Bacteriobiota and Chemical Changes during the Ripening of Traditional Fermented “Pirot ‘Ironed’ Sausage”
Abstract
1. Introduction
2. Materials and Methods
2.1. “Pirot ‘Ironed’ Sausages”
2.2. Metabarcoding Analysis
2.2.1. DNA Extraction, Library Preparation, and NGS Sequencing
2.2.2. Raw Data Processing and Taxonomy Annotation
2.2.3. Statistical Analyses
2.3. Culturable Bacteriobiota
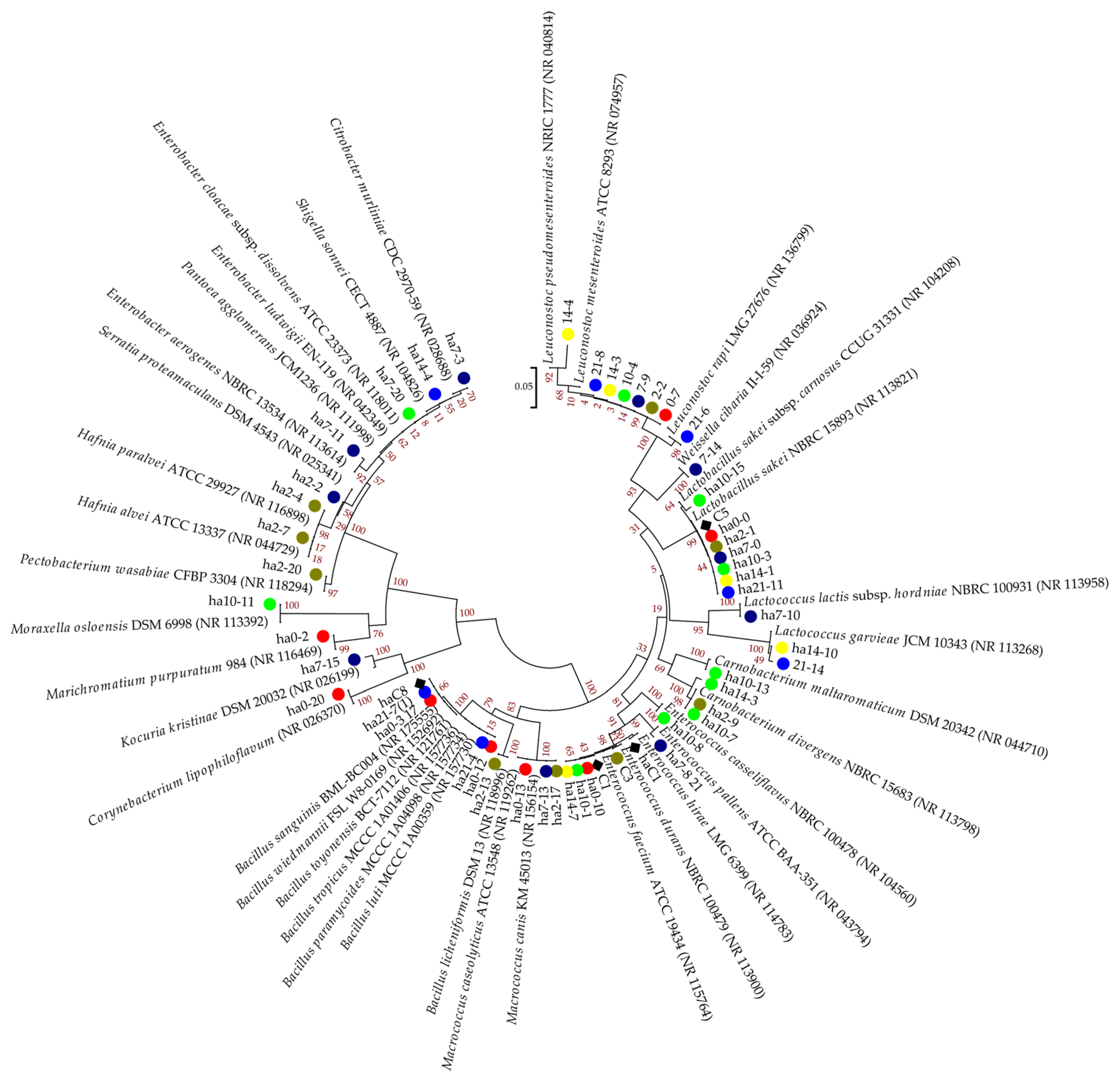
Phylogenetic Analysis
2.4. Physical-Chemical and Technological Parameters during the Ripening
Statistical Processing Analysis
3. Results
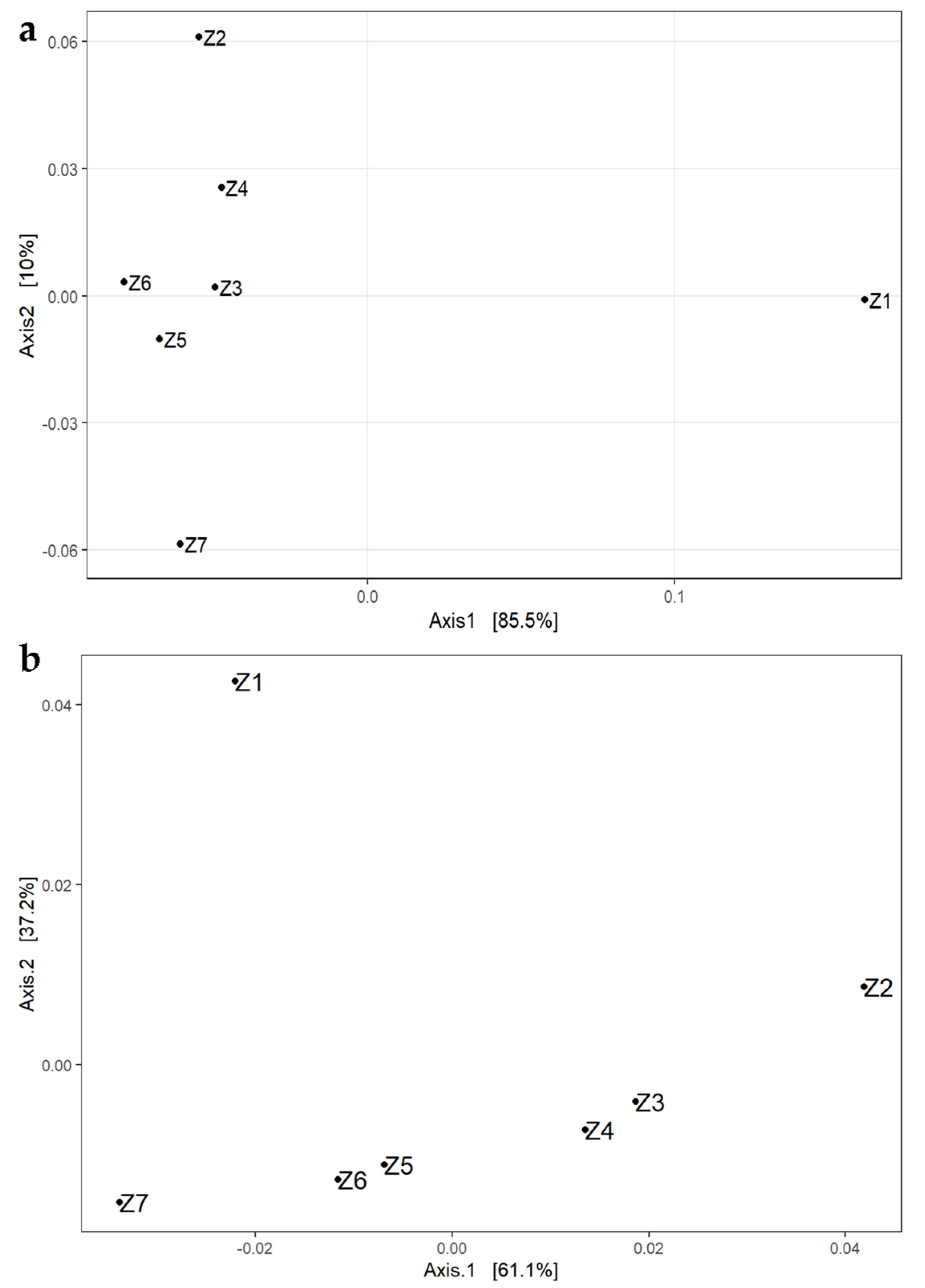
3.1. Total Bacteriobiota Diversity during the Ripening
3.1.1. The Analysis of Alpha and Beta Diversities
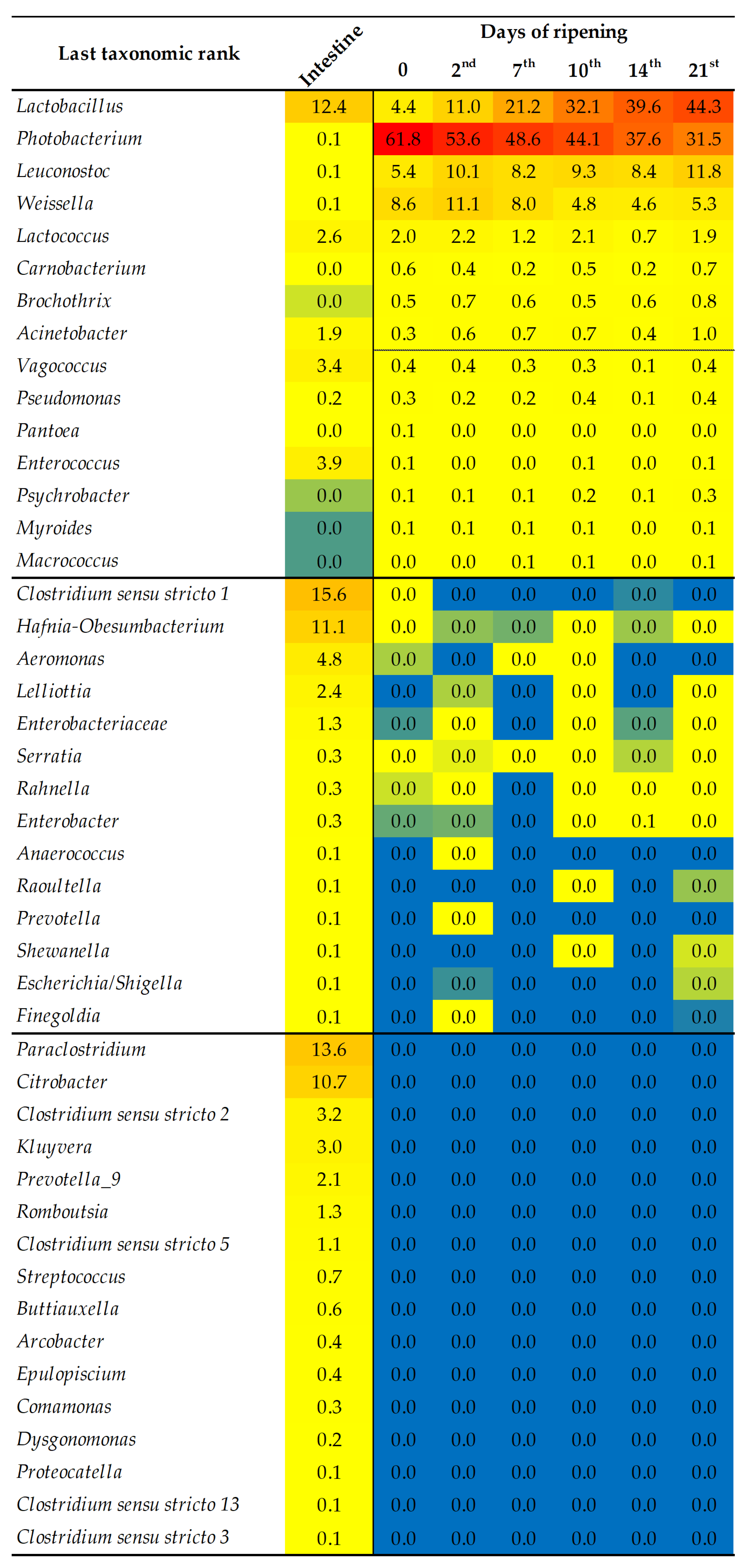
3.1.2. A Taxonomic Annotation of the Total Bacteriobiota
3.2. An Analysis of the Culturable Bacterial Communities
3.3. Physical-Chemical and Technological Parameters during the Ripening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutkinks, R. Meat Fermentation, 1st ed.; Blackwell Publishing Professional: Ames, IA, USA, 2006; pp. 207–303. [Google Scholar]
- Samelis, J.; Metaxopoulos, J.; Vlassi, M.; Pappa, A. Stability and safety of traditional Greek salami-a microbiological ecology study. Int. J. Food Microbiol. 1998, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Gomes, A.; Roseiro, L. Polycyclic aromatic hydrocarbons incidence in Portuguese traditional smoked meat products. Food Chem. Toxicol. 2011, 49, 2343–2347. [Google Scholar] [CrossRef]
- Lu, Y.; Young, O.A.; Brooks, J.D. Physicochemical and sensory characteristics of fermented sheepmeat sausage. Food Sci. Nutr. 2014, 2, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Lücke, F.K. Fermented sausages. In Microbiology of Fermented Foods; Blackie Academic and Professional: London, UK, 1998; Volume 2, pp. 441–483. [Google Scholar]
- Teixeira, A.; Silva, S.; Guedes, C.; Rodrigues, S. Sheep and Goat Meat Processed Products Quality: A Review. Foods 2020, 9, 960. [Google Scholar] [CrossRef]
- Laranjo, M.; Elias, M.; Fraqueza, M.J. The Use of Starter Cultures in Traditional Meat Products. J. Food Qual. 2017, 2017, 9546026. [Google Scholar] [CrossRef]
- Papamanoli, E.; Tzanetakis, N.; Litopoulou-Tzanetaki, E.; Kotzekidou, P. Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 2003, 65, 859–867. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef]
- Prica, N.; Živkov-Baloš, M.; Mihaljev, Ž.; Jakšić, C.; Kapetanov, M. Sadržaj nitrita i ukupnog fosfora u proizvodima od mesa na novosadskom tržištu. AVM 2012, 5, 69–75. [Google Scholar] [CrossRef]
- Cardinali, F.; Milanović, V.; Osimani, A.; Aquilanti, L.; Taccari, M.; Garofalo, C.; Polverigiani, S.; Clementi, F.; Franciosi, E.; Tuohy, K.; et al. Microbial dynamics of model Fabriano-like fermented sausages as affected by starter cultures, nitrates and nitrites. Int. J. Food Microbiol. 2018, 278, 61–72. [Google Scholar] [CrossRef]
- Bonomo, M.; Ricciardi, A.; Zotta, T.; Parente, E.; Salzano, G. Molecular and technological characterization of lactic acid bacteria from traditional fermented sausages of Basilicata region (Southern Italy). Meat Sci. 2008, 80, 1238–1248. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Nevado, F.P.; de Guía Córdoba, M. Screening of lactic acid bacteria and bifidobacteria for potential probiotic use in Iberian dry fermented sausages. Meat Sci. 2008, 80, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Monfort, J.M. Bacterial starter cultures for meat fermentation. Food Chem. 1997, 59, 547–554. [Google Scholar] [CrossRef]
