Enzymatic Acylation of Black Rice Anthocyanins and Evaluation of Antioxidant Capacity and Stability of Their Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anthocyanin Extraction
2.3. Anthocyanin Acylation
2.4. Ultraviolet–Visible (UV–Vis) Analysis
2.5. FTIR Analysis
2.6. Thermogravimetric Analysis (TGA)
2.7. Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry/Mass Spectrometry (UHPLC–HR-MS/MS) Analysis
2.8. Evaluation of the Lipophilicity, Antioxidant Activity and Stability
2.8.1. Determination of the Lipophilicity
2.8.2. Antioxidant Assays
2.8.3. Stability Assays
2.9. Statistical Analysis
3. Results and Discussion
3.1. UV–Vis Analysis
3.2. FTIR Analysis
3.3. TGA Thermal Stability
3.4. UHPLC–HR-MS/MS Analysis
3.5. Determination of the Lipophilicity
3.6. Antioxidant Capacity
3.7. Stability Assays
3.7.1. Thermal Stability
3.7.2. pH Stability
3.7.3. Light Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, Z.H.; Qin, P.Y.; Zhang, Y.; Cui, S.H.; Ren, G.X. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Tatsuzawa, F.; Toki, K.; Saito, N.; Shinoda, K.; Shigihara, A.; Honda, T. Anthocyanin occurrence in the root peels, petioles and flowers of red radish (Raphanus sativus L.). Dye. Pigment. 2008, 79, 83–88. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Martin, P.; Kevin, S.G.; Chiara, L.; Lynnette, R.F. In situ and in vitro antioxidant activity of sweet potato anthocyanins. J. Agric. Food Chem. 2004, 52, 1511–1513. [Google Scholar] [CrossRef]
- Li, Y.H.; Kong, Y.; Wu, Z.N.; Xie, F.Y. Grain Quality Characterization before and after Processing. Foods 2023, 12, 3728. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P.; Moontree, T. Ohmic heating-assisted extraction of anthocyanins from black rice bran to prepare a natural food colorant. Innov. Food Sci. Emerg. Technol. 2015, 27, 102–110. [Google Scholar] [CrossRef]
