Screening and Characterization of an α-Amylase Inhibitor from Carya cathayensis Sarg. Peel
Abstract
:1. Introduction
2. Material and Method
2.1. Material and Chemical Reagents
2.2. Preparation of Extracts from CCSP
2.3. α-Amylase Inhibition Activity Assay
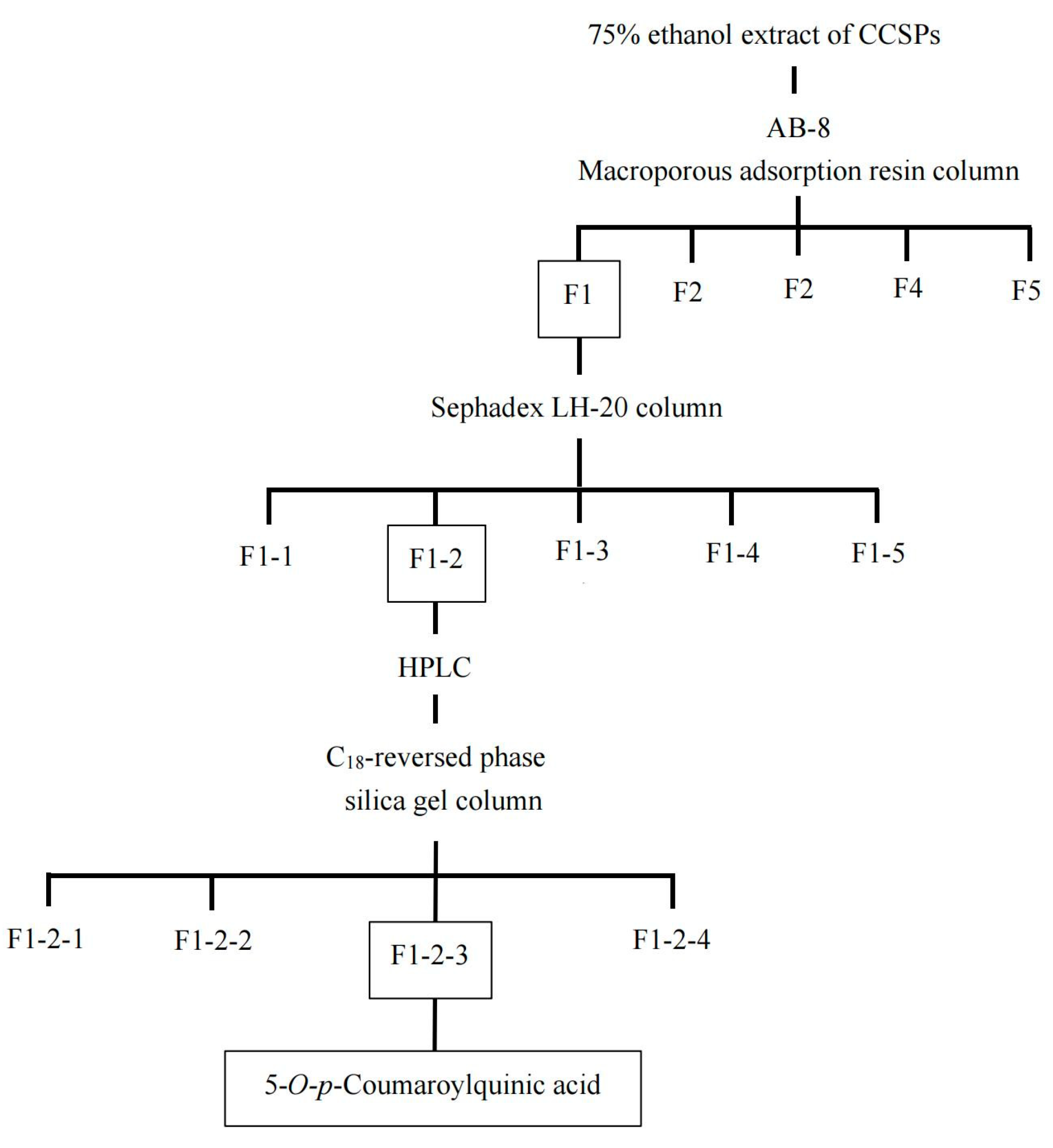
2.4. Isolation of α-Amylase Inhibitor
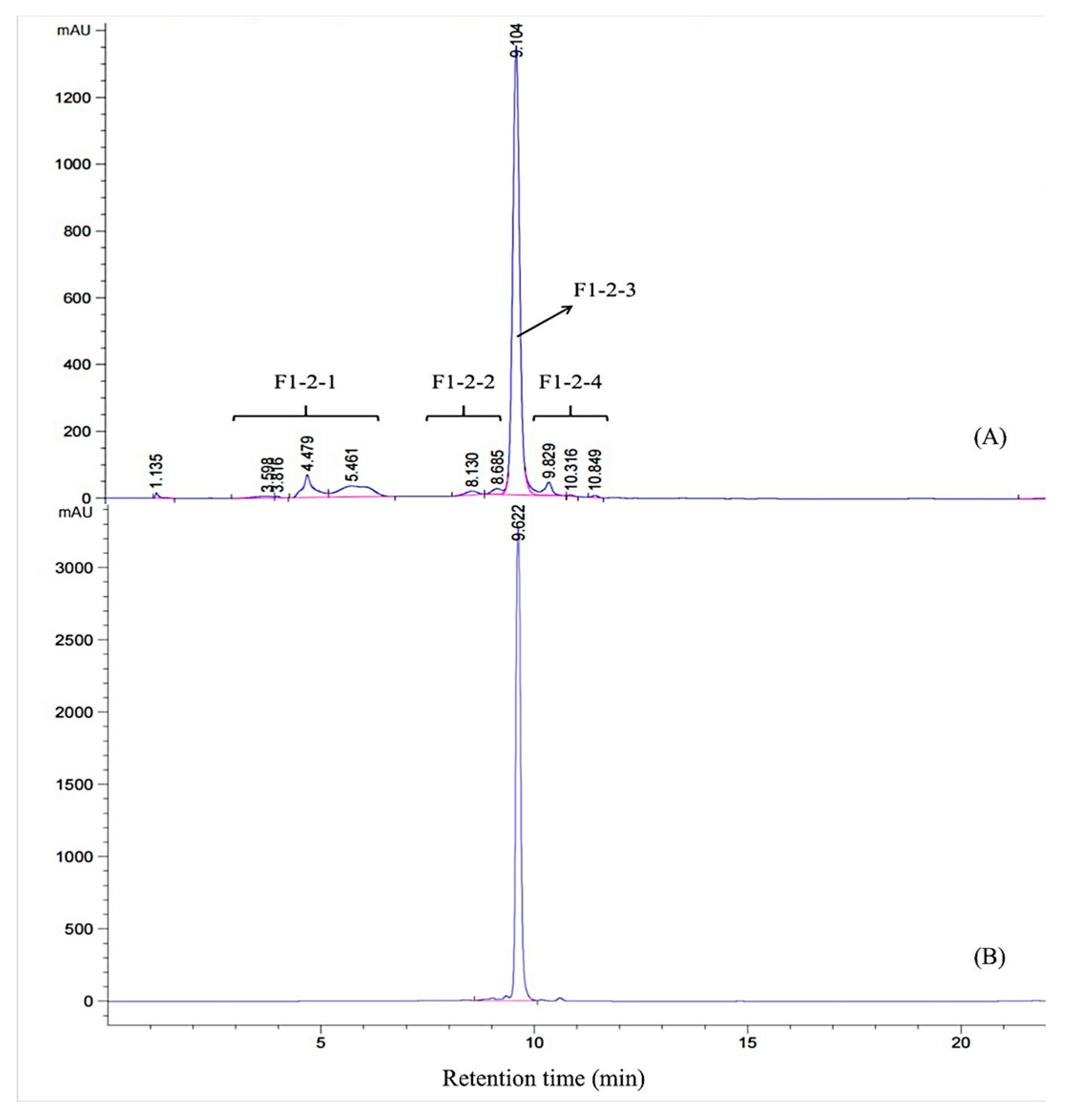
2.5. HPLC Analysis
2.6. Nuclear Magnetic Resonance (NMR) and Q-Exactive Orbitrap Mass Spectrometry Assays
2.7. Inhibitory Kinetics Analysis
2.8. Statistical Analysis
3. Results and Discussion
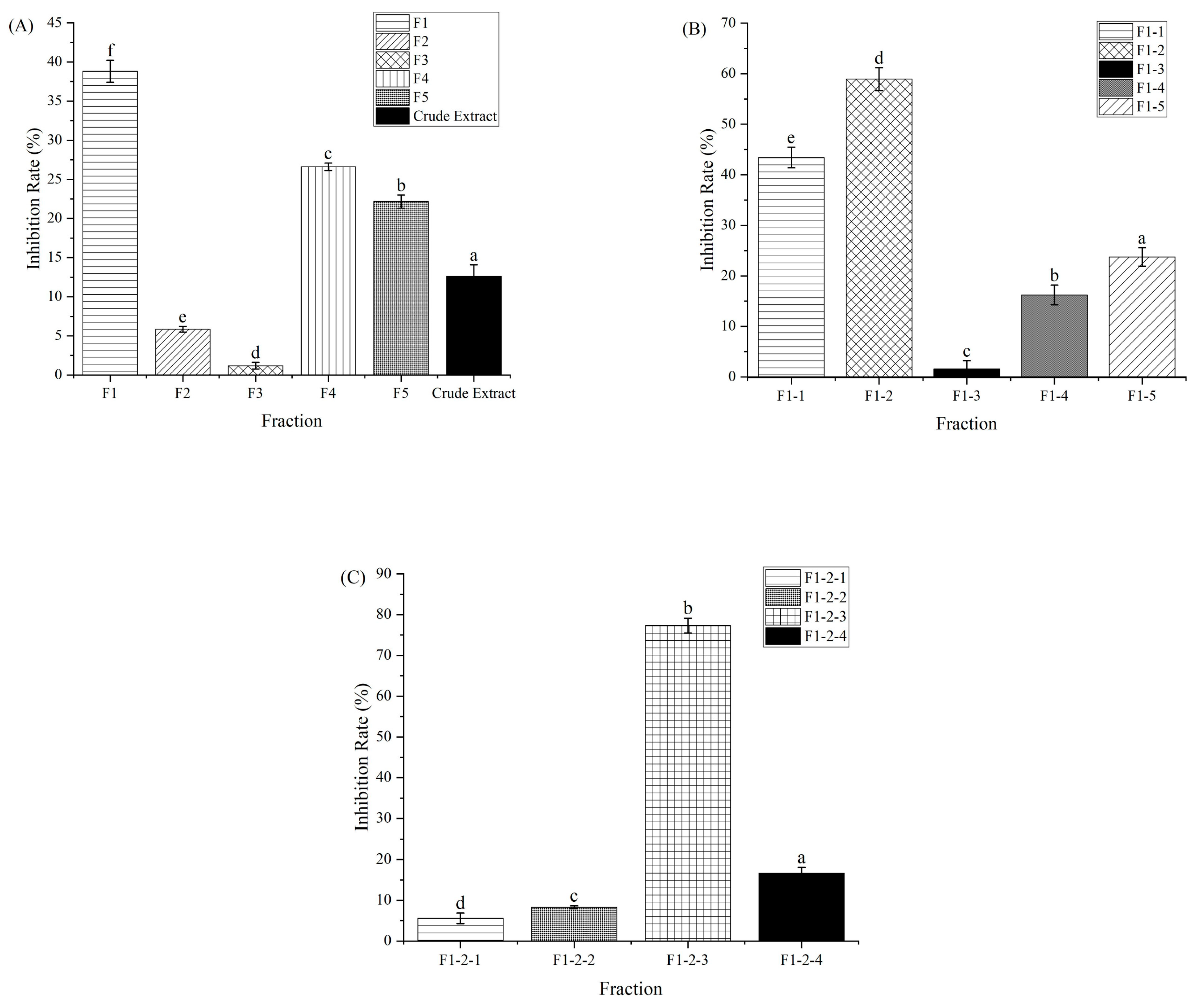
3.1. Purification of α-Amylase Inhibitor from CCSP
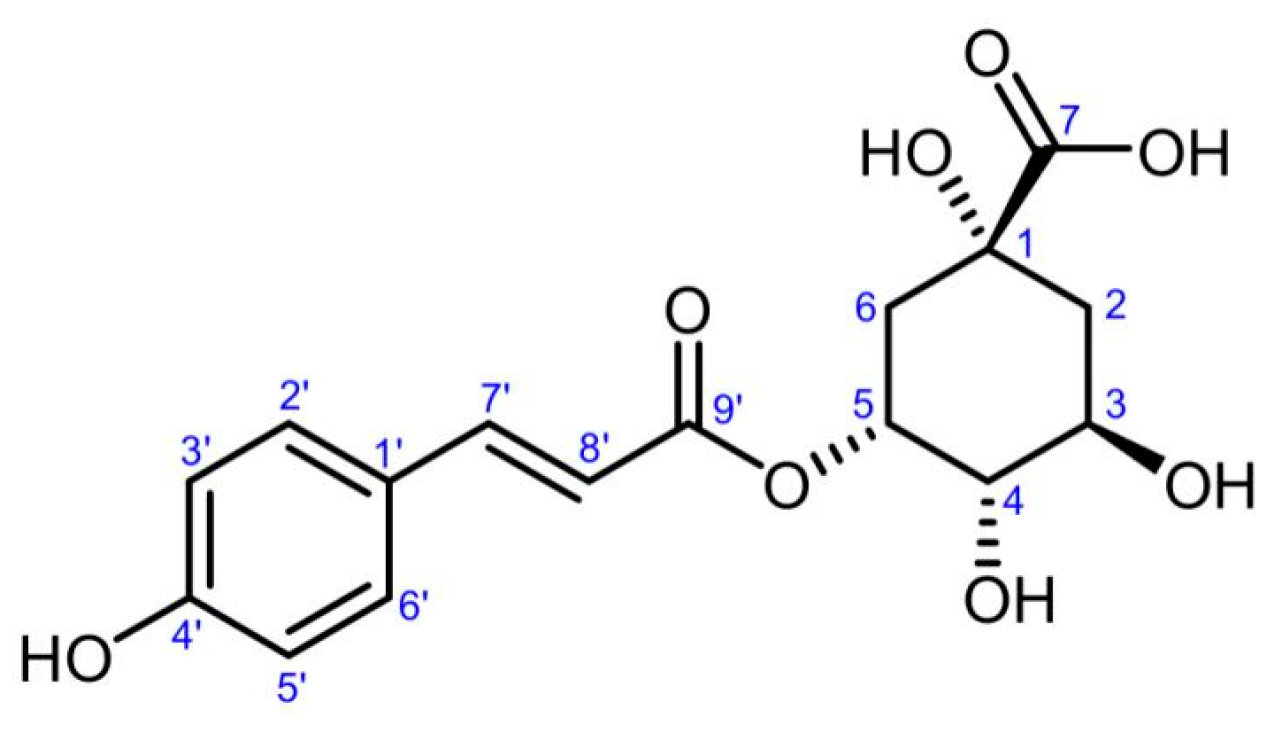
3.2. Identification of Active Compound
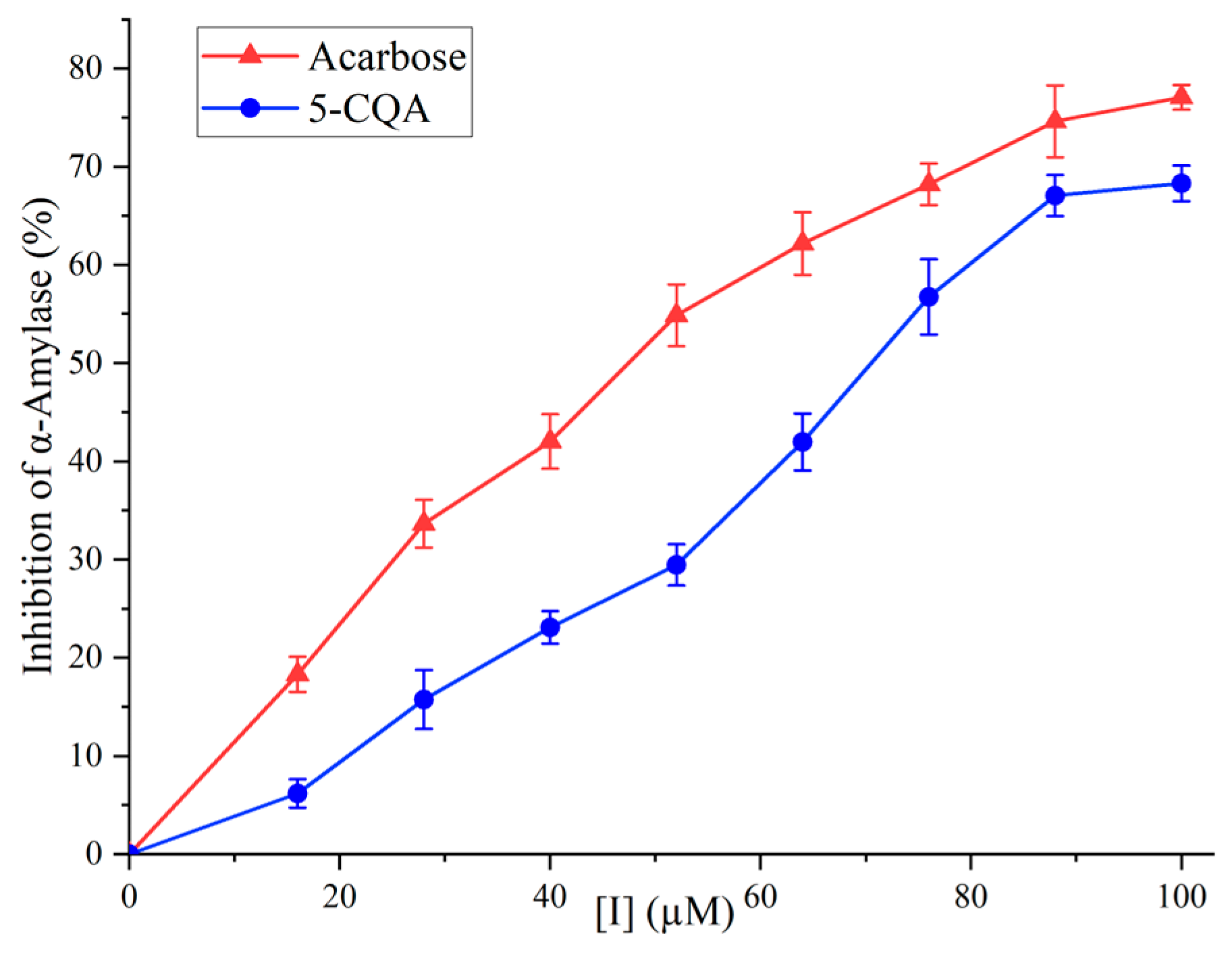
3.3. Inhibitory Activity of 5-CQA against α-Amylase
3.4. Inhibitory Kinetics of 5-CQA against α-Amylase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Liu, X.; Sun, L. Inconsistency between polyphenol-enzyme binding interactions and enzyme inhibition: Galloyl moiety decreases amyloglucosidase inhibition of catechins. Food Res. Int. 2023, 163, 112155. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ng, K.; Zhang, P.; Warner, R.D.; Shen, S.; Tang, H.; Liang, Z.; Fang, Z. In vitro α-glucosidase and α-amylase inhibitory activities of free and bound phenolic extracts from the bran and kernel fractions of five sorghum grain genotypes. Foods 2020, 9, 1301. [Google Scholar] [CrossRef] [PubMed]
