Active and Intelligent Biodegradable Packaging Based on Anthocyanins for Preserving and Monitoring Protein-Rich Foods
Abstract
:1. Introduction
2. Extraction, Structure, and Function of Anthocyanins
2.1. Extraction of Anthocyanins
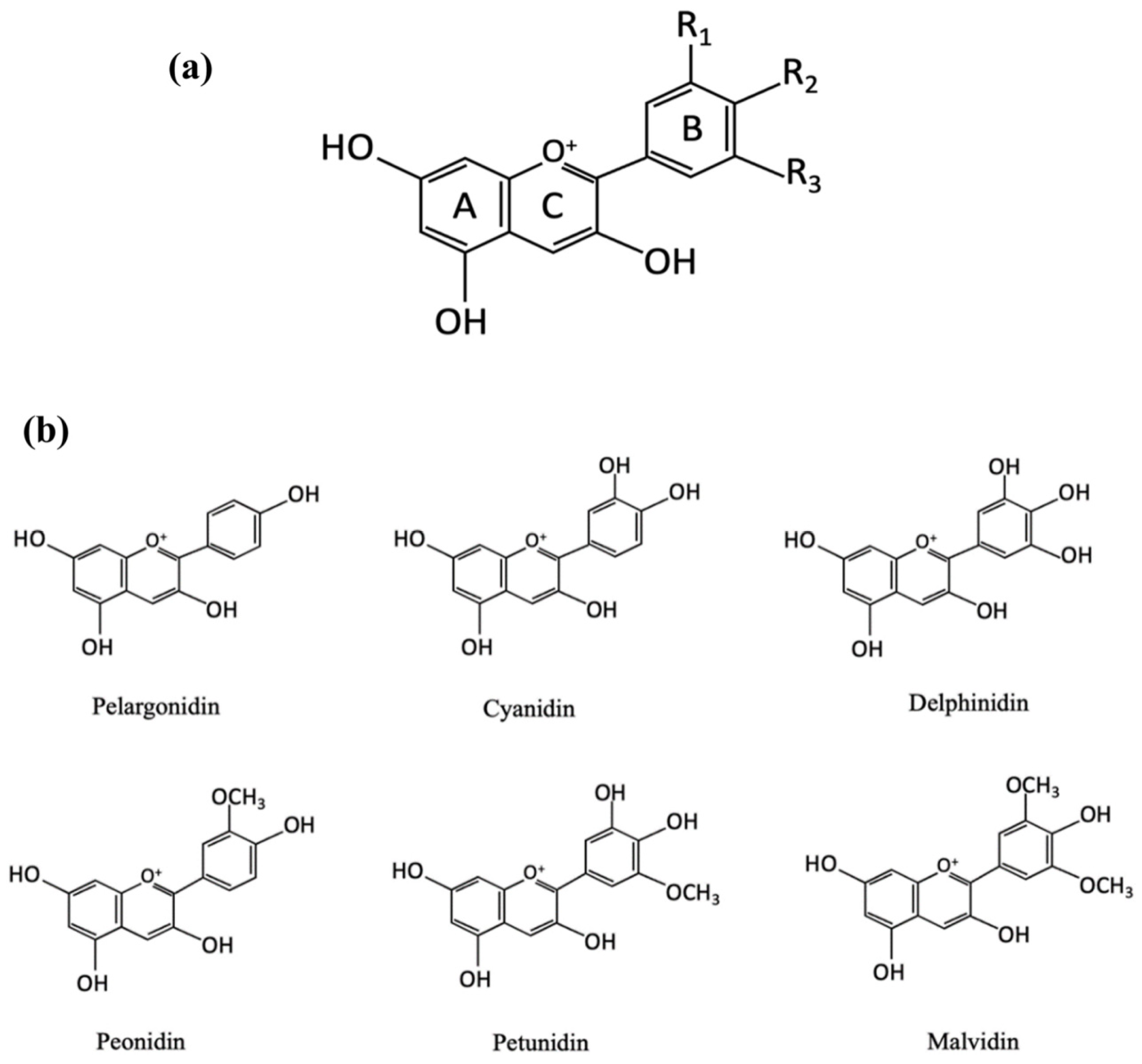
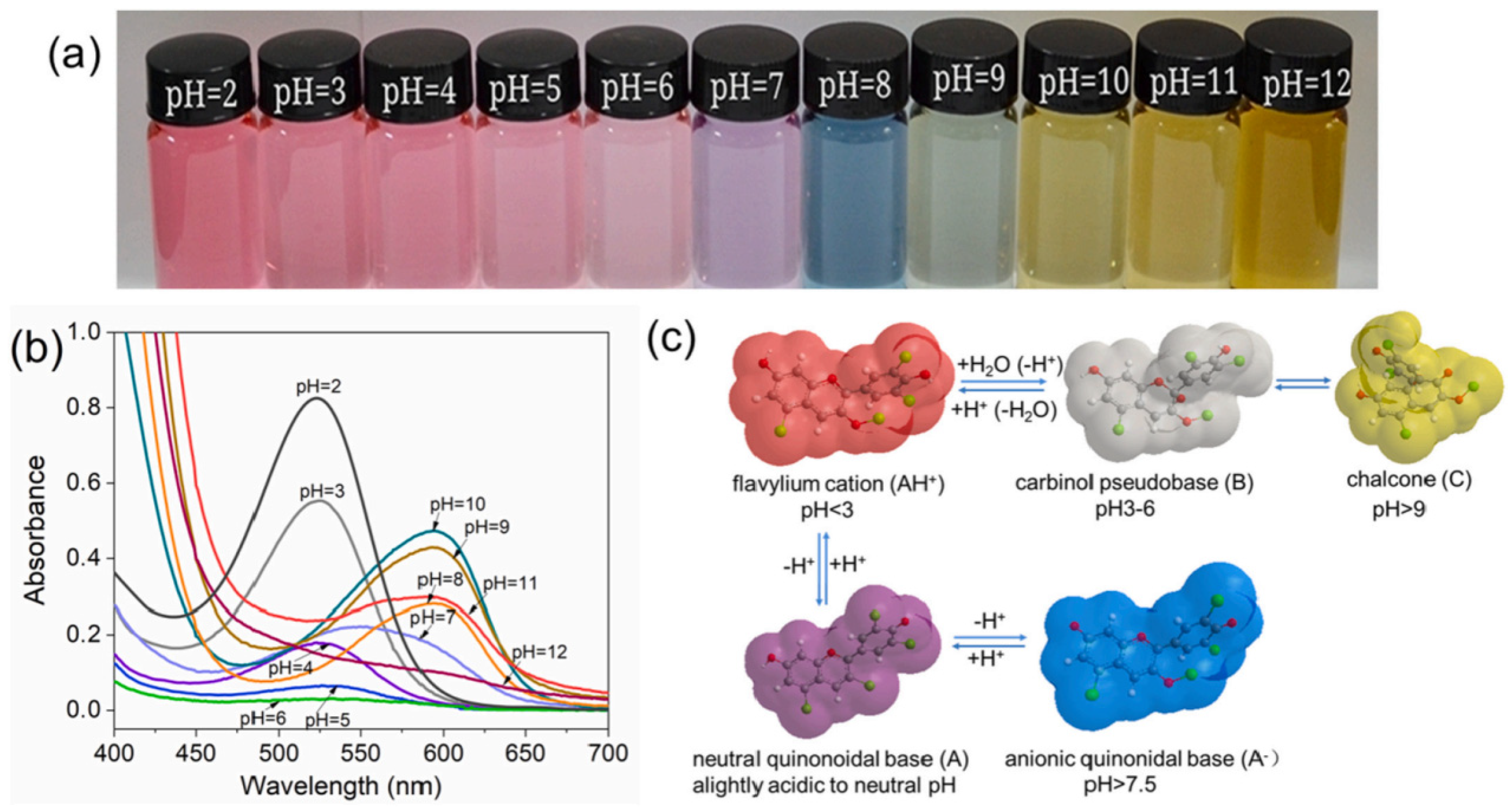
2.2. Chemical Structure and Color Indication Mechanism of Anthocyanins
2.3. Antioxidant Mechanisms of Anthocyanins
2.4. Antimicrobial Mechanisms of Anthocyanins
3. Active and Intelligent Films Containing Anthocyanins
3.1. Cellulose-Based Film
| Substrates | Extracts | Methods | Effects of Anthocyanins | Reference |
|---|---|---|---|---|
| Carrageenan | Jaboticaba peels (50 and 100% w/w based on the polymer) | casting | Improves opacity, UV-vis light barrier against E. coli, scavenges DPPH, | [14] |
| Methylcellulose | Jambolao skins (0, 10, 30, and 50% based on the polymer) | casting | scavenges ABTS and DPPH, increases mechanical and barrier properties | [29] |
| Gelatin, oxidized chitin nanocrystals | Black rice bran (50 and 100% w/w based on the polymer) | casting | UV–vis light barrier and scavenges ABTS, DPPH, and FRAP | [30] |
| Potato starch | Onion (0.1% w/v based on the polymer) | casting | Action against Staphylococcus aureus DSM 20,231, Salmonella bongori DSM 13,772, and Escherichia coli DSM 30083, scavenges DPPH | [38] |
| Cassava starch | Red cabbage (7% v/v based on the polymer) | casting | Improves mechanical strength and hydrophobicity | [49] |
| Quercetin-loaded chitosan, agar, sodium alginate | Purple sweet potato (7% v/v based on the polymer) | casting | UV blocking and water vapor barrier | [50] |
| Starch, agar | Shikonin (1% w/w based on the polymer) | casting | UV-light barrier, mechanical strength, scavenges DPPH and ABTS, action against Listeria monocytogenes | [31] |
| Chitosan | Black rice bran (5, 10, and 20% w/w based on the polymer) | casting | UV–vis light barrier, sensitive and rapid response to pH/NH3, scavenges DPPH, reduces spoilage bacteria | [36] |
| Alginate, carboxymethyl chitosan | Purple cauliflower (10% w/w based on the polymer) | casting | Improves mechanical strength, reduces swelling, improves the sensitivity of the colorimetric response | [10] |
| Hydroxypropyl methylcellulose | Epigallocatechin-3-gallate (0.5, 1, and 2% w/v based on the polymer) | casting | Enhances mechanical strength, superior water vapor barrier, UV protection, detects bacterial growth, kills bacteria on demand | [47] |