- Yue, W.X.; Han, F.L. Effects of monoglucoside and diglucoside anthocyanins from Yan 73 (Vitis vinifera L.) and spine grape (Vitis davidii Foex) skin on intestinal microbiota in vitro. Food Chem. X 2022, 16, 100501. [Google Scholar] [CrossRef]
- Orororo, O.C.; Asagba, S.O.; Tonukari, N.J.; Okandeji, O.J.; Mbanugo, J.J. Effects of Hibiscus sabdarrifa L. anthocyanins on cadmium-induced oxidative stress in Wistar rats. J. Appl. Sci. Environ. Manag. 2018, 22, 465–470. [Google Scholar] [CrossRef]
- Swer, T.L.; Chauhan, K. Stability studies of enzyme aided anthocyanin extracts from Prunus nepalensis L. LWT-Food Sci. Technol. 2018, 102, 181–189. [Google Scholar] [CrossRef]
- de Castro, V.C.; da Silva, P.H.A.; de Oliveira, E.B.; Desobry, S.; Humeau, C. Extraction, identification and enzymatic synthesis of acylated derivatives of anthocyanins from jaboticaba (Myrciaria cauliflora) fruits. Int. J. Food Sci. Technol. 2014, 49, 196–204. [Google Scholar] [CrossRef]
- Fei, P.; Zeng, F.S.; Zheng, S.Y.; Chen, Q.L.; Hu, Y.H.; Cai, J. Acylation of Blueberry Anthocyanins with Maleic Acid: Improvement of the Stability and Its Application Potential in Intelligent Color Indicator. Pack. Mater. Dye. Pigment. 2020, 184, 108852. [Google Scholar] [CrossRef]
- Gonzalez, E.; Fougerousse, A.; Brouillard, R. Two diacylated malvidin glycosides from Petunia hybrida flowers. Phytochemistry 2001, 58, 1257–1262. [Google Scholar] [CrossRef]
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. J. Food Sci. Technol. 2021, 59, 831–845. [Google Scholar] [CrossRef]
- Lv, X.L.; Sun, H.L.; Ji, Z.Y. Acylation of anthocyanins from black rice and their stability properties. Adv. Mater. Res. 2011, 204–210, 750–754. [Google Scholar] [CrossRef]