- Riboulet-Bisson, E.; Sturme, M.H.J.; Jeffery, I.B.; O’Donnell, M.M.; Neville, B.A.; Forde, B.M.; Claesson, M.J.; Harris, H.; Gardiner, G.E.; Casey, P.G.; et al. Effect of Lactobacillus salivarius Bacteriocin Abp118 on the Mouse and Pig Intestinal Microbiota. PLoS ONE 2012, 7, e31113. [Google Scholar] [CrossRef]
- Adiguzel, C.G.; Atasever, M. Phenotypic and Genotypic Characterization of lactic acid bacteria isolated from Turkish dry fermented sausage. Rom. Biotechnol. Lett. 2009, 14, 4130–4138. [Google Scholar]
- Urso, R.; Comi, G.; Cocolin, L. Ecology of lactic acid bacteria in Italian fermented sausages: Isolation, identification and molecular characterization. Syst. Appl. Microbiol. 2006, 29, 671–680. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Bogdanovic, S.; Jelusic, A.; Beric, T.; Nikolic, I.; Danilovic, B.; Stankovic, S.; DimkiC, I. Genetic polymorphism of lactic acid bacteria isolated from “Pirot ‘ironed’ sausage” from Serbia. Arch. Biol. Sci. 2019, 71, 95–102. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bushnell, B.; Rood, J.; Singer, E. BBMerge—Accurate paired shotgun read merging via overlap. PLoS ONE 2017, 12, e0185056. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLOS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef] [PubMed]
- Pavoine, S.; Dufour, A.-B.; Chessel, D. From dissimilarities among species to dissimilarities among communities: A double principal coordinate analysis. J. Theor. Biol. 2004, 228, 523–537. [Google Scholar] [CrossRef]
- Wright, E.S. DECIPHER: Harnessing local sequence context to improve protein multiple sequence alignment. BMC Bioinform. 2015, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.P. Phangorn: Phylogenetic analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Živković, S.; Berić, T.; Ivanović, Z.; Gavrilović, V.; Stanković, S.; Fira, D. Characterization and evaluation of two Bacillus strains, SS-12.6 and SS-13.1, as potential agents for the control of phytopathogenic bacteria and fungi. Biol. Control. 2013, 65, 312–321. [Google Scholar] [CrossRef]
- Simunovic, S.; Djordjevic, V.; Bogdanovic, S.; Dimkic, I.; Stankovic, S.; Novakovic, S.; Tomasevic, I. Changes in chemical attributes during ripening of traditional fermented sausage, “Pirot ironed”. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kopaonik, Serbia, 22–25 September 2019. [Google Scholar]
- Tomasevic, I.; Tomovic, V.; Milovanovic, B.; Lorenzo, J.; Đorđević, V.; Karabasil, N.; Djekic, I. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 2019, 148, 5–12. [Google Scholar] [CrossRef]
- Tomasevic, I.; Tomovic, V.; Ikonic, P.; Lorenzo Rodriguez, J.M.; Barba, F.J.; Djekic, I.; Zivkovic, D. Evaluation of poultry meat colour using computer vision system and colourimeter: Is there a difference? Br. Food J. 2019, 121, 1078–1087. [Google Scholar] [CrossRef]
- De Huidobro, F.R.; Miguel, E.; Blázquez, B.; Onega, E. A comparison between two methods (Warner–Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef]
- Comi, G.; Urso, R.; Iacumin, L.; Rantsiou, K.; Cattaneo, P.; Cantoni, C.; Cocolin, L. Characterisation of naturally fermented sausages produced in the North East of Italy. Meat Sci. 2005, 69, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Mazzette, R.; De Santis, E.; Corona, A.; Cosseddu, A. Evolution and identification of lactic acid bacteria isolated during the ripening of Sardinian sausages. Meat Sci. 2005, 69, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Li, D.; Zhang, W.; Jiang, M.; Chen, X.H.; Dong, M.S. Comparative analysis of the bacterial diversity of Chinese fermented sausages using high-throughput sequencing. LWT 2021, 150, 111975. [Google Scholar] [CrossRef]
