Effect of pH, Reducing Sugars, and Protein on Roasted Sunflower Seed Aroma Volatiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Roasting Methods
2.1.1. Pretreatments
2.1.2. Roasting
2.2. Headspace Volatile Quantification
2.2.1. Sample Preparation
2.2.2. SIFT-MS
2.3. Color
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
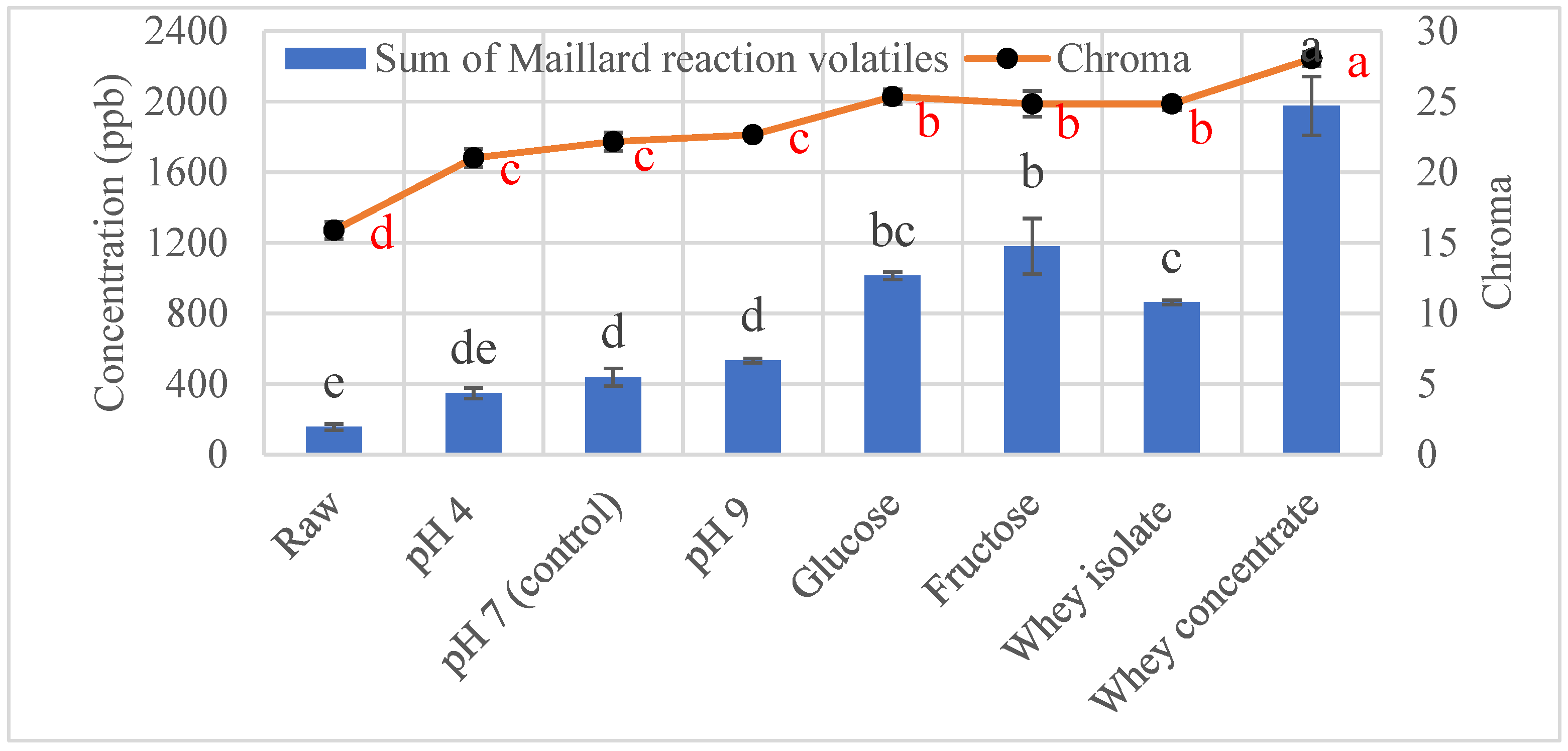
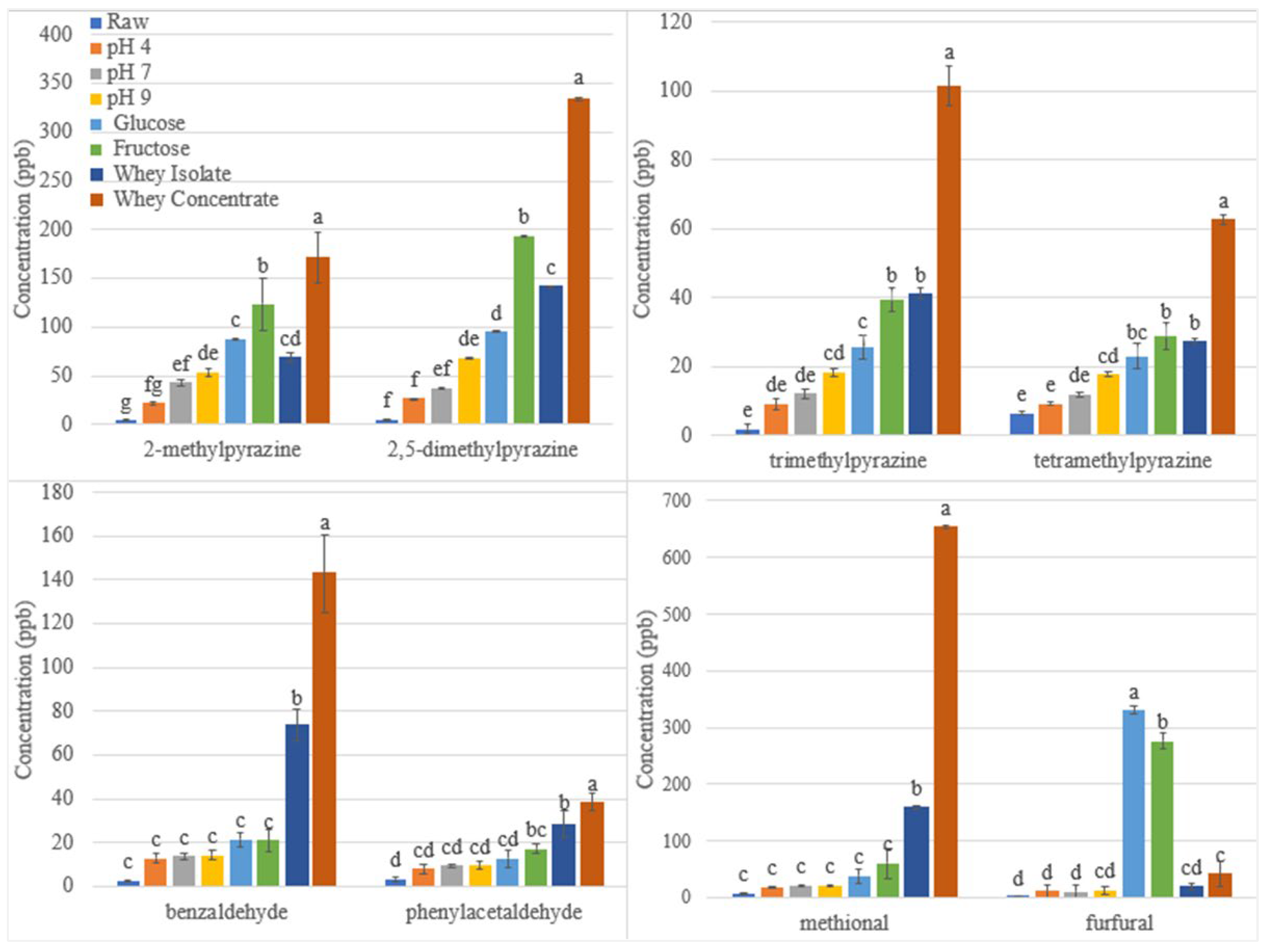
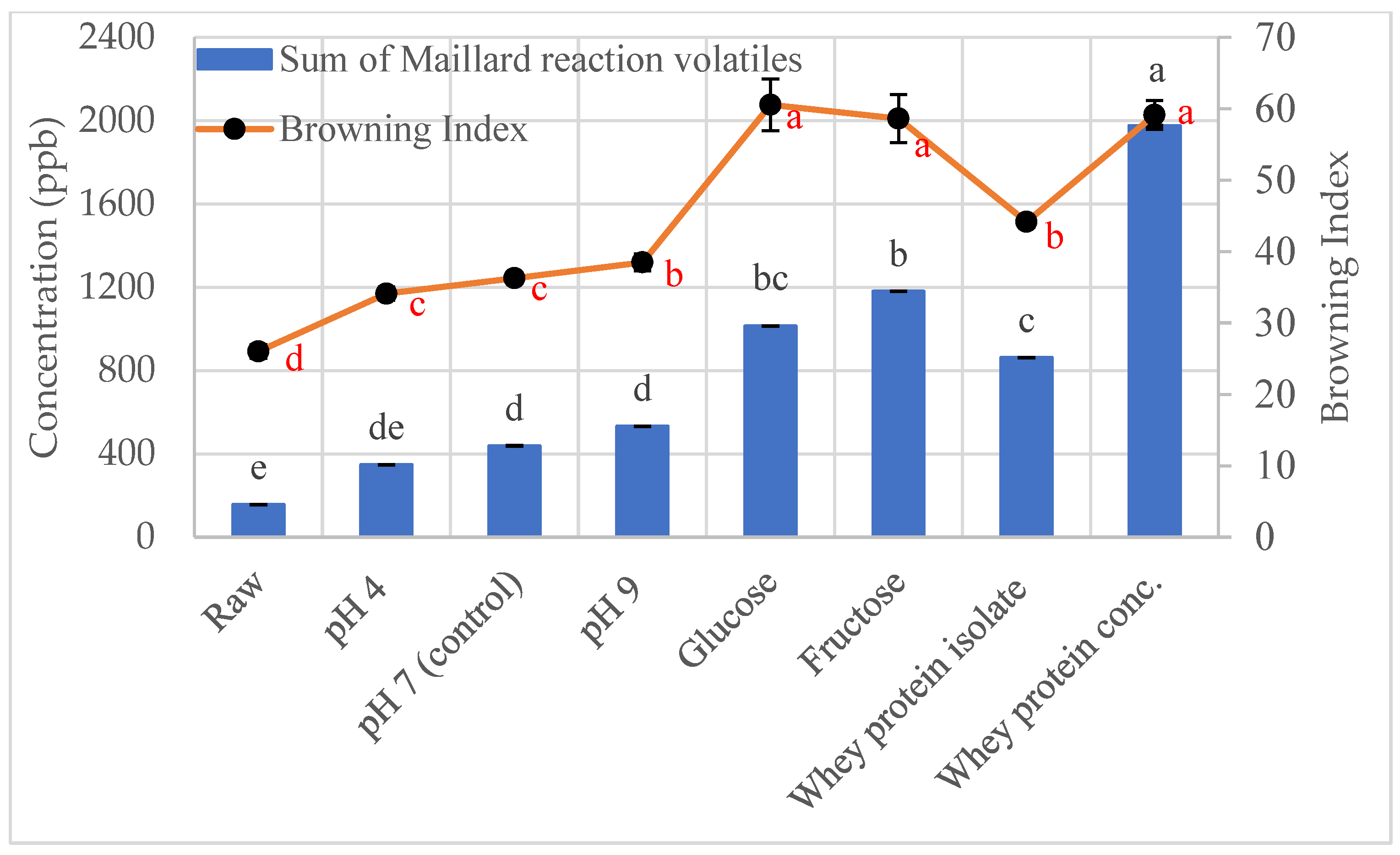
3.1. Effect of Roasting on Maillard Reaction Volatiles
3.1.1. Effect of pH on Maillard Reaction Volatiles in Roasted Sunflower Seeds
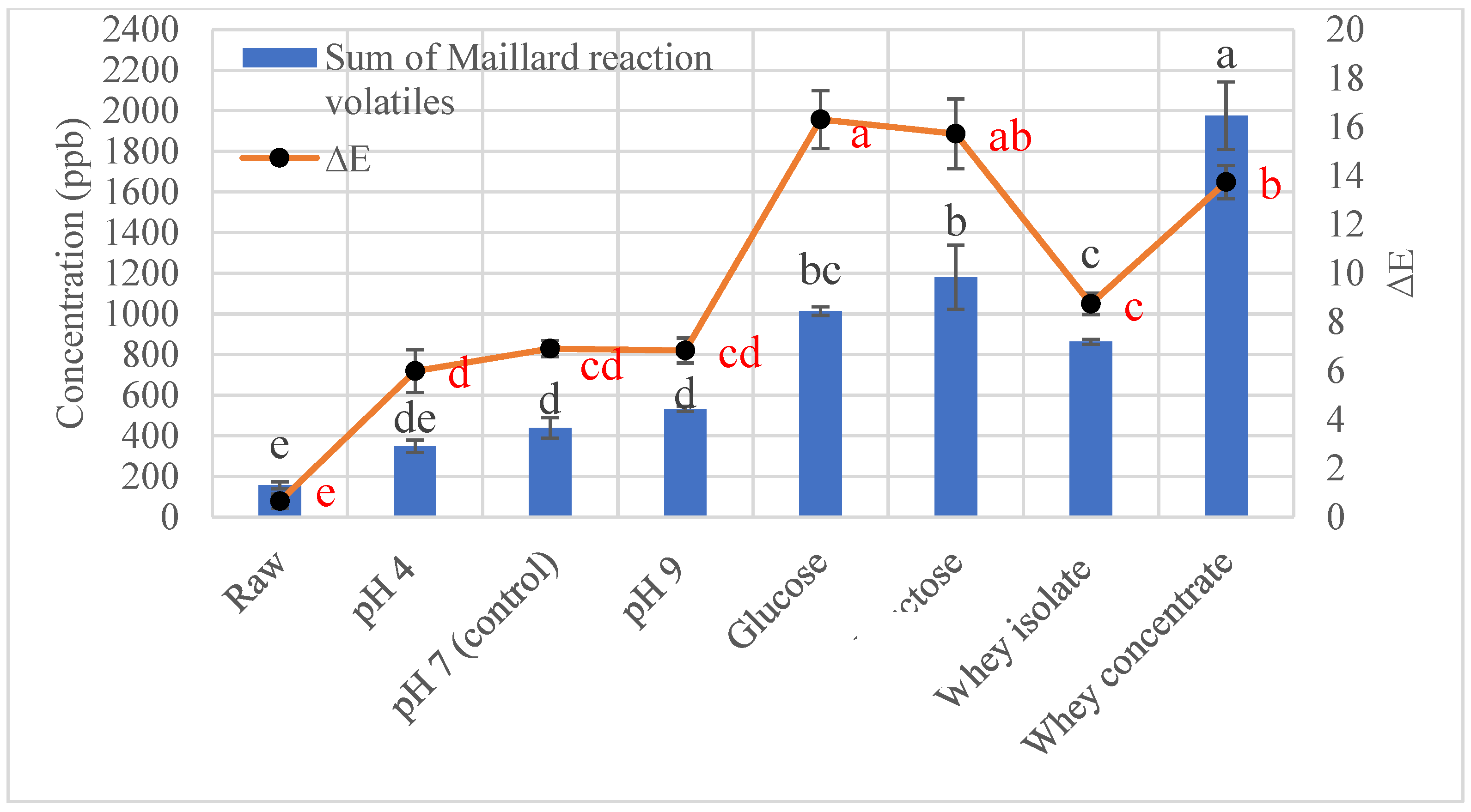
3.1.2. Effect of Reducing Sugars on Maillard Reaction Volatiles in Roasted Sunflower Seeds
3.1.3. Effect of Protein on Maillard Reaction Volatiles in Roasted Sunflower Seeds
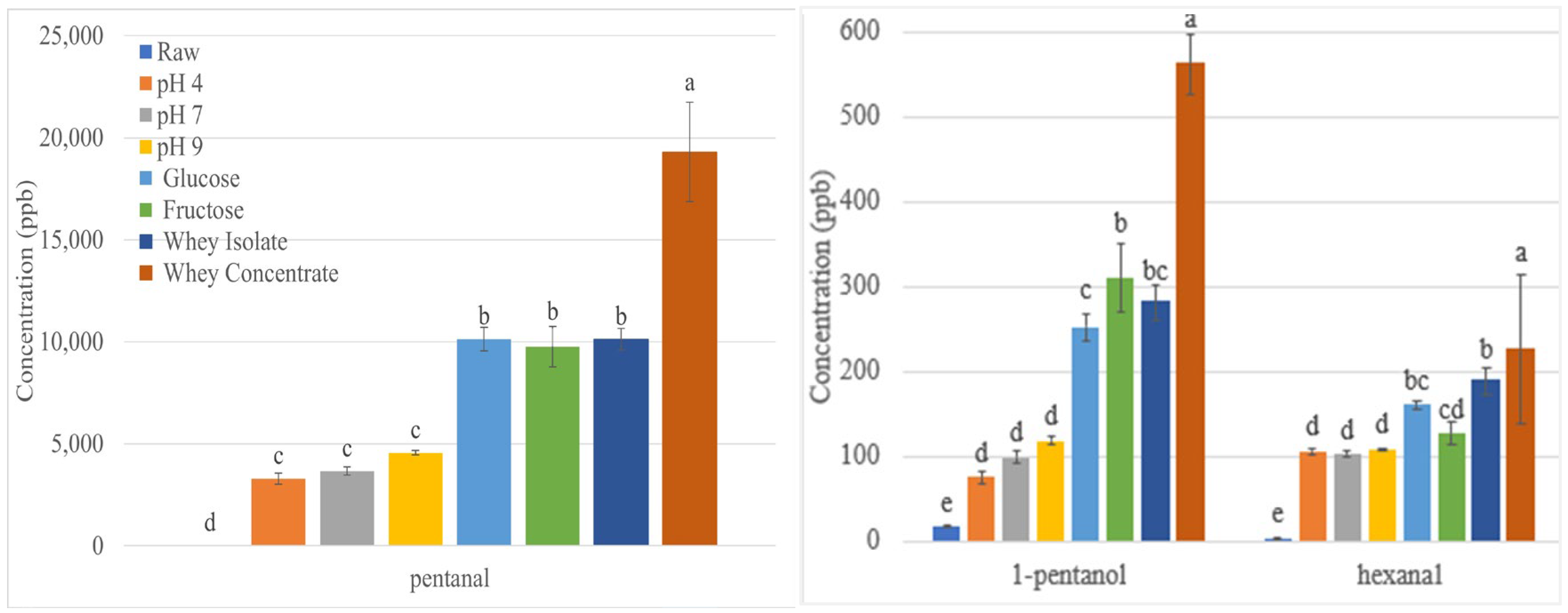
3.2. Effect of Roasting on Lipid Oxidation Volatiles
3.2.1. Effect of pH on Lipid Oxidation Volatiles in Roasted Sunflower Seeds
3.2.2. Effect of Reducing Sugars on Lipid Oxidation Volatiles in Roasted Sunflower Seeds
3.2.3. Effect of Whey Protein on Lipid Oxidation Volatiles in Roasted Sunflower Seeds
3.3. Color
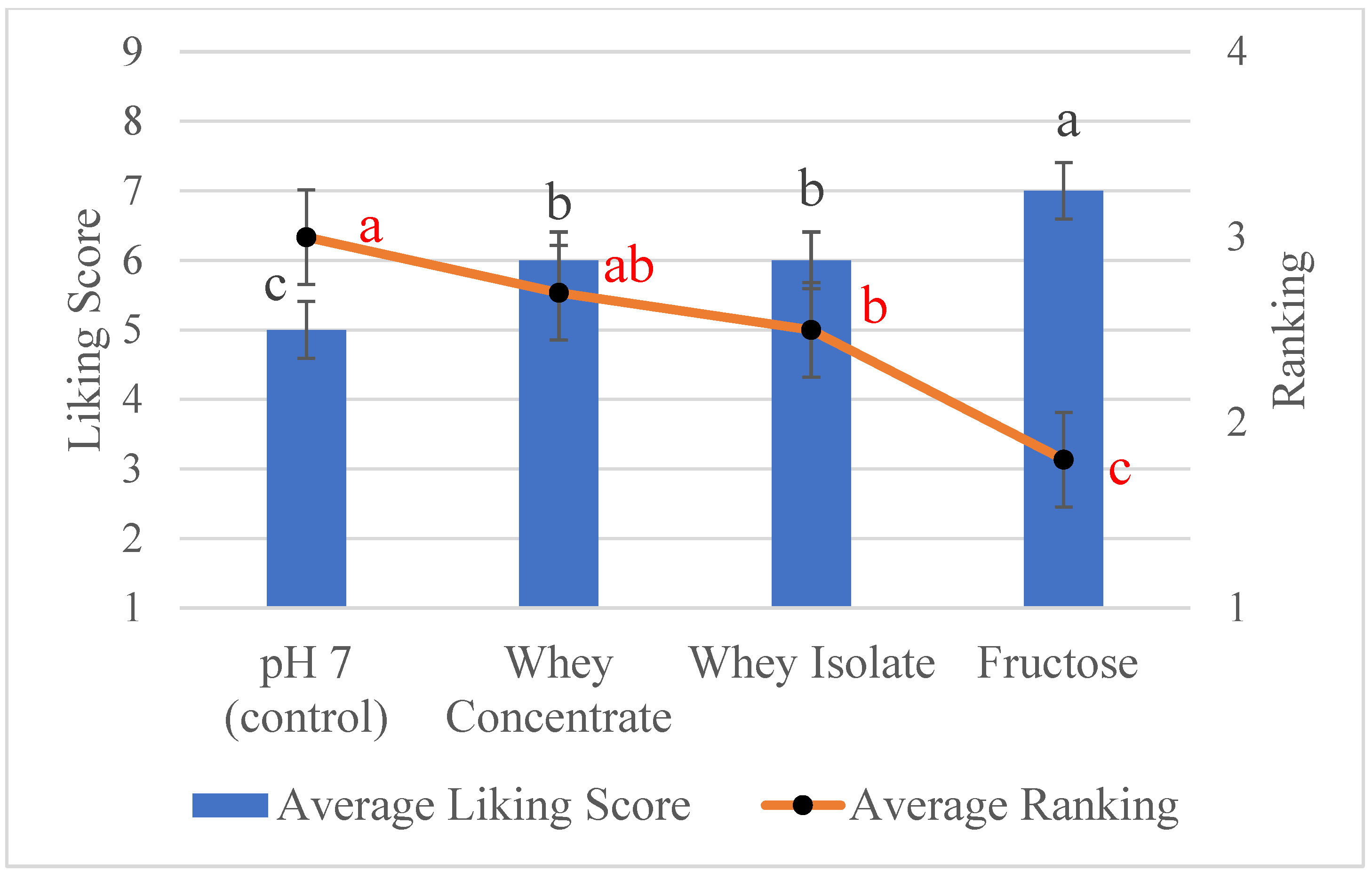
3.4. Sensory
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Volatile | pH 4 | pH 9 | 20% Glucose | 20% Fructose | 17% Whey Isolate | 17% Whey Concentrate |
|---|---|---|---|---|---|---|
| 1,4-butyrolactone | 0 | 0 | 0.05 | 0 | 0.33 | 0.03 |
| 1-octanol | 0 | 0.02 | 0.42 | 0 | 0.66 | 0.06 |
| 1-pentanol | 0 | 0 | 1.33 | 0.88 | 1.79 | 3.64 |
| 2,3-diethyl-5-methylpyrazine | 0 | 0.07 | 0.02 | 0.03 | 0.05 | 0.04 |