- Farazi, M.; Houghton, M.J.; Murray, M.; Williamson, G. A systematic review of the inhibitory effect of extracts from edible parts of nuts on α-glucosidase activity. Food Funct. 2023, 14, 5962–5976. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Zhao, Y. Phenolic compounds in whole grain sorghum and their health benefits. Foods 2021, 10, 1921. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, G.; Muniyandi, K.; Manoharan, A.L.; Nataraj, G.; Thangaraj, P. Understanding the bioaccessibility, α-amylase and α-glucosidase enzyme inhibition kinetics of Allmania nodiflora (L.) R.Br. ex Wight polyphenols during in vitro simulated digestion. Food Chem. 2021, 372, 131294. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Eremia, S.A.V.; Radu, G.L. Artemisia abrotanum and Symphytum officinale polyphenolic compounds-rich extracts with potential application in diabetes management. Metabolites 2023, 13, 354. [Google Scholar] [CrossRef]
- Taylor, R.H.; Jenkins, D.J.; Barker, H.M.; Fielden, H.; Goff, D.V.; Misiewicz, J.J.; Lee, D.A.; Allen, H.B.; MacDonald, G.; Wallrabe, H. Effect of acarbose on the 24-hour blood glucose profile and pattern of carbohydrate absorption. Diabetes Care 1982, 5, 92–96. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review: Antidiabetic effects of phenolic acids. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Cui, J.; Gu, X.; Wang, F.; Ouyang, J.; Wang, J. Purification and structural characterization of an α-glucosidase inhibitory polysaccharide from apricot (Armeniaca sibirica L. Lam.) pulp. Carbohydr. Polym. 2015, 121, 309–314. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; He, Y.; Xu, Y.; Li, L.; Luo, Z. UPLC-Triple-TOF/MS characterization of phenolic constituents and the influence of natural deep eutectic solvents on extraction of Carya cathayensis Sarg. peels: Composition, extraction mechanism and in vitro biological activities. Food Chem. 2022, 370, 131042. [Google Scholar] [CrossRef]
- Lin, Q.; Qiu, C.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Tian, Y.; Jin, Z. The inhibitory mechanism of amylase inhibitors and research progress in nanoparticle-based inhibitors. Crit. Rev. Food Sci. Nutr. 2022, 14, 761–773. [Google Scholar] [CrossRef]
- Nasab, S.B.; Homaei, A.; Karami, L. Kinetic of α-amylase inhibition by Gracilaria corticata and Sargassum angustifolium extracts and zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 23, 101478. [Google Scholar] [CrossRef]
- Kawamura-Konishi, Y.; Watanabe, N.; Saito, M.; Nakajima, N.; Sakaki, T.; Katayama, T.; Enomoto, T. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga Sargassum patens. J. Agric. Food Chem. 2012, 60, 5565–5570. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Wang, X.; Zhang, C.; Feng, G.; Peng, X. Inhibition mechanism of α-amylase/α-glucosidase by silibinin, its synergism with acarbose, and the effect of milk proteins. J. Agric. Food Chem. 2021, 69, 10515–10526. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lian, W.; Li, T.; Rao, Z.; Li, Y.; Qian, H.; Zhang, H.; Qi, X.; Wang, L. Characterization of promising natural blue pigment from Vaccinium bracteatum thunb. leaves: Insights of the stability and the inhibition of α-amylase. Food Chem. 2020, 326, 126962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z.; Liu, G.; Wu, Y.; Ouyang, J. Inhibitory effect of chestnut (Castanea mollissima Blume) inner skin extract on the activity of α-amylase, α-glucosidase, dipeptidyl peptidase IV and in vitro digestibility of starches. Food Chem. 2020, 324, 126847. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Tech. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.S.; Dzuvor, C.K.O.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, Y.; Yi, X.; Wang, X.; He, X. Chemical constituent and antioxidant activity of the husk of Chinese hickory. J. Funct. Foods 2016, 23, 378–388. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Belwal, T.; Li, L.; Luo, Z. Phytosterols extraction from hickory (Carya cathayensis Sarg.) husk with a green direct citric acid hydrolysis extraction method. Food Chem. 2020, 315, 126217. [Google Scholar] [CrossRef] [PubMed]
- Lazar, L.; Talmaciu, A.I.; Volf, I.; Popa, V.I. Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochem. 2016, 32, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, T.; Shintani, T.; Sato, H. α-Amylase inhibitory activity from nut seed skin polyphenols. 1. Purification and characterization of almond seed skin polyphenols. J. Agric. Food Chem. 2013, 61, 4570–4576. [Google Scholar] [CrossRef]
- Yonemoto, R.; Shimada, M.; Gunawan-Puteri, M.D.; Kato, E.; Kawabata, J. α-Amylase inhibitory triterpene from Abrus precatorius leaves. J. Agric. Food Chem. 2014, 62, 8411–8414. [Google Scholar] [CrossRef]
- Borah, P.K.; Sarkar, A.; Duary, R.K. Water-soluble vitamins for controlling starch digestion: Conformational scrambling and inhibition mechanism of human pancreatic α-amylase by ascorbic acid and folic acid. Food Chem. 2019, 288, 395–404. [Google Scholar] [CrossRef]