- Dini, C.; Zaro, M.J.; Viña, S.Z. Bioactivity and functionality of anthocyanins: A review. Curr. Bioact. Compd. 2019, 15, 507–523. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, H.; Zhang, Z.Q.; Yang, S.L.; Li, H.R. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Yan, Z.; Li, C.Y.; Zhang, L.X.; Liu, Q.; Ou, S.Y.; Zeng, X.X. Enzymatic Acylation of Anthocyanin Isolated from Black Rice with Methyl Aromatic Acid Ester as Donor: Stability of the Acylated Derivatives. J. Agric. Food Chem. 2016, 64, 1137–1143. [Google Scholar] [CrossRef]
- Xie, J.H.; Hao, X.; Shang, Y.Q.; Chen, W. Improvement of stability and lipophilicity of pelargonidin-3-glucoside by enzymatic acylation with aliphatic dicarboxylic acid. Food Chem. 2022, 389, 133077. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem. 2010, 122, 1205–1211. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Zhang, P.L.; Liu, S.; Zhao, Z.G.; You, L.J.; Harrison, M.D.; Zhang, Z.Y. Enzymatic acylation of cyanidin-3-glucoside with fatty acid methyl esters improves stability and antioxidant activity. Food Chem. 2020, 343, 128482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Chen, J.; Wang, Z.Q.; Shen, R.M.; Cui, N.; Sun, A.D. Direct Acylation of Cyanidin-3-Glucoside with Lauric Acid in Blueberry and Its Stability Analysis. Int. J. Food Prop. 2016, 19, 1–12. [Google Scholar] [CrossRef]
- Wang, P.K.; Liu, J.N.; Zhuang, Y.H.; Fei, P. Acylating blueberry anthocyanins with fatty acids: Improvement of their lipid solubility and antioxidant activities. Food Chem. X 2022, 15, 100420. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kortesniemi, M.; Yang, B.R.; Zheng, J. Enzymatic Acylation of Anthocyanins Isolated from Alpine Bearberry (Arctostaphylos alpina) and Lipophilic Properties, Thermostability, and Antioxidant Capacity of the Derivatives. J. Agric. Food Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Ben Sassi, C.; Marcet, I.; Rendueles, M.; Díaz, M.; Fattouch, S. Egg yolk protein as a novel wall material used together with gum Arabic to encapsulate polyphenols extracted from Phoenix dactylifera L pits. LWT-Food Sci. Technol. 2020, 131, 109778. [Google Scholar] [CrossRef]