| dimethylpyrazine isomers | 0.03 | 0.03 | 0.10 | 0.03 | 0.01 | 0 |
| 2-decenal | 0.40 | 0 | 0.23 | 0.24 | 0.15 | 0.09 |
| 2-heptenal | 0 | 0 | 0 | 0 | 0.07 | 0.04 |
| 2-isopropyl-3-methoxypyrazine | 0 | 0 | 0 | 0 | 0 | 0 |
| 2-methylpyrazine | 0.24 | 0.21 | 0.29 | 0.36 | 0.54 | 0.27 |
| 2-pentylfuran | 0.40 | 0.31 | 0.20 | 0.42 | 0.55 | 0.83 |
| 3-methyl-2-ethylpyrazine isomers | 0.53 | 0.28 | 0.31 | 0.18 | 0.30 | 0 |
| hydroxymethylfurfural (HMF) | 0.44 | 0.28 | 0.58 | 0.28 | 0.29 | 0.58 |
| Pinene isomers | 0.52 | 0.27 | 0.54 | 0.46 | 0.43 | 0.60 |
| benzaldehyde | 1.74 | 1.13 | 0.44 | 0.23 | 2.41 | 2.02 |
| decanal | 0.59 | 0.29 | 1.69 | 0.83 | 0.42 | 0.37 |
| furfural | 0.19 | 0.08 | 0.53 | 0.19 | 0.39 | 0.31 |
| hexanal | 0 | 0 | 0.56 | 0.09 | 6.78 | 25.5 |
| methional | 0.19 | 0.08 | 0.17 | 0 | 0.23 | 0.22 |
| nonanal | 0.75 | 0.40 | 2.76 | 1.16 | 0.81 | 1.79 |
| octanal | 0.10 | 0.04 | 0.49 | 0.50 | 0.50 | 1.09 |
| pentanal | 0.21 | 0.06 | 0.48 | 0.25 | 1.97 | 8.57 |
| phenylacetaldehyde | 0.86 | 0.92 | 0.42 | 0.47 | 0.39 | 0.23 |
| pyrazine | 0.08 | 0.12 | 0.20 | 0.08 | 0.23 | 0.04 |
| tetramethylpyrazine | 0.07 | 0.03 | 0.09 | 0.04 | 0.06 | 0 |
| trans-2-undecenal | 0.06 | 0 | 0 | 0.13 | 0.07 | 0.37 |
| trimethylpyrazine | 0.18 | 0.20 | 0.21 | 0.22 | 0.22 | 0.18 |
References
- National Sunflower Association. All about Sunflower. 2023. Available online: https://www.sunflowernsa.com/all-about/ (accessed on 14 November 2023).
- Ezra, L.; Yudo, B.; Msafiri, R. The Effect of dehulling on physicochemical properties of seed oil cake quality of sunflower. TAJAS 2014, 13, 41–47. [Google Scholar]