| Zein | Blueberry (1, 5, and 10% w/w based on the polymer) | casting | Scavenges DPPH and ABTS and action against E. coli and S. aureus | [32] |
| Chitosan, cassava starch | Mulberry anthocyanin (1.7% w/w based on the polymer) | casting | Reduces oxygen and water vapor transmittance, scavenges DPPH, action against E. coli and S. aureus | [51] |
| Gelatin, carrageenan | Shikonin (10% w/w based on the polymer) | casting | UV blocking and action against E. coli and L. monocytogenes | [52] |
| Cellulose nanofiber | Brassica oleracea (6% w/w based on the polymer) | casting | UV blocking, improves the physicochemical properties, scavenges DPPH and ABTS, | [48] |
| Gelatin | Alizarin (20% w/w based on the polymer) | casting | rapid response to pH/NH3, light barrier, hydrophobicity, scavenges ABTS, action against E. coli and S. aureus | [23] |
| Cellulose acetate | Perilla frutescens (10% w/w based on the polymer) | electrospinning | Scavenges DPPH, enhances hydrophobicity, action against E. coli and S. aureus | [26] |
| Locust bean gum, polyvinyl alcohol, chitosan, κ-carrageenan | Purple sweet potato, Purple cabbage (1% w/w based on the polymer) | casting | Improves light barrier, scavenges DPPH, ammonia sensitivity | [8] |
| Potato starch | Blueberry (7.5% w/w based on the polymer) | casting | Improves mechanical properties and is ammonia-responsive | [53] |
| Gelatin, zein | Blueberry (5% w/v based on the polymer) | electrospinning | Fe2+ enhances the color response of anthocyanins | [7] |
| Gelatin | Coleus scutellarioides (10, 20, and 30% v/v based on the polymer) | casting | Increases film flexibility, decreases tensile strength, UV-vis light transmittance | [54] |
| Gelatin | Haskap berries (0, 0.5, 1, 2, and 3% w/w based on the polymer) | casting | increases water vapor, UV-vis light barrier, improves tensile strength, scavenges DPPH | [55] |
3.2. Chitosan-Based Film
3.3. Starch-Based Film
3.4. Gelatin-Based Film
3.5. Film-Forming Methods
4. Application in Protein-Rich Foods
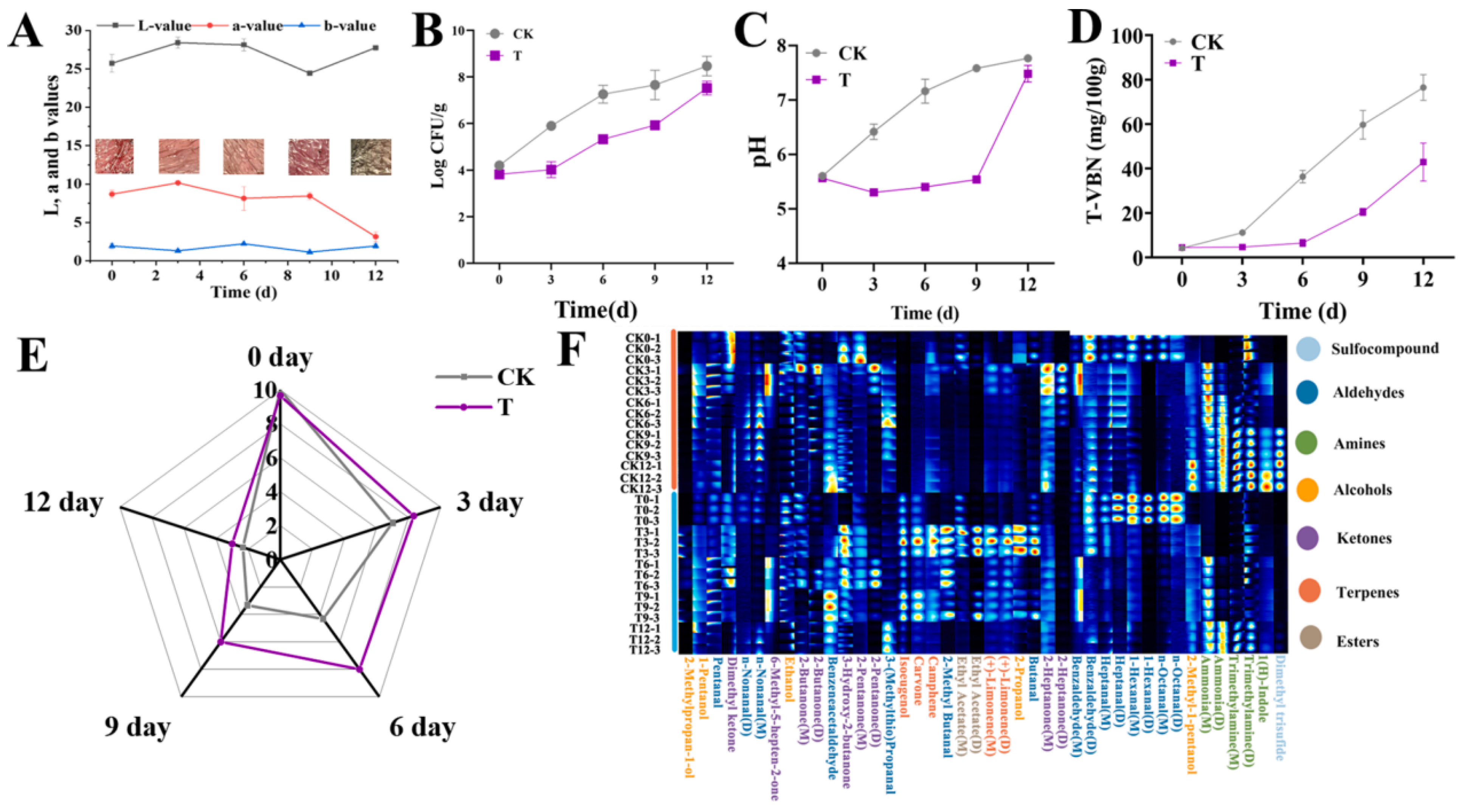
4.1. Fresh Meat
4.2. Aquatic Products
4.3. Milk
5. Summary and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel composite films based on sodium alginate and gallnut extract with enhanced antioxidant, antimicrobial, barrier and mechanical properties. Food Hydrocoll. 2021, 113, 1–11. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent packaging for real-time monitoring of food-quality: Current and future developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernandez, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural food colorants and preservatives: A review, a demand, and a challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.F.; Lu, W.W.; Qin, Y.Y.; Cheng, G.G.; Yuan, M.L.; Li, L. An intelligent pH indicator film based on cassava starch/polyvinyl alcohol incorporating anthocyanin extracts for monitoring pork freshness. J. Food Process Pres. 2021, 45, e15822. [Google Scholar] [CrossRef]
- Gao, R.C.; Hu, H.L.; Shi, T.; Bao, Y.L.; Sun, Q.C.; Wang, L.; Ren, Y.H.; Jin, W.G.; Yuan, L. Incorporation of gelatin and Fe increases the pH-sensitivity of zein-anthocyanin complex films used for milk spoilage detection. Curr. Res. Food Sci. 2022, 5, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Liu, J.; Kan, J.; Liu, J. Active/intelligent packaging films developed by immobilizing anthocyanins from purple sweetpotato and purple cabbage in locust bean gum, chitosan and κ-carrageenan-based matrices. Int. J. Biol. Macromol. 2022, 211, 238–248. [Google Scholar] [CrossRef]
- You, P.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: Konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int. J. Biol. Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, I.L.; Lin, C.; Hang, Y.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Intelligent films of marine polysaccharides and purple cauliflower extract for food packaging and spoilage monitoring. Carbohyd. Polym. 2023, 299, 120133. [Google Scholar] [CrossRef]
- Ahmed, M.; Bose, I.; Goksen, G.; Roy, S. Himalayan sources of anthocyanins and its multifunctional applications: A review. Foods 2023, 12, 2203. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects-A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- AlMohammed, H.I.; Khalaf, A.K.; Albalawi, A.E.; Alanazi, A.D.; Baharvand, P.; Moghaddam, A.; Mahmoudvand, H. Chitosan-based nanomaterials as valuable sources of anti-leishmanial agents: A systematic review. Nanomaterials 2021, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.B.; Barreto, E.R.C.; Moraes, C.C.; Morais, M.M.; Rosa, G.S.D. Promising new material for food packaging: An active and intelligent carrageenan film with natural jaboticaba additive. Foods 2022, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A.A. Natural anthocyanins: Sources, extraction, characterization, and suitability for smart packaging. Food Packag. Shelf. 2022, 33, 100872. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, H.; Zhao, J.; Humayun, M.; Wu, S.; Wang, C.; Zhi, Z.; Pang, J. A novel strategy to formulate edible active-intelligent packaging films for achieving dynamic visualization of product freshness. Food Hydrocolloids 2022, 133, 107998. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Bi, Y.H.; Chi, X.W.; Zhang, R.; Lu, Y.H.; Wang, Z.Y.; Dong, Q.; Ding, C.X.; Yang, R.L.; Jiang, L. Highly efficient extraction of mulberry anthocyanins in deep eutectic solvents: Insights of degradation kinetics and stability evaluation. Innov. Food Sci. Emerg. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Jovanovic, M.S.; Krgovic, N.; Zivkovic, J.; Stevic, T.; Zdunic, G.; Bigovic, D.; Savikin, K. Ultrasound-assisted natural deep eutectic solvents extraction of bilberry anthocyanins: Optimization, bioactivities, and storage stability. Plants 2022, 11, 2680. [Google Scholar] [CrossRef]
- Jovanovic, M.S.; Krgovic, N.; Radan, M.; Cujic-Nikolic, N.; Mudric, J.; Lazarevic, Z.; Savikin, K. Natural deep eutectic solvents combined with cyclodextrins: A novel strategy for chokeberry anthocyanins extraction. Food Chem. 2023, 405, 134816. [Google Scholar] [CrossRef]
- Myint, K.Z.; Yu, Q.; Qing, J.; Zhu, S.; Shen, J.; Xia, Y. Botanic antimicrobial agents, their antioxidant properties, application and safety issue. Food Packag. Shelf. 2022, 34, 100924. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhang, H.; Dong, M.; Li, L.; Zhangsun, H.; Wang, L. Dual-functional intelligent gelatin based packaging film for maintaining and monitoring the shrimp freshness. Food Hydrocolloids 2022, 124, 107258. [Google Scholar] [CrossRef]
- Adaku, C.; Skaar, I.; Berland, H.; Byamukama, R.; Jordheim, M.; Andersen, O.M. Anthocyanins from mauve flowers of Erlangea tomentosa (Bothriocline longipes) based on erlangidin-The first reported natural anthocyanidin with C-ring methoxylation. Phytochem. Lett. 2019, 29, 225–230. [Google Scholar] [CrossRef]
- Duan, M.X.; Yu, S.; Sun, J.S.; Jiang, H.X.; Zhao, J.B.; Tong, C.L.; Hu, Y.Q.; Pang, J.; Wu, C.H. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, H.; Qi, D.; Xia, L.; Li, L.; Li, X.; Jiang, S. Multifunctional colorimetric cellulose acetate membrane incorporated with Perilla frutescens (L.) Britt. anthocyanins and chamomile essential oil. Carbohyd. Polym. 2022, 278, 118914. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems-An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhou, T.T.; Wei, C.H.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Da Silva Filipini, G.; Romani, V.P.; Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocolloids 2020, 109, 106139. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent gelatin/oxidized chitin nanocrystals nanocomposite films containing black rice bran anthocyanins for fish freshness monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. Starch and agar-based color-indicator films integrated with shikonin for smart packaging application of shrimp. ACS Food Sci. Technol. 2021, 1, 1963–1969. [Google Scholar] [CrossRef]
- Kong, J.; Ge, X.; Sun, Y.; Mao, M.; Yu, H.; Chu, R.; Wang, Y. Multi-functional pH-sensitive active and intelligent packaging based on highly cross-linked zein for the monitoring of pork freshness. Food Chem. 2023, 404, 134754. [Google Scholar] [CrossRef] [PubMed]
- Moura-Alves, M.; Esteves, A.; Ciríaco, M.; Silva, J.A.; Saraiva, C. Antimicrobial and antioxidant edible films and coatings in the shelf-life improvement of chicken meat. Foods 2023, 12, 2308. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Bomser, J.A.; Schwartzt, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Kang, J.; Guo, X.; Sun, M.; Li, H.; Bai, H.; Cui, H.; Shi, L. pH-responsive chitosan-based film containing oregano essential oil and black rice bran anthocyanin for preserving pork and monitoring freshness. Food Chem. 2023, 403, 134393. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Multilayer photonic films based on interlocked chiral-nematic cellulose nanocrystals in starch/chitosan. Carbohyd. Polym. 2022, 275, 118709. [Google Scholar] [CrossRef]
- Boccalon, E.; Viscusi, G.; Lamberti, E.; Fancello, F.; Zara, S.; Sassi, P.; Marinozzi, M.; Nocchetti, M.; Gorrasi, G. Composite films containing red onion skin extract as intelligent pH indicators for food packaging. Appl. Surf. Sci. 2022, 593, 153319. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Li, L.; Wang, Q.; Jiang, S.; Chen, M.; Li, X.; Shaotong, J. pH-responsive antibacterial film based polyvinyl alcohol/poly (acrylic acid) incorporated with aminoethyl-phloretin and application to pork preservation. Food Res. Int. 2021, 147, 110532. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S. Antimicrobial assessment of polyphenolic extracts from onion (Allium cepa L.) skin of fifteen cultivars by sonication-assisted extraction method. Heliyon 2020, 6, e05478. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: Freshness indicators based on anthocyanin extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Ren, L.; Mei, J.; Xu, Y.; Li, J. Preparation of pH-sensitive polylactic acid-naringin coaxial electrospun fiber membranes for maintaining and monitoring salmon freshness. Int. J. Biol. Macromol. 2021, 188, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocolloids 2022, 129, 107665. [Google Scholar] [CrossRef]