- Juárez-Castelán, C.; García-Cano, I.; Escobar-Zepeda, A.; Azaola-Espinosa, A.; Alvarez-Cisneros, Y.M.; Ponce-Alquicira, E. Evaluation of the bacterial diversity of Spanish-type chorizo during the ripening process using high-throughput sequencing and physicochemical characterization. Meat Sci. 2018, 150, 7–13. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Zhao, H. Unraveling microbial community diversity and succession of Chinese Sichuan sausages during spontaneous fermentation by high-throughput sequencing. J. Food Sci. Technol. 2019, 56, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Fuka, M.M.; Tanuwidjaja, I.; Maksimovic, A.Z.; Zunabovic-Pichler, M.; Kublik, S.; Hulak, N.; Domig, K.J.; Schloter, M. Bacterial diversity of naturally fermented game meat sausages: Sources of new starter cultures. LWT 2020, 118, 108782. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.-J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef]
- Ammor, M.S.; Mayo, B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: An update. Meat Sci. 2007, 76, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, C.-J.; Kunz, B. Identification of lactic acid bacteria isolated from kimchi and studies on their suitability for application as starter culture in the production of fermented sausages. Meat Sci. 2006, 72, 437–445. [Google Scholar] [CrossRef]
- Milicevic, B.; Danilovic, B.; Zdolec, N.; Kozachinski, L.; Dobranic, V.; Savic, D. Microbiota of the fermented sausages: Influence to product quality and safety. Bulg. J. Agric. Sci. 2014, 20, 1061–1078. [Google Scholar]
- Danilović, B.; Joković, N.; Petrović, L.; Veljović, K.; Tolinački, M.; Savić, D. The characterisation of lactic acid bacteria during the fermentation of an artisan Serbian sausage (Petrovská Klobása). Meat Sci. 2011, 88, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Tremonte, P.; Sorrentino, E.; Pannella, G.; Tipaldi, L.; Sturchio, M.; Masucci, A.; Maiuro, L.; Coppola, R.; Succi, M. Detection of different microenvironments and Lactobacillus sakei biotypes in Ventricina, a traditional fermented sausage from central Italy. Int. J. Food Microbiol. 2017, 242, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef] [PubMed]
- Mangia, N.; Murgia, M.A.; Garau, G.; Sanna, M.G.; Deiana, P. Influence of selected lab cultures on the evolution of free amino acids, free fatty acids and Fiore Sardo cheese microflora during the ripening. Food Microbiol. 2008, 25, 366–377. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef]
- Robinson, R.K. Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: London, UK; Corporate Drive: Burlington, MA, USA, 2014. [Google Scholar]
- Fuertes-Perez, S.; Hauschild, P.; Hilgarth, M.; Vogel, R.F. Biodiversity of Photobacterium spp. isolated from meats. Front. Microbiol. 2019, 10, 2399. [Google Scholar] [CrossRef]
- Hilgarth, M.; Fuertes, S.; Ehrmann, M.; Vogel, R.F. Photobacterium carnosum sp. nov., isolated from spoiled modified atmosphere packaged poultry meat. Syst. Appl. Microbiol. 2018, 41, 44–50. [Google Scholar] [CrossRef]