- Sharma, P.; Chaturvedi, N.; Walia, M. Effect of some processing on the proximate and antinutrients composition of Helianthus Annus seed. Int. J. Biotechnol. 2013, 3, 67–72. [Google Scholar]
- Soleimanieh, S.; Eshaghi, M.; Vanak, Z. The effect of roasting method and conditions on physic chemical and sensory properties of sunflower seed kernels. Int. J. Biosci. 2015, 6, 7–17. [Google Scholar]
- Mosayebi, M.; Kashaninejad, M.; Najafian, L. Optimizing physiochemical and sensory properties of infrared-hot air roasted sunflower kernels using response surface methodology. J. Food Qual. 2018, 4186050. [Google Scholar] [CrossRef]
- Tenyang, N.; Ponka, R.; Tiencheu, B.; Djikeng, F.; Azaera, T.; Karuna, M.; Prasad, R.; Womeni, H. Effect of boiling and roasting on proximate composition, lipid oxidation, fatty acid profile, and mineral content of two sesame varieties commercialized and consumed in Far-North region of Cameroon. Food Chem. 2017, 221, 1308–1316. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Zhang, M.; Hayat, K.; Yu, J.; Xia, S.; Zhai, Y.; Zhang, X. Improving the Flavor and Oxidation Resistance of Processed Sunflower Seeds with Maillard Peptides. Food Biosci. 2019, 12, 809–819. [Google Scholar] [CrossRef]
- Guo, S.; Na Jom, K.; Ge, Y. Influence of roasting condition on flavor profile of sunflower seeds a flavoromics approach. Sci. Rep. 2019, 9, 11295. [Google Scholar] [CrossRef]
- Zhu, M.; Shen, X.; Chen, J.; Yang, T.; Hou, R. Determination of Volatile Compounds of Chinese Traditional Aromatic Sunflower Seeds (Helianthus annulus L.). Int. J. Food Eng. 2015, 11, 85–95. [Google Scholar] [CrossRef]
