Rheo-Fermentation Dough Properties, Bread-Making Quality and Aroma Characteristics of Red Bean (Vigna angularis) Sourdough Induced by LAB Weissella confusa QS813 Strain Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Red Bean Sourdough Preparation
2.2.1. LAB Strain, Growth Media and Inoculum Preparation
2.2.2. Red Bean Sourdough Fermentation
2.2.3. Physicochemical Changes in Red Bean Sourdough
2.3. Dough and Bread Preparation
2.4. Bread Dough and Bread Analyses
2.4.1. Rheo-Fermentation Properties of Bread Doughs
2.4.2. Dynamic Rheology Properties of Bread Doughs
2.4.3. Molecular Weight Distribution of Gluten in Bread Dough by Size-Exclusion High Performance Liquid Chromatography (SE-HPLC)
2.4.4. Sulfhydryl (SH) Content in Bread Doughs
2.4.5. Baking Loss, Specific Volume and Moisture Content of Bread
2.4.6. Texture Profile Analysis of Bread
2.4.7. Gas Chromatography–Mass Spectrometry Analysis of Bread
2.4.8. Sensory Evaluation of Bread
2.5. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Properties of Red Bean Sourdough
3.2. The Effect of In Situ-Formed EPS in Sourdough on Rheo-Fermentation Properties of Bread Dough
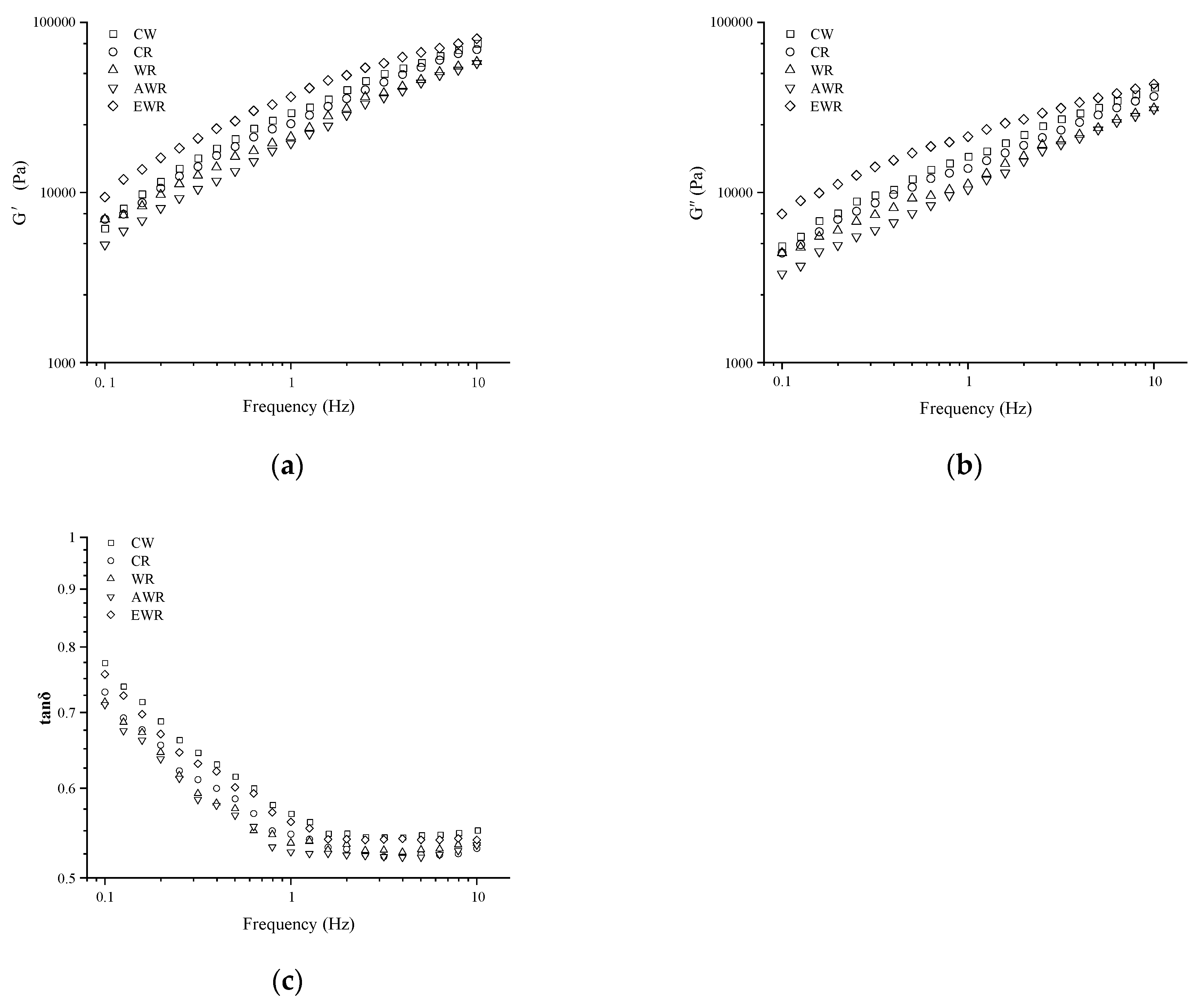
3.3. The Effect of In Situ-Formed EPS in Sourdough on Dynamic Rheology Properties of Bread Dough
3.4. Size Distribution of Gluten Proteins in Bread Doughs
3.5. Free SH Content in Bread Dough
3.6. Quality Characteristics of Red Bean Sourdough Bread
3.7. The Aroma Compounds in Red Bean Sourdough Bread Crumb
3.8. Principal Component Analysis (PCA) of Aroma Compounds of Red Bean Sourdough Bread Crumb
3.9. Sensory Evaluation of Bread
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Yang, T.; Liu, R.; Redden, B.; Maalouf, F.; Zong, X. Food legume production in China. Crop J. 2017, 5, 115–126. [Google Scholar] [CrossRef]
- Honda, Y.; Saito, Y.; Mishima, T.; Katsumi, N.; Matsumoto, K.; Enomoto, T.; Miwa, S. Characterization of physicochemical and digestive properties of starches from various ‘dainagon’ adzuki beans (Vigna angularis) cultivated in Japan. Int. J. Biol. Macromol. 2020, 148, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Monnet, A.K.; Laleg, K.; Michon, C.; Micard, V. Legume enriched cereal products: A generic approach derived from material science to predict their structuring by the process and their final properties. Trends Food Sci. Technol. 2019, 86, 131–143. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2020, 108, 71–83. [Google Scholar] [CrossRef]
