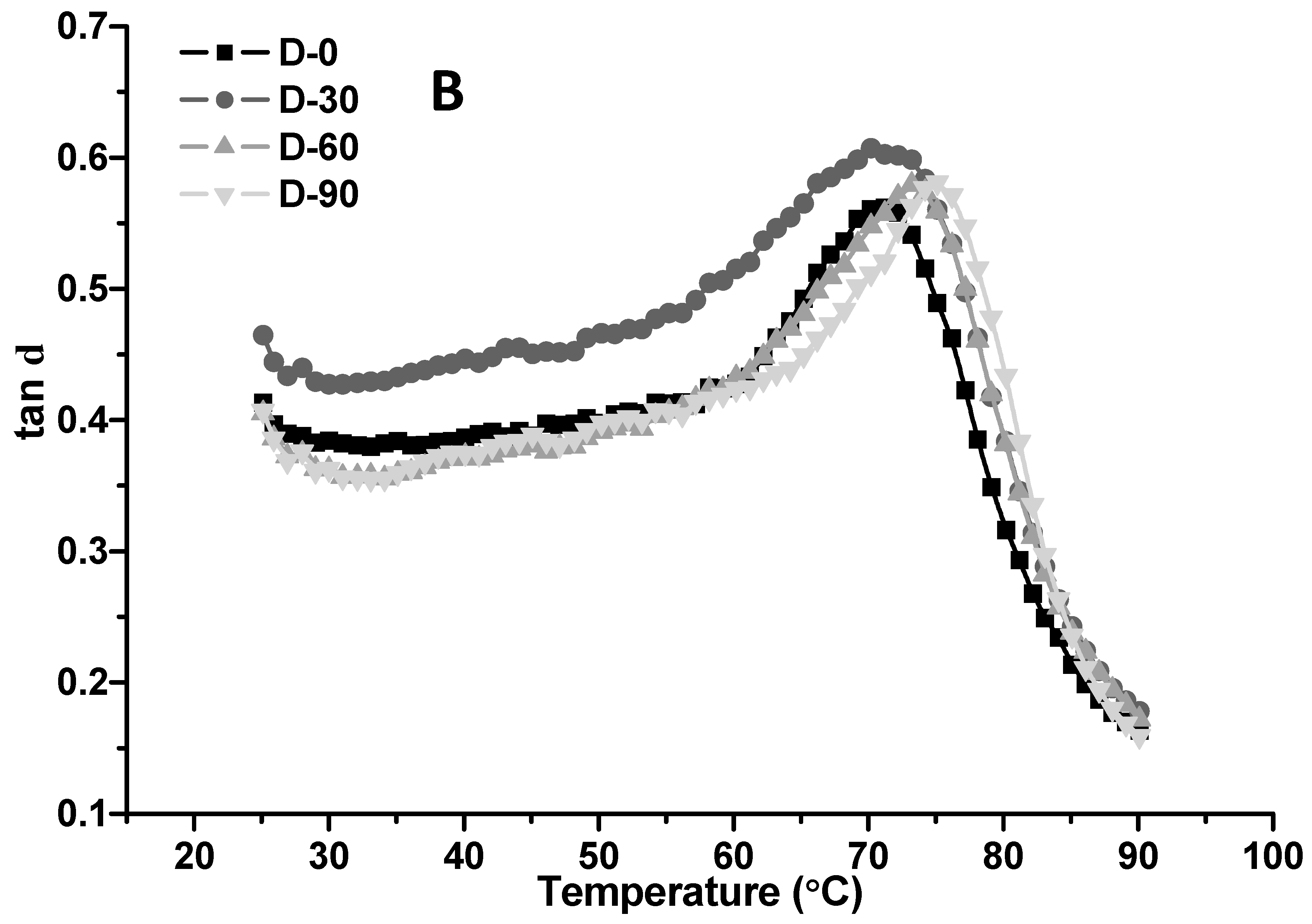
3.5.3. Temperature Sweep
3.6. Creep and Recovery Measurements
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Tay, S.L.; Koh, A.H.S.; Zhou, W. Dough and bread made from high-and low-protein flours by vacuum mixing: Part 2. Yeast activity, dough proofing and bread quality. J. Cereal Sci. 2017, 77, 275–283. [Google Scholar] [CrossRef]
- Stanke, M.; Zettel, V.; Schütze, S.; Hitzmann, B. Measurement and mathematical modeling of the relative volume of wheat dough during proofing. J. Food Eng. 2014, 131, 58–64. [Google Scholar] [CrossRef]
- Primo-Martín, C.; van Dalen, G.; Meinders, M.B.J.; Don, A.; Hamer, R.H.; van Vliet, T. Bread crispness and morphology can be controlled by proving conditions. Food Res. Int. 2010, 43, 207–217. [Google Scholar] [CrossRef]
- Doppler, F.; Jelonkiewicz, L.; Rezaei, M.N.; Lesens, C.; Toussaint, R.; Durand-Dubief, M. Viability of Saccharomyces Cerevisiae during baking of bread dough by flow cytometry. J. Microbiol. Methods 2022, 200, 106556. [Google Scholar] [CrossRef] [PubMed]
- Olojede, A.O.; Sanni, A.I.; Banwo, K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT-Food Sci. Technol. 2020, 120, 108875. [Google Scholar] [CrossRef]
- Montemurro, M.; Coda, R.; Rizzello, C.G. Recent advances in the use of sourdough biotechnology in pasta making. Foods 2019, 8, 129. [Google Scholar] [CrossRef]
- Jayaram, V.B.; Cuyvers, S.; Verstrepen, K.J.; Delcour, J.A.; Courtin, C.M. Succinic acid in levels produced by yeast (Saccharomyces cerevisiae) during fermentation strongly impacts wheat bread dough properties. Food Chem. 2014, 151, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Franco, W.; Pérez-Díaz, I.M.; Connelly, L.; Diaz, J.T. Isolation of exopolysaccharide-producing yeast and lactic acid bacteria from quinoa (Chenopodium quinoa) sourdough fermentation. Foods 2020, 9, 337. [Google Scholar] [CrossRef]
- Kelly, C.P.; Feighery, C.F.; Gallagher, R.B.; Weir, D.G. Diagnosis and treatment of gluten-sensitive enteropathy. Adv. Intern. Med. 1990, 35, 341–363. [Google Scholar]
- Li, J.; Zhu, Y.; Yadav, M.P.; Li, J. Effect of various hydrocolloids on the physical and fermentation properties of dough. Food Chem. 2019, 271, 165–173. [Google Scholar] [CrossRef]
- Witczak, T.; Juszczak, L.; Ziobro, R.; Korus, J. Rheology of gluten-free dough and physical characteristics of bread with potato protein. J. Food Process. Eng. 2016, 40, e12491. [Google Scholar] [CrossRef]