- Damasceno, E.; Almeida, R.; Carvalho, S.; Vieira, S.; Mano, V.; Guimarães, L. Nano-Encapsulation of Lippia origanoides Kunth. Essential Oil by Chitosan-CAFFEIC Acid Nanogel. Química Nova 2019, 43, 16–23. [Google Scholar] [CrossRef]
- Xie, F.Y.; Bi, W.W.; Wang, X.J.; Zhang, X.L.; Zhang, X.N.; Zhao, G.X.; Liu, Q.Q. Extraction and Identification of Black Rice Polyphenolic Compounds by Reversed Phase High Performance Liquid Chromatography–Electrospray Ionization Mass Spectrometry. J. Food Process. Preserv. 2017, 41, e13119. [Google Scholar] [CrossRef]
- Paulina, S.; Anna, D.; Janina, G. Interaction between Mimic Lipid Membranes and Acylated and Nonacylated Cyanidin and Its Bioactivity. J. Agric. Food Chem. 2016, 64, 7414–7422. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.Y.; Zheng, J.; Yang, B.R. Enzymatic Acylation of Blackcurrant (Ribes nigrum) Anthocyanins and Evaluation of Lipophilic Properties and Antioxidant Capacity of Derivatives. Food Chem. 2018, 281, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Baublis, A.; Spomer, A.; Berber-Jiménez, M.D. Anthocyanin pigments: Comparison of extract stability. J. Food Sci. 1994, 59, 1219–1221. [Google Scholar] [CrossRef]
- Shi, Z.U.; Francis, F.R.J.; Daun, H.E. Quantitative comparison of the stability of anthocyanins from Brassica oleracea and Tradescantia pallida in non-sugar drink model and protein model systems. J. Food Sci. 1992, 57, 768–770. [Google Scholar] [CrossRef]
- Fenger, J.A.; Moloney, M.; Robbin, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural antioxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019, 10, 6740–6751. [Google Scholar] [CrossRef] [PubMed]