- Alfonzo, A.; Miceli, C.; Nasca, A.; Franciosi, E.; Ventimiglia, G.; Di Gerlando, R.; Tuohy, H.; Francesca, N.; Moschetti, G.; Settanni, L. Monitoring of wheat lactic acid bacteria from the field until the first step of dough fermentation. Food Microbiol. 2017, 62, 256–269. [Google Scholar] [CrossRef]
- Ribotta, P.D.; Ausar, S.F.; Beltramo, D.M.; León, A.E. Interactions of hydrocolloids and sonicated-gluten proteins. Food Hydrocoll. 2005, 19, 93–99. [Google Scholar] [CrossRef]
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Tang, X.; Liu, R.; Huang, W.; Zhang, B.; Wu, Y.; Zhuang, J.; Omedi, J.O.; Wang, F.; Zheng, J. Impact of in situ formed exopolysaccharides on dough performance and quality of Chinese steamed bread. LWT—Food Sci. Technol. 2018, 96, 519–525. [Google Scholar] [CrossRef]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2017, 110, 52–61. [Google Scholar] [CrossRef]
- Tang, X.; Liu, N.; Huang, W.; Cheng, X.; Wang, F.; Zhang, B.; Chen, J.; Jiang, H.; Omedi, J.O.; Li, Z. Syneresis rate, water distribution and microstructure of wheat starch gel during freeze-thaw process: Role of a high molecular weight dextran produced by Weissella confusa QS813 from traditional sourdough. Cereal Chem. 2018, 95, 117–129. [Google Scholar] [CrossRef]
- Zhang, B.; Omedi, J.O.; Zheng, J.; Huang, W.; Jia, C. Exopolysaccharides in sourdough fermented by Weissella confusa QS813 protected protein matrix and quality of frozen gluten-red bean dough during freeze-thaw cycles. Food Biosci. 2021, 43, 101180. [Google Scholar] [CrossRef]
- Omedi, J.O.; Huang, W.; Su, X.; Liu, R.; Tang, X.; Xu, Y.; Rayas-Duarte, P. Effect of five lactic acid bacteria starter type on angiotensin-I converting enzyme inhibitory activity and emulsifying properties of soy flour sourdoughs with and without wheat bran supplementation. J. Cereal Sci. 2016, 69, 57–63. [Google Scholar] [CrossRef]