- Yin, W.; Shi, R.; Li, S.; Ma, X.; Wang, A. Changes in key aroma-active compounds and sensory characteristics of sunflower oils induced by seed roasting. J. Food Sci. 2021, 87, 699–713. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, H.; Woo, K.; Lee, J.; Jeong, H. Biological Activities of Maillard Reaction Products (MRPs) in Sugar-Amino Acid Model System. Food Chem. 2011, 16, 221–227. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of pyrazines in maillard model systems: Effects of structures of lysine—Containing dipeptides/tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazine generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Lund, M.; Ray, C. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed]
- Bemis-young, G.; Huang, J.; Bernhard, R. Effect of pH on pyrazine formation in glucose-glycine model systems. Food Chem. 1993, 46, 383–387. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, A. The effect of pH on formation of aroma compounds produced by heating a model system containing L-ascorbic acid with L-threonine/L-serine. Food Chem. 2010, 119, 214–219. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Wang, P.; Yang, N.; Li, W.; Champagne, P. Experimental and kinetic study on production of furfural and HMF from glucose. Catalysts 2021, 11, 11. [Google Scholar] [CrossRef]
- Nakama, A.; Kim, E.; Shinohara, K.; Omura, H. Formation of furfural derivatives in amino carbonyl reactions. Biosci. Biotechnol. Biochem. 1993, 57, 1757–1759. [Google Scholar] [CrossRef]