- Dong, W.; Wang, D.; Hua, R.; Long, Y.; Lv, L. Chemical composition, structural and functional properties of soluble dietary fiber obtained from coffee peel using different extraction methods. Food Res. Int. 2020, 136, 109497. [Google Scholar] [CrossRef]
- Nagar, S.; Pigott, M.; Kukula-Koch, W.; Sheridan, H. Unravelling novel phytochemicals and anticholinesterase activity in Irish cladonia portentosa. Molecules 2023, 28, 4145. [Google Scholar] [CrossRef]
- Chen, J.; Mangelinckx, S.; Ma, L.; Wang, Z.; Li, W.; De Kimpe, N. Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast α-glucosidase and PTP1B inhibitory activity. Fitoterapia 2014, 99, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Foo, L.Y.; McNabb, W.C.; Molan, A.L. Phenolic glycosides of forage legume Onobrychis viciifolia. Phytochemistry 2000, 55, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory effects of edible seaweeds, polyphenolics and alginates on the activities of porcine pancreatic α-amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-alucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Gao, Y.; Zhang, X.; Sun, Y.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X. Effects of Oolong tea polyphenols, EGCG, and EGCG3″Me on pancreatic α-amylase activity in vitro. J. Agric. Food Chem. 2014, 62, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Loukili, E.; Bouchal, B.; Bouhrim, M.; Abrigach, F.; Genva, M.; Zidi, K.; Bnouham, M.; Bellaoui, M.; Hammouti, B.; Addi, M.; et al. Chemical composition, antibacterial, antifungal and antidiabetic activities of ethanolic extracts of Opuntia dillenii fruits collected from Morocco. J. Food Qual. 2022, 2022, 9471239. [Google Scholar] [CrossRef]