- Zhang, B.; Wendan, Y.; Wang, F.; Omedi, J.O.; Liu, R.; Huang, J.; Zhang, L.; Zou, Q.; Huang, W.; Li, S. Use of Kluyveromyces marxianus prefermented wheat bran as a source of enzyme mixture to improve dough performance and bread biochemical properties. Cereal Chem. 2019, 96, 142–153. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Huang, W.; Liu, J.G.; Tilley, M.; Yao, Y. Rheology, Microstructure and Baking Characteristics of Frozen Dough Containing Rhizopus chinensis Lipase and Transglutaminase. Cereal Chem. 2011, 88, 596–601. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, T.; Yu, W.; Yang, N.; Jin, Z.; Xu, X. Structural, thermal and rheological properties of gluten dough: Comparative changes by dextran, weak acidification and their combination. Food Chem. 2020, 330, 127154. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.; Tmoa, S.J.; Nakai, S. Determination of SH- and SS-Groups in Some Food Proteins Using Ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef]
- Takeda, K.; Matsumura, Y.; Shimizu, M. Emulsifying and surface properties of wheat gluten under acidic conditions. J. Food Sci. 2001, 66, 393–399. [Google Scholar] [CrossRef]
- Su, X.; Wu, F.; Zhang, Y.; Yang, N.; Chen, F.; Jin, Z.; Xu, X. Effect of organic acids on bread quality improvement. Food Chem. 2019, 278, 267–275. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Huang, W.; Ma, Z.; Zhang, F.; Wang, F.; Zou, Q.; Zheng, J. Hydration, water distribution and microstructure of gluten during freeze thaw process: Role of a high molecular weight dextran produced by Weissella confusa QS813. Food Hydrocoll. 2019, 90, 377–384. [Google Scholar] [CrossRef]
- Don, C.; Mann, G.; Bekes, F.; Hamer, R.J. HMW-GS affect the properties of glutenin particles in GMP and thus flour quality. J. Cereal Sci. 2006, 44, 127–136. [Google Scholar] [CrossRef]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, L.; Xu, D.; Li, D.; Yang, N.; Chen, F.; Jin, Z.; Xu, X. Effects of dextran with different molecular weights on the quality of wheat sourdough breads. Food Chem. 2018, 256, 373–379. [Google Scholar] [CrossRef]
- Gray, J.A.; Bemiller, J.N. Bread Staling: Molecular Basis and Control. Compr. Rev. Food Sci. Food Saf. 2002, 2, 1–21. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef]
- Cho, I.H.; Peterson, D.G. Chemistry of bread aroma: A review. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]