- Scalone, G.; Cucu, T.; Dekimpe, N.; DeMeulenaer, B. Influence of free amino acids, oligopeptides and polypeptides on formation of pyrazines in Maillard model systems. J. Agric. Food Chem. 2015, 63, 5364–5372. [Google Scholar] [CrossRef]
- Fayek, N.; Xiao, J.; Farag, M. A multifunctional study of naturally occurring pyrazines in biological systems; formation mechanisms, metabolism, food applications and functional properties. Crit. Rev. Food Sci. Nutr. 2021, 63, 5322–5338. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Hu, X.; Liao, X.; Ni, Y.; Li, Q. Effects of sugar in batter formula and baking conditions on HMF and furfural formation in sponge cake models. Food Res. Int. 2012, 1, 439–445. [Google Scholar] [CrossRef]
- Parker, J.K. Thermal Generation of Aroma. In Flavor Development, Analysis, and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Food Science Technology and Nutrition: Warsaw, Poland, 2015; pp. 151–185. [Google Scholar]
- Zhang, L.; Chen, J.; Zhang, J.; Sagymbek, A.; Li, Q.; Gao, Y.; Du, S.; Yu, X. Lipid oxidation in fragrant rapeseed oil: Impact of seed roasting on the generation of key volatile compounds. Food Chem. 2022, 16, 100491. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Shen, X.; Peng, H.; Zhou, Q.; Yun, J.; Sun, Y.; Ho, C.; Cai, H.; Hou, R. Identification of rancidity markers in roasted sunflower seeds produced from raw material stored for different periods of time. Food Sci. Technol. 2020, 118, 108721. [Google Scholar] [CrossRef]
- Yamauchi, R.; Goto, Y.; Kato, K.; Veno, Y. Prooxidant effect of dihydroxyacetone and reducing sugar on the autoxidation of methyl linoleate in emulsions. Agric. Biol. Chem. 1984, 4, 843–848. [Google Scholar] [CrossRef]