- Gu, R.; Yun, H.; Chen, L.F.; Wang, Q.; Huang, X.J. Regenerated cellulose films with amino-terminated hyperbranched polyamic anchored nanosilver for active food packaging. ACS Appl. Bio Mater. 2020, 3, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohyd. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.W.; Lu, H.T.; Ho, Y.C.; Lu, K.Y.; Wang, P.; Mi, F.L. A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Mat. Sci. Eng. C Mater. 2021, 118, 111396. [Google Scholar] [CrossRef]
- Wagh, R.V.; Khan, A.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Cellulose nanofiber-based multifunctional films integrated with carbon dots and anthocyanins from Brassica oleracea for active and intelligent food packaging applications. Int. J. Biol. Macromol. 2023, 233, 123567. [Google Scholar] [CrossRef]
- González, C.M.O.; Schelegueda, L.I.; Ruiz-Henestrosa, V.M.P.; Campos, C.A.; Basanta, M.F.; Gerschenson, L.N. Cassava starch films with anthocyanins and betalains from agroindustrial by-products: Their use for intelligent label development. Foods 2022, 11, 3361. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, Y.; Lu, D.; Gao, W.; Zhao, Q.; Shi, X. Multifunctional intelligent film integrated with purple sweet potato anthocyanin and quercetin-loaded chitosan nanoparticles for monitoring and maintaining freshness of shrimp. Food Packag. Shelf. 2023, 35, 101022. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Alomgir Hossen, M.; Dai, J.; Qin, W.; Liu, Y. Facile fabrication of sandwich-like anthocyanin/chitosan/lemongrass essential oil films via 3D printing for intelligent evaluation of pork freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of gelatin/carrageenan-based color-indicator film integrated with shikonin and propolis for smart food packaging applications. ACS Appl. Bio Mater. 2020, 4, 770–779. [Google Scholar] [CrossRef]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; et al. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control 2022, 131, 108441. [Google Scholar] [CrossRef]