- Nieminen, T.T.; Dalgaard, P.; Björkroth, J. Volatile organic compounds and Photobacterium phosphoreum associated with spoilage of modified-atmosphere-packaged raw pork. Int. J. Food Microbiol. 2016, 218, 86–95. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Gałązka-Czarnecka, I.; Otlewska, A.; Czyżowska, A.; Nowak, A. Aronia melanocarpa (Michx.) Elliot, Chaenomeles superba Lindl. and Cornus mas L. Leaf Extracts as Natural Preservatives for Pork Meat Products. Molecules 2021, 26, 3009. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Ren, H.; Zhan, Y. Comparison of bacterial diversity profiles and microbial safety assessment of salami, Chinese dry-cured sausage and Chinese smoked-cured sausage by high-throughput sequencing. LWT 2018, 90, 108–115. [Google Scholar] [CrossRef]
- Hilgarth, M.; Fuertes-Pèrez, S.; Ehrmann, M.; Vogel, R. An adapted isolation procedure reveals Photobacterium spp. as common spoilers on modified atmosphere packaged meats. Lett. Appl. Microbiol. 2018, 66, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Wang, Y.; Li, L.; Huang, J.; Yang, X.; Chen, S.; Zhao, Y. Contribution of microbial community to flavor formation in tilapia sausage during fermentation with Pediococcus pentosaceus. LWT 2022, 154, 112628. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhou, X.; Wang, S.; Li, P. Salt Replacement Changed the Bacterial Community Composition and Physicochemical Characteristics of Sodium-Reduced Fermented Sausages during Fermentation and Ripening. Foods 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Fessard, A.; Remize, F. Why Are Weissella spp. Not Used as Commercial Starter Cultures for Food Fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef]
- Jeong, S.E.; Chun, B.H.; Kim, K.H.; Park, D.; Roh, S.W.; Lee, S.H.; Jeon, C.O. Genomic and metatranscriptomic analyses of Weissella koreensis reveal its metabolic and fermentative features during kimchi fermentation. Food Microbiol. 2018, 76, 1–10. [Google Scholar] [CrossRef]
- Čolo, J.; Mihajlović, S.; Tolinački, M.; Alkić, M.; Popović, D.; Kojić, M.; Terzić-Vidojević, A. Characterization of lactic acid bacteria isolated from bosnian artisanal dry fermented sausage (sudžuk) during fermentation. Genetika 2015, 47, 819–832. [Google Scholar] [CrossRef]
- Snauwaert, I.; Papalexandratou, Z.; De Vuyst, L.; Vandamme, P. Characterization of strains of Weissella fabalis sp. nov. and Fructobacillus tropaeoli from spontaneous cocoa bean fermentations. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 5, 1709–1716. [Google Scholar] [CrossRef]
- Maksimovic, A.Z.; Zunabovic-Pichler, M.; Kos, I.; Mayrhofer, S.; Hulak, N.; Domig, K.J.; Fuka-Mrkonjić, M. Microbiological hazards and potential of spontaneously fermented game meat sausages: A focus on lactic acid bacteria diversity. LWT 2018, 89, 418–426. [Google Scholar] [CrossRef]
- Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Mauriello, G.; Villani, F.; Ercolini, D. Spoilage-Related Activity of Carnobacterium maltaromaticum Strains in Air-Stored and Vacuum-Packed Meat. Appl. Environ. Microbiol. 2011, 77, 7382–7393. [Google Scholar] [CrossRef]
- Nieminen, T.T.; Vihavainen, E.; Paloranta, A.; Lehto, J.; Paulin, L.; Auvinen, P.; Solismaa, M.; Bjorkroth, K.J. Characterization of psychrotrophic bacterial communities in modified atmosphere-packed meat with terminal restriction length polymorphism. Int. J. Food Microbiol. 2011, 144, 360–366. [Google Scholar] [CrossRef]