- Molaeafard, S.; Jamei, R.; Marjani, A.P. Co-pigmentation of anthocyanins extracted from sour cherry (Prunus cerasus L.) with some organic acids: Color intensity, thermal stability, and thermodynamic parameters. Food Chem. 2020, 339, 128070. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhuang, Y.H.; Hu, Y.H.; Xue, S.; Li, H.; Chen, L.; Fei, P. Improving the color stability and antioxidation activity of blueberry anthocyanins by enzymatic acylation with p-coumaric acid and caffeic acid. LWT-Food Sci. Technol. 2020, 130, 109673. [Google Scholar] [CrossRef]
- Modesto Junior, E.N.; Martins, M.G.; Pereira, G.A.; Chisté, R.C.; Pena, R.d.S. Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments. Foods 2023, 12, 565. [Google Scholar] [CrossRef]


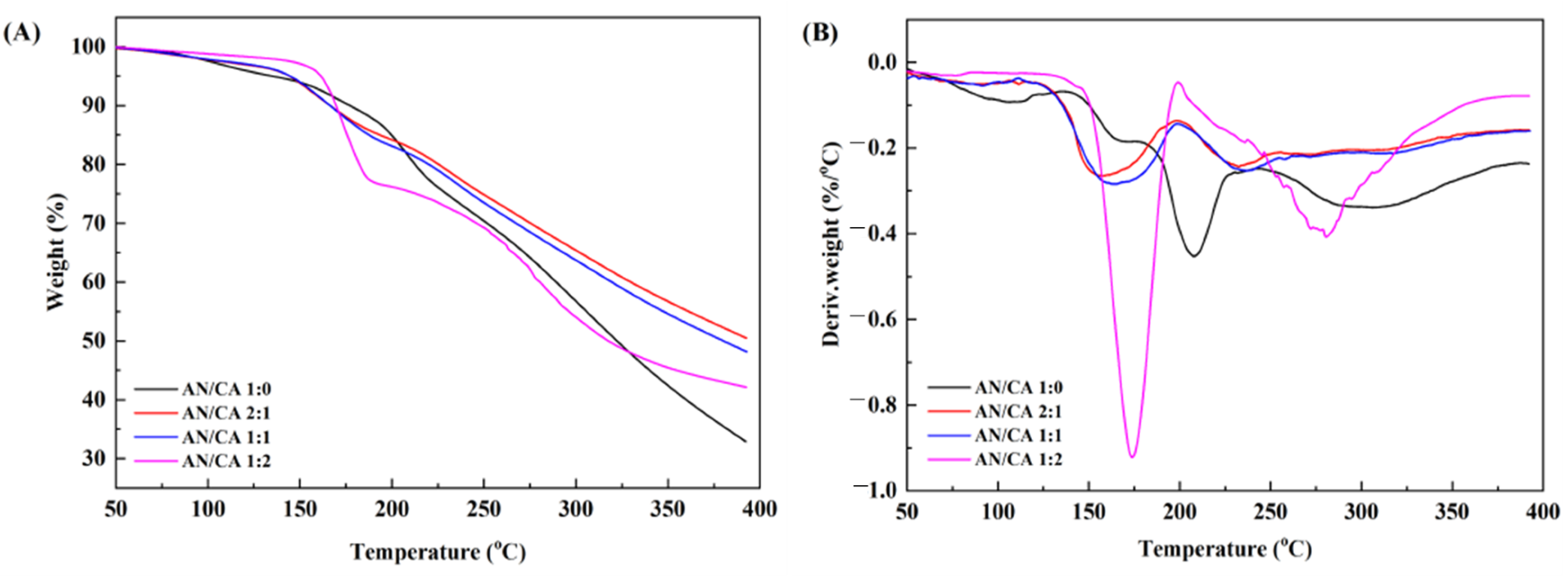



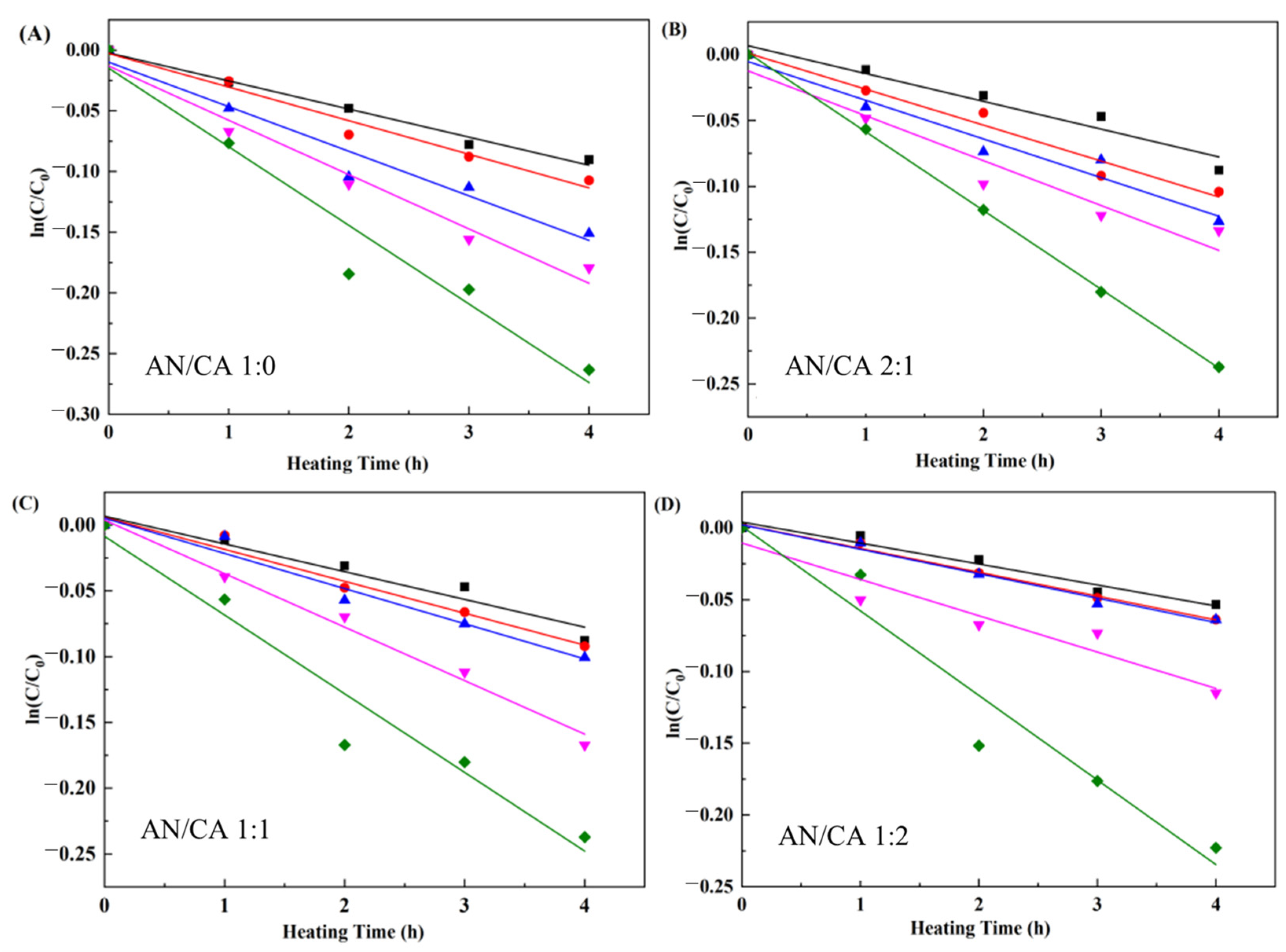
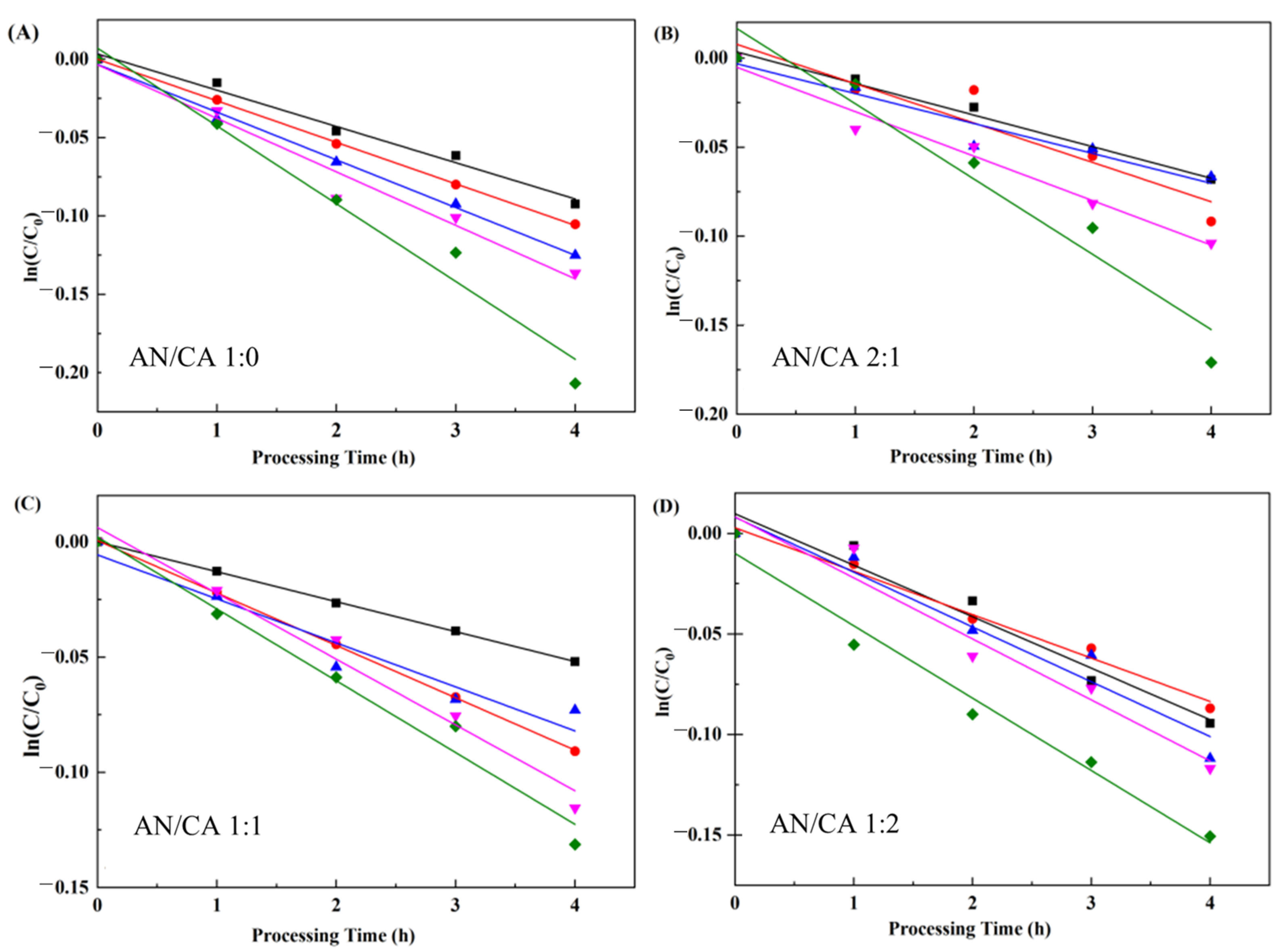

| T (°C) | k (10−2/h) | t1/2 (h) | E0 (kJ/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AN/CA 1:0 | AN/CA 2:1 | AN/CA 1:1 | AN/CA 1:2 | AN/CA 1:0 | AN/CA 2:1 | AN/CA 1:1 | AN/CA 1:2 | AN/CA 1:0 | AN/CA 2:1 | AN/CA 1:1 | AN/CA 1:2 | |
| 50 | 2.47 ± 0.18 a | 1.61 ± 0.43 b | 1.36 ± 0.61 b | 1.12 ± 0.41 b | 28.04 ± 2.12 d | 43.02 ± 0.25 c | 50.93 ± 0.72 b | 61.95 ± 8.26 a | 23.69 ± 1.22 c | 25.52 ± 1.31 c | 34.47 ± 1.53 b | 39.27 ± 1.82 a |
| 60 | 2.91 ± 0.41 a | 2.65 ± 0.35 a | 1.92 ± 0.76 b | 1.46 ± 0.28 b | 23.80 ± 3.16 c | 26.18 ± 3.65 c | 36.16 ± 1.17 b | 47.56 ± 0.75 a | ||||
| 70 | 4.39 ± 0.73 a | 3.37 ± 0.57 b | 2.18 ± 0.89 c | 1.50 ± 0.32 c | 15.80 ± 2.64 d | 20.58 ± 3.77 c | 31.74 ± 1.94 b | 46.14 ± 2.26 a | ||||
| 80 | 5.47 ± 0.92 a | 4.29 ± 0.74 b | 3.83 ± 0.29 b | 3.43 ± 0.46 b | 12.67 ± 2.10 c | 16.16 ± 3.10 b | 18.11 ± 1.40 a | 20.23 ± 3.91 a | ||||
| 90 | 7.51 ± 0.76 a | 5.87 ± 0.15 b | 5.69 ± 0.98 b | 5.58 ± 0.18 b | 9.23 ± 1.44 b | 11.81 ± 0.31 a b | 12.17 ± 2.25 a | 12.43 ± 1.75 a | ||||
| pH Value | k (10−2/h) | t1/2 (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| AN/CA 1:0 | AN/CA 2:1 | AN/CA 1:1 | AN/CA 1:2 | AN/CA 1:0 | AN/CA 2:1 | AN/CA 1:1 | AN/CA 1:2 | |