- Hematian, F.; Baghaei, H.; Nafchi, A.M.; Bolandi, M. Preparation and characterization of an intelligent film based on fish gelatin and anthocyanin to monitor the freshness of rainbow trout fish fillet. Food Sci. Nutr. 2023, 11, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yong, H.M.; Liu, Y.P.; Qin, Y.; Kan, J.; Liu, J. Preparation and characterization of active and intelligent films based on fish gelatin and haskap berries (Lonicera caerulea L.) extract. Food Packag. Shelf. 2019, 22, 100417. [Google Scholar] [CrossRef]
- Kassem, A.; Ayoub, G.M.; Malaeb, L. Antibacterial activity of chitosan nano-composites and carbon nanotubes: A review. Sci. Total Environ. 2019, 668, 566–576. [Google Scholar] [CrossRef]
- Zheng, H.; Deng, W.; Yu, L.; Shi, Y.; Deng, Y.; Wang, D.; Zhong, Y. Chitosan coatings with different degrees of deacetylation regulate the postharvest quality of sweet cherry through internal metabolism. Int. J. Biol. Macromol. 2024, 254, 127419. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuang, S.T. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Tang, H.; Hao, W.; Wu, J.; Sun, Y.; Sun, J.; Liu, Y.; Jiang, L. Development, characterization and application of intelligent/active packaging of chitosan/chitin nanofibers films containing eggplant anthocyanins. Food Hydrocolloids 2023, 139, 108496. [Google Scholar] [CrossRef]
- Luchese, C.L.; Benelli, P.; Spada, J.C.; Tessaro, I.C. Impact of the starch source on the physicochemical properties and biodegradability of different starch-based films. J. Appl. Polym. Sci. 2018, 135, 46564. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; de Farias, P.M.; Satoriva, J.M.; de Andrade, C.J.; Fai, A.E.C. Cassava starch films for food packaging: Trends over the last decade and future research. Int. J. Biol. Macromol. 2023, 225, 658–672. [Google Scholar] [CrossRef]
- Nizam, N.H.M.; Rawi, N.F.M.; Ramle, S.F.M.; Abd Aziz, A.; Abdullah, C.K.; Rashedi, A.; Kassim, M.H.M. Physical, thermal, mechanical, antimicrobial and physicochemical properties of starch based film containing aloe vera: A review. J. Mater. Res. Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- Sun, C.; Wei, Z.H.; Xue, C.H.; Yang, L. Development, application and future trends of starch-based delivery systems for nutraceuticals: A review. Carbohyd Polym. 2023, 308, 120675. [Google Scholar] [CrossRef] [PubMed]