- Sabia, C.; Manicardi, G.; Messi, P.; de Niederhäusern, S.; Bondi, M. Enterocin 416K1, an antilisterial bacteriocin produced by Enterococcus casseliflavus IM 416K1 isolated from Italian sausages. Int. J. Food Microbiol. 2002, 75, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Legrand, R.; Lucas, N.; Dominique, M.; Azhar, S.; Deroissart, C.; Le Solliec, M.-A.; Rondeaux, J.; Nobis, S.; Guérin, C.; Léon, F.; et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice—A new potential probiotic for appetite and body weight management. Int. J. Obes. 2020, 44, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Charmpi, C.; Van Reckem, E.; Sameli, N.; Van Der Veken, D.; De Vuyst, L.; Leroy, F. The Use of Less Conventional Meats or Meat with High pH Can Lead to the Growth of Undesirable Microorganisms during Natural Meat Fermentation. Foods 2020, 9, 1386. [Google Scholar] [CrossRef]
- Rebecchi, A.; Pisacane, V.; Miragoli, F.; Polka, J.; Falasconi, I.; Morelli, L.; Puglisi, E. High-throughput assessment of bacterial ecology in hog, cow and ovine casings used in sausages production. Int. J. Food Microbiol. 2015, 212, 49–59. [Google Scholar] [CrossRef]
- Kim, H.-S.; Ma, B.; Perna, N.T.; Charkowski, A.O. Phylogeny and Virulence of Naturally Occurring Type III Secretion System-Deficient Pectobacterium Strains. Appl. Environ. Microbiol. 2009, 75, 4539–4549. [Google Scholar] [CrossRef]
- Kovacevic, D.; Mastanjevic, K.; Pleadin, J.; Frece, J. Physicochemical, microbiological, and colour attributes of horse salami established during the ripening period. Ital. J. Food Sci. 2016, 28, 96–106. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Michinel, M.; López, M.; Carballo, J. Biochemical Characteristics of Two Spanish Traditional Dry-cured Sausage Varieties: Androlla and Botillo. J. Food Compos. Anal. 2000, 13, 809–817. [Google Scholar] [CrossRef]
- Ikonić, P.; Petrović, L.; Tasić, T.; Džinić, N.; Jokanović, M.; Tomović, V. Physicochemical, biochemical and sensory properties for the characterization of Petrovská klobása (traditional fermented sausage). Acta Period. Technol. 2010, 41, 19–31. [Google Scholar] [CrossRef]
- Vuković, I.; Vasilev, D.; Saičić, S.; Ivanković, S. Ispitivanje važnijih promena u toku zrenja tradicionalne fermentisane kobasice lemeški kulen. Tehnol. Mesa. 2012, 53, 140–147. [Google Scholar]
- Salgado, A.; Fontán, M.C.G.; Franco, I.; López, M.; Carballo, J. Biochemical changes during the ripening of Chorizo de cebolla, a Spanish traditional sausage. Effect of the system of manufacture (homemade or industrial). Food Chem. 2005, 92, 413–424. [Google Scholar] [CrossRef]
- Vesković-Moračanin, S.; Karan, D.; Okanović, Đ.; Jokanović, M.; Džinić, N.; Parunović, N.; Trbović, D. Parametri kvaliteta i karakteristike boje i teksture sremske kobasice fermentisane na tradicionalan način. Tehnol. Mesa. 2011, 52, 245–251. [Google Scholar]
- Herrero, A.M.; Ordóñez, J.A.; de Avila, R.; Herranz, B.; de la Hoz, L.; Cambero, M.I. Breaking strength of dry fermented sausages and their correlation with texture profile analysis (TPA) and physico-chemical characteristics. Meat Sci. 2007, 77, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Fanco, I.; Prieto, B.; Cruz, J.M.; Lopez, M.; Carballo, J. Study of the biochemical changes during processing of Androlla, a Spanish drycured pork sausage. Food Chem. 2002, 78, 339–345. [Google Scholar] [CrossRef]
- Markov, K.; Frece, J.; Čvek, D.; Trontel, A.; Slavica, A.; Kovačević, D. Dominantna mikroflora fermentiranih kobasica od konjskog mesa. Meso 2010, 7, 217–221. [Google Scholar]
- Stajić, S.; Stanišić, N.; Perunović, M.; Živković, D.; Žujović, M. Possibilities for the use of goat meat in the production of tradicional sucuk. Biotechnol. Anim. Husb. 2011, 27, 1489–1497. [Google Scholar] [CrossRef]