- Guerzoni, M.E.; Serrazanetti, D.I.; Vernocchi, P.; Gianotti, A. Physiology and biochemistry of sourdough yeasts. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer Science & Business Media: Boston, MA, USA, 2013; pp. 155–181. [Google Scholar]
- Shehzad, A.; Chiron, H.; Valle, G.D.; Kansou, K.; Ndiaye, A.; Reguerre, A.L. Porosity and stability of bread dough during proofing determined by video image analysis for different compositions and mixing conditions. Food Res. Int. 2010, 43, 1999–2005. [Google Scholar] [CrossRef]
- Smets, B.; Ghillebert, R.; De Snijder, P.; Binda, M.; Swinnen, E.; De Virgilio, C.; Winderickx, J. Life in the midst of scarcity: Adaptations to nutrient availability in Saccharomyces cerevisiae. Curr. Genet. 2010, 56, 1–32. [Google Scholar] [CrossRef]
- Gally, T.; Rouaud, O.; Jury, V.; Havet, M.; Ogé, A.; Le-Bail, A. Proofing of bread dough assisted by ohmic heating. Innov. Food Sci. Emerg. Technol. 2017, 39, 55–62. [Google Scholar] [CrossRef]
- Zhang, D.; Mu, T.; Sun, H. Comparative study of the effect of starches from five different sources on the rheological properties of gluten-free model doughs. Carbohydr. Polym. 2017, 176, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, C.; Albrecht, A.; Elgeti, D.; Jekle, M.; Becker, T. Impact of gas formation kinetics on dough development and bread quality. Food Res. Int. 2015, 76, 860–866. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, B.; Zhang, Y.; Ye, Y.; Gao, K. Improved nutritional and antioxidant properties of hulless barley following solid-state fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum. J. Food Process. Preserv. 2022, 46, e16245. [Google Scholar] [CrossRef]
- He, Y.; Guo, J.; Ren, G.; Cui, G.; Han, S.; Liu, J. Effects of konjac glucomannan on the water distribution of frozen dough and corresponding steamed bread quality. Food Chem. 2020, 330, 127243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mu, T.; Sun, H. Effects of starch from five different botanical sources on the rheological and structural properties of starch-gluten model doughs. Food Res. Int. 2018, 103, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, F.; de Leyn, I.; Eeckhout, M.; Dewettinck, K. Non-linear creep-recovery measurements as a tool for evaluating the viscoelastic properties of wheat flour dough. J. Food Eng. 2011, 107, 50–59. [Google Scholar] [CrossRef]
- Pasqualone, A.; Caponio, F.; Pagani, M.A.; Summo, C.; Paradiso, V.M. Effect of salt reduction on quality and acceptability of durum wheat bread. Food Chem. 2019, 289, 575–581. [Google Scholar] [CrossRef]