- Kanha, N.; Osiriphun, S.; Rakariyatham, K.; Klangpetch, W.; Laokuldilok, T. On-package indicator films based on natural pigments and polysaccharides for monitoring food quality: A review. J. Sci. Food Agric. 2022, 102, 6804–6823. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, D.; Wei, L.F.; Zhu, W.J.; Yan, X.Q.; Zhou, R.; Din, Z.U.; Ding, W.P.; Ma, T.Z.; Cai, J. Structural and mechanistic insights into starch microgel/anthocyanin complex assembly and controlled release performance. Int. J. Biol. Macromol. 2022, 213, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Zhang, Y. Biopolymer-based encapsulation of anthocyanins as reinforced natural colorants for food applications. J. Agric. Food Res. 2023, 11, 100488. [Google Scholar] [CrossRef]
- Li, J.; Zhu, B.F.; Yu, H.D.; Yuan, M.L.; Chen, H.Y.; Qin, Y.Y. Application of pH-indicating film containing blue corn anthocyanins on corn starch/polyvinyl alcohol as substrate for preservation of tilapia. J. Food Meas. Charact. 2022, 16, 4416–4424. [Google Scholar] [CrossRef]
- Bekhit, A.E.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total volatile basic nitrogen and trimethylamine in muscle foods: Potential formation pathways and effects on human health. Compr. Rev. Food Sci. Food Saft. 2021, 20, 3620–3666. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf. 2020, 24, 100506. [Google Scholar] [CrossRef]
- Liu, X.X.; Song, X.S.; Gou, D.J.; Li, H.L.; Jiang, L.; Yuan, M.L.; Yuan, M.W. A polylactide based multifunctional hydrophobic film for tracking evaluation and maintaining beef freshness by an electrospinning technique. Food Chem. 2023, 428, 136784. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.T.; Tsai, I.L.; Ho, Y.C.; Hang, Y.H.; Lin, C.; Tsai, M.L.; Mi, F.L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohyd Polym. 2021, 254, 117410. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Zhong, Y.; Wang, D.; Deng, Y. Active and Intelligent Biodegradable Packaging Based on Anthocyanins for Preserving and Monitoring Protein-Rich Foods. Foods 2023, 12, 4491. https://doi.org/10.3390/foods12244491
Zhu B, Zhong Y, Wang D, Deng Y. Active and Intelligent Biodegradable Packaging Based on Anthocyanins for Preserving and Monitoring Protein-Rich Foods. Foods. 2023; 12(24):4491. https://doi.org/10.3390/foods12244491
Chicago/Turabian StyleZhu, Bifen, Yu Zhong, Danfeng Wang, and Yun Deng. 2023. "Active and Intelligent Biodegradable Packaging Based on Anthocyanins for Preserving and Monitoring Protein-Rich Foods" Foods 12, no. 24: 4491. https://doi.org/10.3390/foods12244491
APA StyleZhu, B., Zhong, Y., Wang, D., & Deng, Y. (2023). Active and Intelligent Biodegradable Packaging Based on Anthocyanins for Preserving and Monitoring Protein-Rich Foods. Foods, 12(24), 4491. https://doi.org/10.3390/foods12244491