- Vu, T.; He, L.; McClements, D.; Decker, E. Effects of water activity, sugars, and protein on lipid oxidative stability of low moisture model crackers. Food Res. Int. 2020, 130, 108844. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Visessanguan, W.; Phongkanpai, V.; Tanaka, M. Antioxidative activity of caramelization products and their preventive effect on lipid oxidation in fish mince. Food Chem. 2005, 90, 231–239. [Google Scholar] [CrossRef]
- Bowman, T.; Barringer, S. Analysis of Factors Affecting Volatile Compound Formation in Roasted Pumpkin Seeds with Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) and Sensory Analysis. J. Food Sci. 2012, 1, C51–C60. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+, and O2+ with a series of volatile carboxylic acids and esters. Int. J. Mass Spectrom. Ion Process 1998, 172, 137–147. [Google Scholar] [CrossRef]
- Spanel, P.; Smith, D. Quantitative selected ion flow tube mass spectrometry: The influence of ionic diffusion and mass discrimination. J. Am. Soc. Mass Spectrom. 2001, 12, 863e872. [Google Scholar] [CrossRef]
- Perkins, M.J.; Langford, V.S. Application of Routine Analysis Procedures to a Direct Mass Spectrometry Technique: Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). Rev. Sep. Sci. 2021, 3, e21003. [Google Scholar] [CrossRef]
- Noseda, B.; Ragaert, P.; Pauwels, D.; Anthirens, T.; Langenhove, H.; Dewulf, J.; Devlieghere, F. Validation of Selective Ion Flow Tube Mass Spectrometry for Fast Quantification of Volatile Bases Produced on Atlantic Cod (Gadusmorhua). J. Agric. Food Chem. 2010, 58, 5213–5219. [Google Scholar] [CrossRef]