- Gobbetti, M.; Gänzle, M. Taxonomy and Biodiversity of Sourdough Yeasts and Lactic Acid Bacteria (Chapter 5). In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer Science & Business Media: Boston, MA, USA, 2013; pp. 105–154. [Google Scholar]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Gaenzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2013, 37, 2–10. [Google Scholar] [CrossRef]
- Bosmans, G.M.; Lagrain, B.; Deleu, L.; Fierens, E.; Hills, B.P.; Delcour, J.A. Assignments of proton populations in dough and bread using NMR relaxometry of starch, gluten, and flour model systems. J. Agric. Food Chem. 2012, 60, 5461–5470. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dickinson, L.C.; Chinachoti, P. Mobility of unfreezable and freezable water in waxy corn starch by 2H and 1H NMR. J. Agric. Food Chem. 1998, 46, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Thitipraphunkul, K.; Piyachomkwan, K.; Sriroth, K. Effect of starch modifications and hydrocolloids on freezable water in cassava starch systems. Starch-Stärke 2008, 60, 61–69. [Google Scholar] [CrossRef]
- Seetapan, N.; Limparyoon, N.; Fuongfuchat, A.; Gamonpilas, C.; Methacanon, P. Effect of freezing rate and starch granular morphology on ice formation and non-freezable water content of flour and starch gels. Int. J. Food Prop. 2016, 19, 1616–1630. [Google Scholar] [CrossRef]
- Wehrle, K.; Arendt, E.K. Rheological changes in wheat sourdough during controlled and spontaneous fermentation. Cereal Chem. 1998, 75, 882–886. [Google Scholar] [CrossRef]
- Abedfar, A.; Hosseininezhad, M.; Rafe, A. Effect of microbial exopolysaccharide on wheat bran sourdough: Rheological, thermal and microstructural characteristics. Int. J. Biol. Macromol. 2020, 154, 371–379. [Google Scholar] [CrossRef]
- Chen, P.; Long, Z.; Ruan, R.; Labuza, T. Nuclear magnetic resonance studies of water mobility in bread during storage. Lebensm. Wiss. Und Technol. 1997, 30, 178–183. [Google Scholar] [CrossRef]
- Esselink, E.; van Aalst, H.; Maliepaard, M.; Henderson, T.M.H.; Hoekstra, N.L.L.; Van Duynhoven, J. Impact of industrial dough processing on structure: A rheology, nuclear magnetic resonance, and electron microscopy study. Cereal Chem. 2003, 80, 419–423. [Google Scholar] [CrossRef]
- Lu, Z.; Seetharaman, K. 1H Nuclear magnetic resonance (NMR) and differential scanning calorimetry (DSC) studies of water mobility in dough systems containing barley flour. Cereal Chem. 2013, 90, 120–126. [Google Scholar] [CrossRef]
- Falade, A.T.; Emmambux, M.N.; Buys, E.M.; Taylor, J.R. Improvement of maize bread quality through modification of dough rheological properties by lactic acid bacteria fermentation. J. Cereal Sci. 2014, 60, 471–476. [Google Scholar] [CrossRef]