| Red Bean Dough | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicochemical Parameters of Sourdough | Physicochemical Properties | Dough Development Properties | Gaseous Release Properties | ||||||||||
| pH | TTA (mL) | LAB (CFU/g) | EPS (g/kg Sourdough) | Lactic Acid (mmol/100 g) | Acetic Acid (mmol/100 g) | FQ | Sample Name | pH | TTA (mL) | Hm (mm) | H’m (mm) | VT (mL) | R (%) |
| 4.34 ± 0.05 | 11.07 ± 0.15 | 2.98×108 | 18.68 ± 0.15 | 106.97 ± 0.16 | 62.66 ± 0.21 | 1.71 | WR | 5.31 ± 0.05 a | 4.66 ± 0.11 c | 68.40 ± 0.22 b | 68.30 ± 0.21 a | 1464.00 ± 1.90 a | 87.16 ± 0.18 c |
| Nd | Nd | Nd | Nd | Nd | Nd | Nd | CW | 5.48 ± 0.08 b | 3.68 ± 0.04 a | 78.70 ± 0.24 d | 73.10 ± 0.15 c | 1540.00 ± 3.00 c | 88.57 ± 0.11 d |
| Nd | Nd | Nd | Nd | Nd | Nd | Nd | CR | 5.46 ± 0.02 b | 4.05 ± 0.06 b | 69.80 ± 0.09 c | 71.70 ± 0.08 b | 1486.00 ± 4.00 a | 84.32 ± 0.08 b |
| Nd | Nd | Nd | Nd | Nd | Nd | Nd | EWR | 5.42 ± 0.04 b | 4.01 ± 0.05 b | 68.70 ± 0.18 b | 68.50 ± 0.18 a | 1473.00 ± 3.20 a | 87.44 ± 0.25 c |
| Nd | Nd | Nd | Nd | Nd | Nd | Nd | AWR | 5.33 ± 0.07 a | 4.61 ± 0.09 c | 67.00 ± 0.15 a | 75.10 ± 0.22 d | 1525.00 ± 2.25 b | 87.44 ± 0.25 c |
| Molecular Weight Distribution (%) | |||||
|---|---|---|---|---|---|
| Samples | Free SH Content (μmol/g) | PP | MP | GMP | PP:MP Ratio |
| CW | 5.11 ± 0.05 bc | 21.01 ± 0.18 a | 47.87 ± 0.08 a | 31.12 ± 0.18 e | 0.4389 |
| CR | 5.08 ± 0.04 bc | 23.94 ± 0.11 b | 53.05 ± 0.05 d | 23.01 ± 0.18 b | 0.4513 |
| WR | 5.12 ± 0.06 bc | 24.45 ± 0.02 c | 50.74 ± 0.18 c | 24.81 ± 0.08 c | 0.4819 |
| AWR | 5.16 ± 0.03 c | 25.96 ± 0.18 d | 52.93 ± 0.15 d | 21.11 ± 0.05 a | 0.4905 |
| EWR | 5.02 ± 0.01 a | 24.28 ± 0.05 c | 50.45 ± 0.11 b | 25.27 ± 0.18 d | 0.4813 |
| Samples | Baking Loss (%) | Specific Volume (mL/g) | Moisture (%), 0 day | Moisture Loss (%/day), 7 days | Hardness (g), 0 day | Staling Rate (g/day), 7 days |
|---|---|---|---|---|---|---|
| CW | 27.12 ± 0.11 b | 8.44 ± 0.05 c | 37.48 ± 0.22 a | 0.78 ± 0.01 b | 300.33 ± 2.21 c | 173.76 ± 1.35 b |
| CR | 26.23 ± 0.06 a | 7.01 ± 0.09 a | 38.84 ± 0.13 b | 0.81 ± 0.02 b | 302.33 ± 1.64 c | 191.33 ± 4.58 c |
| WR | 27.48 ± 0.12 cd | 8.04 ± 0.06 b | 39.28 ± 0.06 c | 0.73 ± 0.01 a | 243.00 ± 5.12 a | 136.95 ± 0.79 a |
| AWR | 27.81 ± 0.29 d | 8.16 ± 0.09 b | 38.91 ± 0.08 b | 0.77 ± 0.01 b | 275.00 ± 3.24 b | 137.71 ± 1.37 a |