- Agila, A.; Barringer, S. Volatile Profile of Cashews (Anacardium occidentale L.) from Different Geographical Origins during Roasting. J. Food Sci. 2011, 5, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Niu, X.; Yang, F.; Xu, Y.; Dai, Y.; Liu, Y.; Zhou, Q. Roasting pretreatment of walnut (Juglans regia L.) Kernels: Improvement of the oil flavor profile and correlation with the chemical composition. Food Funct. 2022, 13, 10956–10969. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Mason, M.; Odell, G. Odor threshold values of pyrazine compounds and assessment of their role in the flavor of foods. J. Food Sci. 1971, 36, 816–818. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, S.; Nie, Y.; Xu, Y. Quantitative Analysis of Pyrazines and Their Perceptual Interactions in Soy Sauce Aroma Type Baijiu. Foods 2021, 10, 441. [Google Scholar] [CrossRef]
- Buttery, R.; Orts, W.; Takeaka, G.; Nam, Y. Volatile Flavor Components of Rice Cakes. J. Agric. Food Chem. 1999, 47, 4353–4356. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.; Tena, N.; Aparicio-Ruiz, R.; Morales, M. Relationship between sensory attributes and volatile compounds qualifying dry cured hams. Meat Sci. 2008, 2, 315–325. [Google Scholar] [CrossRef]
- Huang, Y.; Barringer, S. Monitoring of Cocoa Volatiles Produced During Roasting by Selected Ion Flow Tube- Mass Spectrometry (SIFT-MS). J. Food Sci. 2011, 76, 279–286. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service (USDA). USDA National Nutrient Database for Standard Reference. 2019. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 3 March 2023).