- Fu, Z.-Q.; Che, L.-M.; Li, D.; Wang, L.-J.; Adhikari, B. Effect of partially gelatinized corn starch on the rheological properties of wheat dough. LWT-Food Sci. Technol. 2016, 66, 324–331. [Google Scholar] [CrossRef]
- Tandazo, A.S.; Ozturk, O.K.; Hamaker, B.R.; Campanella, O.H. Rice starch and Co-proteins improve the rheological properties of zein dough. J. Cereal Sci. 2021, 102, 103334. [Google Scholar] [CrossRef]
- Huang, M.; Theng, A.H.P.; Yang, D.; Yang, H. Influence of κ-carrageenan on the rheological behaviour of a model cake flour system. LWT-Food Sci. Technol. 2021, 136, 110324. [Google Scholar] [CrossRef]
- Egelandsdal, B.; Fretheim, K.; Harbitz, O. Dynamic rheological measurements on heat-induced myosin gels: An evaluation of the method’s suitability for the filamentous gels. J. Sci. Food Agric. 1986, 37, 944–954. [Google Scholar] [CrossRef]
- Kim, W.W.; Yoo, B. Rheological behavior of acorn starch dispersions: Effects of concentration and temperature. Int. J. Food Sci. Technol. 2009, 44, 503–509. [Google Scholar] [CrossRef]
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J. Food Eng. 2007, 79, 1033–1047. [Google Scholar] [CrossRef]
- Mezaize, S.; Chevallier, S.; Le-Bail, A.; de Lamballerie, M. Gluten-free frozen dough: Influence of freezing on dough rheological properties and bread quality. Food Res. Int. 2010, 43, 2186–2192. [Google Scholar] [CrossRef]
- Renard, D.; van de Velde, F.; Visschers, R.W. The gap between food gel structure, texture and perception. Food Hydrocoll. 2006, 20, 423–431. [Google Scholar] [CrossRef]
- Rezaei, M.N.; Dornez, E.; Jacobs, P.; Parsi, A.; Verstrepen, K.J.; Courtin, C.M. Harvesting yeast (Saccharomyces cerevisiae) at different physiological phases significantly affects its functionality in bread dough fermentation. Food Microbiol. 2014, 39, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Jekle, M.; Mühlberger, K.; Becker, T. Starch–gluten interactions during gelatinization and its functionality in dough like model systems. Food Hydrocoll. 2016, 54, 196–201. [Google Scholar] [CrossRef]
- Sivaramakrishnan, H.P.; Senge, B.; Chattopadhyay, P. Rheological properties of rice dough for making rice bread. J. Food Eng. 2004, 62, 37–45. [Google Scholar] [CrossRef]
- Wang, F.C.; Sun, X.S. Creep-Recovery of wheat flour doughs and relationship to other physical dough tests and breadmaking performance. Cereal Chem. 2002, 79, 567–571. [Google Scholar] [CrossRef]