- Stajić, S.; Pisinov, B.; Tomasevic, I.; Djekic, I.; Čolović, D.; Ivanović, S.; Živković, D. Use of culled goat meat in frankfurter production—Effect on sensory quality and technological properties. Int. J. Food Sci. Technol. 2019, 55, 14346. [Google Scholar]
- Savić, S.; Bunčić, O.; Uzelac, B.; Tripković, J. Mikroflora “Sremske salame” proizvedene sa i bez starter-kulture. Tehnol. Mesa. 2001, 42, 71–74. [Google Scholar]
- Petrović, L.; Džinić, N.; Ikonić, P.; Tasić, T.; Tomović, V. Quality and Safety Standardization of Traditional Fermented Sausages. Meat Technol. 2021, 52, 34–44. [Google Scholar]
- Salgado, A.; García Fontán, M.C.; Franco, I.; López, M.; Carballo, J. Effect of the type of manufacture (homemade or industrial) on the biochemical characteristics of Chorizo de cebolla (a Spanish traditional sausage). Food Control. 2006, 17, 213–221. [Google Scholar] [CrossRef]
- Soyer, A.; Ertas, A.; Üzümcüoglu, U. Effect of processing conditions on the quality of naturally fermented Turkish sausages (sucuks). Meat Sci. 2005, 69, 135–141. [Google Scholar] [CrossRef]
- Fernández-Fernández, E.; Vázquez-Odériz, M.L.; Romero-Rodríguez, M.A. Colour changes during manufacture of Galician chorizo sausage. Z Leb. Unters 1998, 207, 18–21. [Google Scholar] [CrossRef]
- Škaljac, S.; Jokanović, M.; Tomović, V.; Peulić, T.; Ikonić, P.; Šojić, B.; Ivić, M.; Petrović, L.; Babić, J.; Hromiš, N. Uticaj dimljenja na formiranje boje i sadržaj policikličnih aromatičnih jedinjenja u tradicionalnoj fermentisanoj kobasici. Glas. Hemičara Tehnol. I Ekol. Repub. Srp. 2019, 15, 25–32. [Google Scholar] [CrossRef]
- Bozkurt, H.; Bayram, M. Colour and textural attributes of sucuk during ripening. Meat Sci. 2006, 73, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Pẻrez-Alvarez, J.A.; Sayas-Barberá, M.E.; Fernández-López, J.; Aranda-Catalá, V. Physicochemical characteristics of Spanish-type dry-cured sausage. Food Res. Int. 1999, 32, 599–607. [Google Scholar] [CrossRef]
- Kayaardı, S.; Gök, V. Effect of replacing beef fat with olive oil on quality characteristics of Turkish soudjouk (sucuk). Meat Sci. 2003, 66, 249–257. [Google Scholar] [CrossRef]
- Rosmini, M.R.; Zogbi, A.P. Effect of water, sodium chloride, lactic acid, sodium nitrite, sodium ascorbate and paprika upon lightness (L*) in a dry-cured sausages model system. J. Food Technol. 2005, 3, 555–562. [Google Scholar]
- Gimeno, O.; Astiasaran, I.; Bello, J. Calcium ascorbate as a potential partial substitute NaCl in dry fermented sausages: Effect on colour, texture and hygiene quality at different concentrations. Meat Sci. 2001, 57, 23–29. [Google Scholar] [CrossRef]
- Papadima, S.N.; Bloukas, J.G. Effect of fat level and storage conditions on quality characteristics of traditional Greek sausages. Meat Sci. 1999, 51, 103–113. [Google Scholar] [CrossRef]
- García, M.L.; Dominguez, R.; Galvez, M.D.; Casas, C.; Selgas, M.D. Utilization of cereal and fruit fibres in low fat dry fermented sausages. Meat Sci. 2002, 60, 227–236. [Google Scholar] [CrossRef]
- Saccani, G.; Fornelli, G.; Zanardi, E. characterization of textural properties and changes of myofibrillar and sarcoplasmic proteins in salame Felino during ripening. Int. J. Food Prop. 2013, 16, 1460–1471. [Google Scholar] [CrossRef]
- Severini, C.; De Pilli, T.; Baiano, A. Partial substitution of pork backfat with extra-virgin olive oil in “salami” products: Effects on chemical, physical and sensorial quality. Meat Sci. 2003, 64, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Spaziani, M.; Del Torre, M.; Stecchini, M.L. Changes of physicochemical, microbiological, and textural properties during ripening of Italian low-acid sausages. Proteolysis, sensory and volatile profiles. Meat Sci. 2009, 81, 77–85. [Google Scholar] [CrossRef]