| EWR | 27.31 ± 0.13 bc | 8.01 ± 0.14 b | 38.86 ± 0.05 b | 0.73 ± 0.02 a | 248.00 ± 4.85 a | 135.43 ± 0.81 a |
| Peak Area (×106) | ||||||
|---|---|---|---|---|---|---|
| Type | Compounds | CW | CR | WR | AWR | EWR |
| Acids | Decanoic acid | Nd | 6.12 ± 0.06 b | 3.24 ± 0.03 a | 12.2 ± 0.1 d | 9.80 ± 0.08 c |
| Octanoic acid | 15.2 ± 0.2 b | 17.5 ± 0.3 c | 1.84 ± 0.04 a | 16.0 ± 0.5 b | 15.6 ± 0.3 b | |
| Nonanoic acid | 11.2 ± 0.4 b | 7.82 ± 0.11 a | 55.3 ± 0.5 d | 11.8 ± 0.2 b | 17.8 ± 0.05 c | |
| Hexanoic acid | 36.3 ± 0.3 c | 26.8 ± 0.3 a | 38.7 ± 0.4 d | 37.9 ± 0.6 d | 30.8 ± 0.2 b | |
| Acetic acid | nd | 25.6 ± 0.2 b | 2.57 ± 0.07 a | 57.7 ± 0.5 c | 89.3 ± 0.9 d | |
| Oxalic acid | 7.04 ± 0.14 c | 6.08 ± 0.12 b | 33.5 ± 0.04 e | 5.64 ± 0.08 a | 8.47 ± 0.15 d | |
| 2-Methyl-propanoic acid | 94.7 ± 0.9 e | 36.3 ± 0.4 b | 17.0 ± 0.3 a | 41.7 ± 0.3 c | 50.6 ± 0.5 d | |
| 2-(Aminooxy)-propanoic acid | 2.35 ± 0.04 c | 0.82 ± 0.02 a | 3.71 ± 0.09 d | 9.92 ± 0.08 e | 1.98 ± 0.04 b | |
| 2-(Aminooxy)-butanoic acid | 2.90 ± 0.02 a | 15.7 ± 0.2 c | 27.1 ± 0.4 e | 6.89 ± 0.05 b | 21.8 ± 0.5 d | |
| Sub-total | 170 ± 3 b | 143 ± 2 a | 183 ± 3 c | 200 ± 4 d | 246 ± 3 e | |
| Alcohols | 3-Methyl-1-butanol | 45.1 ± 0.5 c | 77.3 ± 0.8 d | 87.6 ± 0.9 e | 8.38 ± 0.08 b | 2.74 ± 0.03 a |
| 2-Methyl-1-propanol | 8.28 ± 0.06 a | 12.9 ± 0.1 b | 20.2 ± 0.2 c | 22.3 ± 0.3 d | 81.9 ± 0.8 e | |
| Ethanol | 26.2 ± 0.3 a | 376 ± 4 c | 441 ± 5 d | 373 ± 4 c | 318 ± 3 b | |
| 5-Methyl-2-(1-methylethyl)-cyclohexanol | nd | 58.2 ± 0.6 d | 54.8 ± 0.6 c | 7.50 ± 0.12 a | 47.1 ± 0.5 b | |
| Phenylethyl alcohol | 208 ± 5 a | 205 ± 3 a | 195 ± 6 a | 229 ± 3 b | 230 ± 5 b | |
| Benzyl alcohol | 862 ± 9 d | 24.3 ± 0.3 a | 24.7 ± 0.2 a | 29.0 ± 0.3 b | 33.5 ± 0.5 c | |
| Z-4-Dodecenol | nd | 37.5 ± 0.5 c | 34.6 ± 0.3 b | 31.4 ± 0.6 a | 31.9 ± 0.2 a | |
| Levomenthol | nd | 14.6 ± 0.3 a | 17.3 ± 0.5 b | 65.9 ± 0.6 c | 18.1 ± 0.3 b | |
| 2,3-Butanediol | 47.3 ± 0.3 a | 54.4 ± 0.5 b | 63.6 ± 0.6 c | 47.7 ± 0.3 a | 68.8 ± 0.8 d | |
| Sub-total | 1200 ± 16 e | 861 ± 10 c | 939 ± 11 d | 814 ± 8 a | 832 ± 10 b | |
| Aldehydes | Nonanal | 28.8 ± 0.3 c | 15.6 ± 0.2 a | 21.3 ± 0.6 b | nd | 15.7 ± 0.3 a |
| Hexanal | 5.43 ± 0.05 e | 4.23 ± 0.04 d | 0.80 ± 0.06 a | 1.15 ± 0.02 b | 2.04 ± 0.01 c | |