- Weipert, D. The benefits of basic rheometry in studying dough rheology. Cereal Chem. 1990, 67, 311–317. [Google Scholar]

| Sample | ΔH (J/g) | CF (%) | CNF (%) |
|---|---|---|---|
| D-0 | 91.72 ± 1.75 a | 27.46 ± 0.53 a | 44.24 ± 0.53 a |
| D-30 | 92.12 ± 2.01 a | 27.58 ± 0.71 a | 43.92 ± 0.71 a |
| D-60 | 92.80 ± 0.87 a | 27.78 ± 0.26 a | 42.72 ± 0.26 ab |
| D-90 | 93.96 ± 0.25 a | 28.13 ± 0.15 a | 41.39 ± 0.15 b |
| Sample | T21 | T22 | T23 | A21 | A22 | A23 |
|---|---|---|---|---|---|---|
| D-0 | 4.50 ± 0.00 b | 31.44 ± 0.00 c | - | 18.52 ± 0.06 d | 81.48 ± 0.14 a | - |
| D-30 | 4.82 ± 0.00 a | 38.72 ± 0.21 b | - | 21.86 ± 0.17 c | 78.14 ± 0.32 b | - |
| D-60 | 4.82 ± 0.00 a | 41.50 ± 0.00 a | 2171.12 ± 0.30 a | 23.16 ± 0.20 b | 76.82 ± 0.09 c | 0.03 ± 0.01 b |
| D-90 | 4.82 ± 0.00 a | 41.50 ± 0.00 a | 766.34 ± 0.00 b | 24.00 ± 0.32 a | 75.84 ± 0.27 d | 0.19 ± 0.01 a |
| Sample | LVR (%) | z′ | K | R2 |
|---|---|---|---|---|
| D-0 | 0.066 ± 0.003 c | 0.157 ± 0.008 b | 4.861 ± 0.039 a | 0.912 ± 0.004 b |
| D-30 | 0.075 ± 0.004 c | 0.166 ± 0.002 ab | 4.805 ± 0.026 ab | 0.999 ± 0.000 a |
| D-60 | 0.104 ± 0.003 b | 0.188 ± 0.012 a | 4.810 ± 0.018 ab | 0.992 ± 0.006 a |
| D-90 | 0.123 ± 0.005 a | 0.190 ± 0.004 a | 4.732 ± 0.032 b | 0.999 ± 0.000 a |
| Sample | Creep Phase | Steady State | ||
|---|---|---|---|---|
| Jmax × 105 (1/Pa) | η0 × 10−6 (Pa·s) | Je/Jmax (%) | Jv/Jmax (%) | |
| D-0 | 2.64 ± 2.10 d | 1.82 ± 0.52 a | 21.40 ± 1.37 a | 78.60 ± 2.34 c |
| D-30 | 3.20 ± 1.05 c | 1.69 ± 0.25 a | 19.44 ± 0.91 ab | 80.56 ± 1.95 ab |
| D-60 | 5.46 ± 1.28 b | 0.79 ± 0.04 b | 18.77 ± 1.22 b | 81.23 ± 2.07 ab |
| D-90 | 7.56 ± 1.21 a | 0.74 ± 0.11 b | 15.46 ± 1.79 c | 84.54 ± 1.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D. Effect of Proofing on the Rheology and Moisture Distribution of Corn Starch-Hydroxypropylmethylcellulose Gluten-Free Dough. Foods 2023, 12, 695. https://doi.org/10.3390/foods12040695
Zhang D. Effect of Proofing on the Rheology and Moisture Distribution of Corn Starch-Hydroxypropylmethylcellulose Gluten-Free Dough. Foods. 2023; 12(4):695. https://doi.org/10.3390/foods12040695
Chicago/Turabian StyleZhang, Duqin. 2023. "Effect of Proofing on the Rheology and Moisture Distribution of Corn Starch-Hydroxypropylmethylcellulose Gluten-Free Dough" Foods 12, no. 4: 695. https://doi.org/10.3390/foods12040695
APA StyleZhang, D. (2023). Effect of Proofing on the Rheology and Moisture Distribution of Corn Starch-Hydroxypropylmethylcellulose Gluten-Free Dough. Foods, 12(4), 695. https://doi.org/10.3390/foods12040695