- González-Fernández, C.; Santos, E.M.; Rovira, J.; Jaime, I. The effect of sugar concentration and starter culture on instrumental and sensory textural properties of chorizo-Spanish dry-cured sausage. Meat Sci. 2006, 74, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Madruga, M.S.; Bressan, M.C. Goat meats: Description, rational use, certification, processing and technical developments. Small Rumin. Res. 2011, 98, 39–45. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Salvador, A.; Flores, M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010, 86, 251–257. [Google Scholar] [CrossRef] [PubMed]

| Sample | Intestine/Day of Ripening | OBS | Chao1 | SE Chao1 | ACE | SE ACE | Shannon | Simpson | Inv Simpson | Fisher | Taxa Hierarchy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z1 | Small bovine intestine | 279 | 279.5 | 0.99 | 280.0 | 7.75 | 3.8 | 1.0 | 24.0 | 33.9 | OTU |
| 95 | 95.0 | 0.00 | 95.0 | 4.64 | 2.8 | 0.9 | 10.9 | 10.1 | Genus | ||
| 42 | 42.0 | 0.00 | 42.0 | 2.76 | 2.0 | 0.8 | 5.6 | 4.1 | Family | ||
| 8 | 8.0 | 0.00 | 8.0 | 1.22 | 0.8 | 0.5 | 2.0 | 0.7 | Phylum | ||
| Z2 | 0 | 126 | 126.3 | 0.66 | 127.1 | 5.28 | 2.7 | 0.9 | 6.9 | 14.3 | OTU |
| Z3 | 2nd | 132 | 133.7 | 2.20 | 133.5 | 5.52 | 2.8 | 0.9 | 8.5 | 14.7 | |
| Z4 | 7th | 118 | 121.0 | 3.18 | 120.8 | 5.37 | 2.7 | 0.9 | 8.4 | 13.5 | |
| Z5 | 10th | 132 | 139.0 | 6.65 | 136.6 | 5.06 | 2.8 | 0.9 | 8.5 | 14.5 | |
| Z6 | 14th | 121 | 121.8 | 1.26 | 122.7 | 5.36 | 2.5 | 0.9 | 6.9 | 13.0 | |
| Z7 | 21st | 127 | 128.9 | 2.26 | 130.0 | 5.07 | 2.6 | 0.9 | 7.6 | 14.2 | |
| Z2 | 0 | 38 | 38.0 | 0.00 | 38.0 | 3.01 | 1.4 | 0.6 | 2.4 | 3.7 | Genus |
| Z3 | 2nd | 47 | 47.3 | 0.74 | 47.6 | 3.38 | 1.5 | 0.7 | 3.0 | 4.6 | |
| Z4 | 7th | 40 | 40.0 | 0.16 | 40.3 | 3.15 | 1.5 | 0.7 | 3.3 | 4.0 | |
| Z5 | 10th | 38 | 38.0 | 0.16 | 38.5 | 2.50 | 1.5 | 0.7 | 3.2 | 3.6 | |
| Z6 | 14th | 40 | 40.1 | 0.44 | 41.1 | 3.15 | 1.4 | 0.7 | 3.2 | 3.8 | |
| Z7 | 21st | 40 | 40.2 | 0.62 | 41.1 | 3.00 | 1.5 | 0.7 | 3.2 | 3.9 | |
| Z2 | 0 | 26 | 26.0 | 0.00 | 26.0 | 2.43 | 1.3 | 0.6 | 2.4 | 2.5 | Family |
| Z3 | 2nd | 31 | 31.0 | 0.16 | 31.3 | 2.62 | 1.4 | 0.6 | 2.8 | 2.9 | |
| Z4 | 7th | 28 | 28.0 | 0.16 | 28.3 | 2.50 | 1.4 | 0.7 | 3.1 | 2.7 | |
| Z5 | 10th | 23 | 23.0 | 0.00 | 23.0 | 1.98 | 1.4 | 0.7 | 3.1 | 2.1 | |
| Z6 | 14th | 27 | 27.1 | 0.49 | 28.6 | 2.61 | 1.3 | 0.7 | 3.1 | 2.5 | |
| Z7 | 21st | 25 | 25.3 | 0.73 | 26.5 | 2.51 | 1.4 | 0.7 | 3.1 | 2.3 | |
| Z2 | 0 | 7 | 7.0 | 0.00 | 7.0 | 0.93 | 0.9 | 0.5 | 2.1 | 0.6 | Phylum |
| Z3 | 2nd | 7 | 7.0 | 0.00 | 7.0 | 1.20 | 0.9 | 0.6 | 2.3 | 0.6 | |
| Z4 | 7th | 7 | 7.0 | 0.00 | 7.0 | 1.31 | 1.0 | 0.6 | 2.4 | 0.6 | |
| Z5 | 10th | 7 | 7.0 | 0.00 | 7.0 | 0.93 | 0.8 | 0.5 | 2.2 | 0.6 | |
| Z6 | 14th | 8 | 8.0 | 0.00 | 8.0 | 1.22 | 0.9 | 0.6 | 2.2 | 0.6 | |
| Z7 | 21st | 6 | 6.0 | 0.00 | 6.0 | 0.91 | 0.7 | 0.5 | 1.9 | 0.5 |
| Isolates Origin | No. of Total Isolates/Unique Species | Total Number of Unique Species for Sample(s) |
|---|---|---|
| Z1 (intestine) | 8 | 5 |
| Z2 (0 day) | 28 | 8 |
| Z3 (2nd day) | 26 | 9 |
| Z4 (7th day) | 28 | 10 |
| Z5 (10th day) | 24 | 8 |
| Z6 (14th day) | 20 | 7 |
| Z7 (21st day) | 23 | 6 |
| ∑ | 157 | 32 |
| Z1 Z2 Z3 Z4 Z5 Z6 Z7 | 1 | Latilactobacillus sakei (basonym Lactobacillus sakei) |
| Z2 Z3 Z4 Z5 Z6 Z7 | 1 | Leuconostoc mesenteroides |