| Succindialdehyde | 6.42 ± 0.07 a | nd | nd | 15.2 ± 0.4 b | nd | |
| Benzaldehyde | 17.4 ± 0.1 b | 15.8 ± 0.3 a | 17.2 ± 0.4 b | 16.9 ± 0.3 b | 18.4 ± 0.2 c | |
| Sub-total | 58.0 ± 0.5 d | 35.6 ± 0.3 b | 39.7 ± 2 c | 33.2 ± 0.9 a | 36.1 ± 0.6 b | |
| Esters | Ethyl acetate | 13.8 ± 0.2 a | nd | 70.6 ± 0.8 b | nd | nd |
| Octanoic acid ethyl ester | 15.7 ± 0.2 a | 43.1 ± 0.3 d | 25.4 ± 0.4 b | 26.1 ± 0.5 b | 27.6 ± 0.3 c | |
| cis-4-Hydroxy-3-methylundecanoic acid lactone | 3.81 ± 0.05 a | nd | nd | nd | nd | |
| Dibutyl phthalate | nd | 7.03 ± 0.07 a | 12.4 ± 0.2 d | 9.59 ± 0.10 c | 7.29 ± 0.15 b | |
| Decanoic acid ethyl ester | 22.6 ± 0.3 d | 3.87 ± 0.05 b | 8.00 ± 0.08 d | 1.90 ± 0.02 a | 6.80 ± 0.07 c | |
| 4-Ethoxy-benzoic acid ethyl ester | 21.9 ± 0.5 a | nd | nd | nd | nd | |
| 3-Hydroxy-butanoic acid methyl ester | nd | 1.63 ± 0.06 a | nd | nd | 6.39 ± 0.03 b | |
| 2-Butoxy-1-methyl-2-oxoethyl butanoic acid ester | nd | 13.4 ± 0.2 b | 13.7 ± 0.3 b | 0.60 ± 0.01 a | 13.5 ± 0.4 b | |
| Ethyl oxamate | nd | 2.30 ± 0.06 c | 1.25 ± 0.03 a | 1.67 ± 0.06 b | 9.11 ± 0.08 d | |
| δ-Decalactone | nd | nd | 25.2 ± 0.4 a | nd | nd | |
| Sub-total | 78 ± 5 e | 72 ± 3 d | 93.0 ± 0.9 c | 39.8 ± 0.3 a | 70.8 ± 0.5 b | |
| Hydrocarbons | Undecane | nd | 6.30 ± 0.05 c | 4.92 ± 0.03 b | 3.52 ± 0.08 a | 7.14 ± 0.09 d |
| Tetradecane | nd | nd | nd | 37.2 ± 0.5 a | nd | |
| Tridecane | 44.0 ± 0.3 d | 16.6 ± 0.5 bc | 2.08 ± 0.07 a | 15.7 ± 0.6 b | 17.6 ± 0.5 c | |
| Dodecane | nd | nd | 10.7 ± 0.1 b | 1.58 ± 0.04 a | 28.6 ± 0.3 c | |
| 5-Ethyldecane | nd | nd | nd | 8.76 ± 0.1 b | 2.03 ± 0.06 a | |
| 3,3-Dimethyl-hexane | nd | 6.53 ± 0.07 b | nd | 10.5 ± 0.1 c | 1.08 ± 0.02 a | |
| 2,5,9-Trimethyl-decane | 4.64 ± 0.05 a | 5.38 ± 0.06 b | nd | 22.7 ± 0.3 d | 10.1 ± 0.1 c | |
| Sub-total | 9.04 ± 0.11 a | 34.8 ± 0.3 c | 17.7 ± 0.2 b | 99.9 ± 1.1 e | 66.6 ± 0.5 d | |
| Heterocyclic compounds | Indole | nd | 11.9 ± 0.2 a | 12.4 ± 0.3 a | 12.4 ± 0.2 a | 14.1 ± 0.4 b |
| Maltol | 1.20 ± 0.06 a | 1.07 ± 0.07 a | 1.13 ± 0.02 a | 12.9 ± 0.3 b | 12.6 ± 0.1 b | |
| 2-Pentyl-furan | 4.26 ± 0.06 a | nd | nd | nd | nd | |
| Furfural | 7.02 ± 0.09 a | nd | nd | nd | nd | |
| Sub-total | 12.5 ± 0.4 a | 12.9 ± 0.2 a | 13.5 ± 0.3 d | 25.3 ± 0.4 b | 26.7 ± 0.5 c | |