- Hui, X.; Wu, G.; Han, D.; Stipkovits, L.; Wu, X.; Tang, S.; Brennan, M.A.; Brennan, C.S. The effects of bioactive compounds from blueberry and blackcurrant powders on the inhibitory activities of oat bran pastes against α-amylase and α-glucosidase linked to type 2 diabetes. Food Res. Int. 2020, 138, 109756. [Google Scholar] [CrossRef]
- Yi, J.; Zhao, T.; Zhang, Y.; Tan, Y.; Han, X.; Tang, Y.; Chen, G. Isolated compounds from Dracaena angustifolia Roxb and acarbose synergistically/additively inhibit α-glucosidase and α-amylase: An in vitro study. BMC Complement. Med. Ther. 2022, 22, 177. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Tech. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Janeček, S.; Svensson, B. Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochim. Biophys. Acta 2001, 1546, 1–20. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Singh, A.K.; Rana, H.K.; Singh, V.; Chand Yadav, T.; Varadwaj, P.; Pandey, A.K. Evaluation of antidiabetic activity of dietary phenolic compound chlorogenic acid in streptozotocin induced diabetic rats: Molecular docking, molecular dynamics, in silico toxicity, in vitro and in vivo studies. Comput. Biol. Med. 2021, 134, 104462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.X.; Yang, W.H.; Sun, W.X.; Chen, S.G.; Liu, D.H.; Kong, X.L.; Tian, J.H.; Ye, X.Q. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Claasen, B.; Fischer, M.; Buchweitz, M. Interaction of structurally diverse phenolic compounds with porcine pancreatic α-amylase. J. Agric. Food Chem. 2019, 67, 11108–11118. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New insights into the inhibition mechanism of betulinic acid on α-glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Azam, U.; Mehreen, S.; Naseer, M.M. Synthetic α-glucosidase inhibitors as promising anti-diabetic agents: Recent developments and future challenges. Eur. J. Med. Chem. 2023, 249, 115119. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.B.; Lee, H.S.; Lee, S.Y.; Baek, J.S.; Kim, D.; Moon, T.W.; Robyt, J.F.; Park, K.H. Comparative study of the inhibition of alpha-glucosidase, alpha-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine-glucose. Arch. Biochem. Biophys. 1999, 371, 277–283. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Zhang, H.; Dai, H.Q.; Zhao, P.; Mao, Y.F.; Chen, K.X.; Chen, Z.X. Inhibition of starch digestion: The role of hydrophobic domain of both α-amylase and substrates. Food Chem. 2021, 341, 128211. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Gourinath, S.; Dey, S.; Srinivasan, A.; Singh, T.P. Substrate-inhibitor interactions in the kinetics of alpha-amylase inhibition by ragi alpha-amylase/trypsin inhibitor (RATI) and its various N-terminal fragments. Biochemistry 2001, 40, 4229–4233. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Yoon, H.G.; Kim, Y.W.; Lee, H.S.; Kim, J.W.; Kweon, K.S.; Oh, B.H.; Park, K.H. Molecular and enzymatic characterization of a maltogenic amylase that hydrolyzes and transglycosylates acarbose. Eur. J. Biochem. 