| 3 | 2.04 ± 0.37 a | 1.50 ± 0.28 b | 1.88 ± 0.37 a | 1.93 ± 0.30 a | 34.00 ± 2.48 c | 46.10 ± 0.8 a | 36.67 ± 0.77 b | 35.84 ± 2.42 c |
| 4 | 2.65 ± 0.45 a | 1.88 ± 0.40 b | 2.10 ± 0.69 a b | 2.24 ± 0.03 a b | 26.15 ± 0.45 c | 36.97 ± 0.99 a | 32.96 ± 0.32 b | 31.00 ± 0.32 b |
| 5 | 3.33 ± 0.34 a | 1.91 ± 0.25 c | 2.30 ± 0.37 b | 2.42 ± 0.07 b | 20.84 ± 1.98 d | 36.23 ± 1.23 a | 30.18 ± 2.56 b | 28.66 ± 0.66 c |
| 6 | 3.64 ± 0.54 a | 2.20 ± 0.11 b | 2.41 ± 0.37 b | 2.56 ± 0.67 b | 19.08 ± 2.54 c | 31.59 ± 1.20 a | 28.77 ± 3.01 b | 27.09 ± 2.26 b |
| 7 | 4.48 ± 0.50 a | 3.32 ± 0.84 b | 3.81 ± 0.52 b | 4.40 ± 0.83 a | 15.49 ± 1.61 c | 23.39 ± 3.84 a | 18.20 ± 2.53 b | 15.77 ± 1.68 c |
| Samples | k (10−2/h) | t1/2 (h) |
|---|---|---|
| AN/CA 1:0 | 6.42 ± 0.44 a | 10.43 ± 0.06 c |
| AN/CA 2:1 | 4.73 ± 0.33 b | 14.65 ± 0.07 b |
| AN/CA 1:1 | 3.11 ± 0.24 c | 22.28 ± 0.07 a |
| AN/CA 1:2 | 2.90 ± 0.43 c | 23.90 ± 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Wang, X.; Wu, Z.; Li, Y.; Xu, F.; Xie, F. Enzymatic Acylation of Black Rice Anthocyanins and Evaluation of Antioxidant Capacity and Stability of Their Derivatives. Foods 2023, 12, 4505. https://doi.org/10.3390/foods12244505
Kong Y, Wang X, Wu Z, Li Y, Xu F, Xie F. Enzymatic Acylation of Black Rice Anthocyanins and Evaluation of Antioxidant Capacity and Stability of Their Derivatives. Foods. 2023; 12(24):4505. https://doi.org/10.3390/foods12244505
Chicago/Turabian StyleKong, Yue, Xinhui Wang, Zenan Wu, Yanhui Li, Fu Xu, and Fengying Xie. 2023. "Enzymatic Acylation of Black Rice Anthocyanins and Evaluation of Antioxidant Capacity and Stability of Their Derivatives" Foods 12, no. 24: 4505. https://doi.org/10.3390/foods12244505
APA StyleKong, Y., Wang, X., Wu, Z., Li, Y., Xu, F., & Xie, F. (2023). Enzymatic Acylation of Black Rice Anthocyanins and Evaluation of Antioxidant Capacity and Stability of Their Derivatives. Foods, 12(24), 4505. https://doi.org/10.3390/foods12244505