| Ketones | Acetoin | 139.6 ± 2 d | 60.8 ± 0.5 b | 85 ± 0.02 c | 55.8 ± 0.9 a | 53.4 ± 0.9 a |
| Sub-total | 139.6 ± 2 d | 60.8 ± 0.5 b | 85 ± 0.02 c | 55.8 ± 0.9 a | 53.4 ± 0.9 a | |
| Total | 1667 ± 25 d | 1220 ± 18 b | 1371 ± 13 c | 1268 ± 11 a | 1332 ± 24 b | |
| Samples | Color | Taste | Appearance | Overall Acceptance |
|---|---|---|---|---|
| CW | 6.60 ± 0.18 a | 7.15 ± 0.25 c | 7.01 ± 0.12 b | 7.1 ± 0.01 ab |
| CR | 6.90 ± 0.14 ab | 6.81 ± 0.19 b | 6.53 ± 0.13 a | 6.8 ± 0.03 a |
| WR | 7.00 ± 0.25 b | 7.13 ± 0.16 c | 7.39 ± 0.01 c | 7.3 ± 0.05 b |
| AWR | 6.90 ± 0.11 ab | 6.16 ± 0.05 a | 6.91 ± 0.05 b | 7.0 ± 0.01 ab |
| EWR | 6.90 ± 0.13 ab | 7.01 ± 0.12 c | 7.0 ± 0.05 b | 7.1 ± 0.02 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Huang, J.; Zhang, B.; Omedi, J.O.; Chen, C.; Zhou, L.; Liang, L.; Zou, Q.; Zheng, J.; Zeng, Y.; et al. Rheo-Fermentation Dough Properties, Bread-Making Quality and Aroma Characteristics of Red Bean (Vigna angularis) Sourdough Induced by LAB Weissella confusa QS813 Strain Fermentation. Foods 2023, 12, 605. https://doi.org/10.3390/foods12030605
Huang C, Huang J, Zhang B, Omedi JO, Chen C, Zhou L, Liang L, Zou Q, Zheng J, Zeng Y, et al. Rheo-Fermentation Dough Properties, Bread-Making Quality and Aroma Characteristics of Red Bean (Vigna angularis) Sourdough Induced by LAB Weissella confusa QS813 Strain Fermentation. Foods. 2023; 12(3):605. https://doi.org/10.3390/foods12030605
Chicago/Turabian StyleHuang, Chengye, Jing Huang, Binle Zhang, Jacob Ojobi Omedi, Cheng Chen, Liyuan Zhou, Li Liang, Qibo Zou, Jianxian Zheng, Yongqing Zeng, and et al. 2023. "Rheo-Fermentation Dough Properties, Bread-Making Quality and Aroma Characteristics of Red Bean (Vigna angularis) Sourdough Induced by LAB Weissella confusa QS813 Strain Fermentation" Foods 12, no. 3: 605. https://doi.org/10.3390/foods12030605
APA StyleHuang, C., Huang, J., Zhang, B., Omedi, J. O., Chen, C., Zhou, L., Liang, L., Zou, Q., Zheng, J., Zeng, Y., & Huang, W. (2023). Rheo-Fermentation Dough Properties, Bread-Making Quality and Aroma Characteristics of Red Bean (Vigna angularis) Sourdough Induced by LAB Weissella confusa QS813 Strain Fermentation. Foods, 12(3), 605. https://doi.org/10.3390/foods12030605