1998, 253, 251–262. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food polyphenols and type II diabetes mellitus: Pharmacology and mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]

| Concentration of 5-CQA (mM) | Michaelis Constant (Km) (µM) | Maximum Velocity (Vmax) (µM·min−1) | Constant for Competitive Inhibition (Kic) (µM) | Constant for Noncompetitive Inhibition (Kin) (µM) |
|---|---|---|---|---|
| 0 | 0.64 | 144.30 | 0.38 ± 0.0022 | 0.38 ± 0.0024 |
| 0.20 | 0.65 | 110.62 | ||
| 0.40 | 0.64 | 79.94 | ||
| 0.60 | 0.64 | 58.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Huang, G.; Liu, H.; Chen, W.; Zhao, J.; Jia, Z.; Tao, F. Screening and Characterization of an α-Amylase Inhibitor from Carya cathayensis Sarg. Peel. Foods 2023, 12, 4425. https://doi.org/10.3390/foods12244425
Zhang X, Huang G, Liu H, Chen W, Zhao J, Jia Z, Tao F. Screening and Characterization of an α-Amylase Inhibitor from Carya cathayensis Sarg. Peel. Foods. 2023; 12(24):4425. https://doi.org/10.3390/foods12244425
Chicago/Turabian StyleZhang, Xiaosan, Guangrong Huang, Hua Liu, Wenwei Chen, Jing Zhao, Zhenbao Jia, and Fei Tao. 2023. "Screening and Characterization of an α-Amylase Inhibitor from Carya cathayensis Sarg. Peel" Foods 12, no. 24: 4425. https://doi.org/10.3390/foods12244425
APA StyleZhang, X., Huang, G., Liu, H., Chen, W., Zhao, J., Jia, Z., & Tao, F. (2023). Screening and Characterization of an α-Amylase Inhibitor from Carya cathayensis Sarg. Peel. Foods, 12(24), 4425. https://doi.org/10.3390/foods12244425