| Z1 Z2 Z5 Z6 | 1 | Enterococcus faecium |
| Z1 Z2 Z7 | 1 | Bacillus toyonensis/wiedmannii/sanguinis |
| Z3 Z5 Z6 | 1 | Carnobacterium divergens |
| Z2 Z7 | 1 | Bacillus tropicus/paramycoides/luti |
| Z3 Z4 | 1 | Macrococcus canis |
| Z6 Z7 | 1 | Lactococcus garvieae |
| Z1 | 2 | Enterococcus hirae; Enterococcus durans |
| Z2 | 3 | Corynebacterium lipophiloflavum; Marichromatium purpuratum; Macrococcus caseolyticus |
| Z3 | 5 | Pectobacterium wasabiae; Hafnia alvei; Serratia proteamaculans; Bacillus licheniformis; Hafnia paralvei |
| Z4 | 7 | Kocuria kristinae; Pantoea agglomerans/Enterobacter ludwigii/Enterobacter cloacae subsp. dissolvens; Enterococcus pallens; Weissella cibaria; Citrobacter murliniae; Lactococcus lactis subsp. hordniae; Enterobacter aerogenes |
| Z5 | 4 | Carnobacterium maltaromaticum; Moraxella osloensis; Latilactobacillus sakei subsp. carnosus (basonym Lactobacillus sakei subsp. carnosus); Enterococcus casseliflavus |
| Z6 | 2 | Shigella sonnei; Leuconostoc pseudomesenteroides |
| Z7 | 1 | Leuconostoc rapi |
| Ripening | The Color Parameters | ||
|---|---|---|---|
| L* | a* | b* | |
| The Surface Color of the Sausage with Casing | |||
| 0 day | 33.3 ± 1.71 a | 14.9 ± 0.32 a | 4.4 ± 0.20 a |
| 7th day | 29.6 ± 1.57 ab | 12.2 ± 0.50 b | 2.8 ± 0.41 ab |
| 14th day | 21.4 ± 0.94 c | 9.8 ± 0.82 c | 1.8 ± 0.26 bc |
| 21st day | 22.8 ± 1.81 bc | 8.4 ± 0.50 cd | −0.7 ± 1.02 cd |
| 28th day | 25.0 ± 4.95 bc | 7.0 ± 0.80 d | −0.04 ± 1.55 d |
| The surface color of the sausage without casing | |||
| 0 day | - | - | - |
| 7th day | - | - | - |
| 14th day | 24.3 ± 0.83 a | 10.2 ± 0.50 a | 1.7 ± 0.97 a |
| 21st day | 23.2 ± 1.04 ab | 8.8 ± 0.57 ab | −0.4 ± 0.07 b |
| 28th day | 21.4 ± 0.94 b | 7.7 ± 1.46 b | −0.71 ± 0.36 b |
| The cross-sectional surface color of the sausage | |||
| L* | a* | b* | |
| 0 day | 25.1 ± 1.8 ab | 25.4 ± 0.47 a | 15.9 ± 0.44 a |
| 7th day | 25.3 ± 0.60 a | 25.7 ± 0.92 a | 15.5 ± 1.29 a |
| 14th day | 24.2 ± 0.46 ab | 19.3 ± 0.53 b | 6.0 ± 0.98 b |
| 21st day | 23.9 ± 1.34 ab | 16.3 ± 0.67 c | 1.8 ± 0.37 c |
| 28th day | 22.1 ± 1.15 b | 12.9 ± 1.77 d | 0.5 ± 1.13 c |
| Textural parameters | |||
| Firmness (N) | Toughness (N s) | ||
| 0 day | - | - | |
| 7th day | - | - | |
| 14th day | 20.3 ± 4.12 c | 86.4 ± 14.27 c | |
| 21st day | 31.8 ± 1.98 b | 165.7 ± 5.31 b | |
| 28th day | 42.9 ± 4.98 a | 231.0 ± 20.17 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanović, S.; Stanković, S.; Berić, T.; Tomasevic, I.; Heinz, V.; Terjung, N.; Dimkić, I. Bacteriobiota and Chemical Changes during the Ripening of Traditional Fermented “Pirot ‘Ironed’ Sausage”. Foods 2023, 12, 664. https://doi.org/10.3390/foods12030664
Bogdanović S, Stanković S, Berić T, Tomasevic I, Heinz V, Terjung N, Dimkić I. Bacteriobiota and Chemical Changes during the Ripening of Traditional Fermented “Pirot ‘Ironed’ Sausage”. Foods. 2023; 12(3):664. https://doi.org/10.3390/foods12030664
Chicago/Turabian StyleBogdanović, Svetlana, Slaviša Stanković, Tanja Berić, Igor Tomasevic, Volker Heinz, Nino Terjung, and Ivica Dimkić. 2023. "Bacteriobiota and Chemical Changes during the Ripening of Traditional Fermented “Pirot ‘Ironed’ Sausage”" Foods 12, no. 3: 664. https://doi.org/10.3390/foods12030664
APA StyleBogdanović, S., Stanković, S., Berić, T., Tomasevic, I., Heinz, V., Terjung, N., & Dimkić, I. (2023). Bacteriobiota and Chemical Changes during the Ripening of Traditional Fermented “Pirot ‘Ironed’ Sausage”. Foods, 12(3), 664. https://doi.org/10.3390/foods12030664