- Şen, D.; Gökmen, V. Kinetic modeling of Millard and caramelization reactions in sucrose rich and low moisture foods applied for roasted nuts and seeds. Food Chem. 2022, 395, 133583. [Google Scholar] [CrossRef]
- Agcam, E. A kinetic approach to explain hydroxymethylfurfural and furfural formations induced by Maillard, caramelization, and ascorbic acid degradation reactions in fruit juice mediums. Food Anal. Methods 2022, 15, 1286–1299. [Google Scholar] [CrossRef]
- Poojary, M.; Lund, M. Chemical Stability of Proteins in Foods: Oxidation and the Maillard Reaction. An. Rev. Food Sci. Technol. 2022, 13, 35–58. [Google Scholar] [CrossRef]
- Mottram, T.; Taylor, A. Controlling Maillard Pathway to Generate Flavors. In Dicarbonyl Intermediates: A Control Factor in the Maillard Reaction; Wang, Y., Ho, C., Eds.; American Chemical Society: Washington, DC, USA, 2010; pp. 27–34. [Google Scholar]
- Mohamed, H.N.; Man, Y.C.; Mustafa, S.; Manap, Y.A. Tentative identification of Volatile Flavor Compounds in Commercial Budu, a Malaysian Fish Sause, using GC-MS. Molecules 2012, 17, 5062–5080. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, M.; Vickers, Z.; Reineccius, G. Flavor of whey protein concentrates and isolates. Int. Dairy J. 2008, 18, 649–657. [Google Scholar] [CrossRef]
- González-Weller, D.; Paz-Montelongo, S.; Bethencourt-Brbuzano, E.; Niebla-Canelo, D.; Alejandro-Vega, S.; Gutiérrez, Á.; Hardison, A.; Carrascosa, C.; Rubio, C. Proteins and Minerals in Whey Supplements. Foods 2023, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Decker, E. The Role of Oxygen in Lipid Oxidation Reactions: A Review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of color change of kiwifruits during hot air and microwave drying. J. Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service (USDA). Grades of Shelled Walnuts. 2017. Available online: https://www.ams.usda.gov/grades-standards/walnuts-shell-grades-and-standards (accessed on 10 June 2023).
- U.S. Department of Agriculture, Agricultural Research Service (USDA). USDA Commodity Requirements Document Peanut Products. 2016. Available online: https://www.ams.usda.gov/sites/default/files/2000007618%20-%20Peanut%20Products%20CRD.pdf (accessed on 10 June 2023).
| Volatile | Reagent | Rate Constant (k) (10−9 cm3/s) | Mass (m/z) | Product |
|---|---|---|---|---|
| 1,4-butyrolactone | NO+ | 3.5 | 116 | C4H6O2.NO+ |
| 1-octanol | NO+ | 2.3 | 129 | C8H17O+ |
| 1-pentanol | O2+ | 2.8 | 70 | C5H10+ |
| 2,3-dimethyl-5-methylpyrazine | O2+ | 2.5 | 150 | C9H14N2+ |
| Dimethylpyrazine isomers | O2+ | 2.7 | 108 | C6N2H8+ |
| 2-decenal | NO+ | 2.1 | 153 | C10H17O+ |
| 2-heptenal | NO+ | 3.9 | 111 | C7H11O+ |
| 2-isopropyl-3-methylpyrazine | H3O+ | 3.0 | 153 | C8H12N2O.H+ |
| 2-methylpyrazine | NO+ | 2.8 | 94 | C5H6N2+ |
| 2-pentylfuran isomers | NO+ | 2.0 | 138 | C9H14O+ |
| 3-methyl-2-ethylpyrazine isomers | NO+ | 2.5 | 152 | C7H10N2.NO+ |
| Pinene isomers | NO+ | 2.3 | 136 | C10H16+ |
| benzaldehyde | O2+ | 2.4 | 106 | C7H6O+ |
| Decanal | NO+ | 3.3 | 155 | C10H19O+ |
| Furfural | NO+ | 3.2 | 96 | C5H4O2+ |
| Hexanal | NO+ | 2.5 | 99 | C6H11O+ |
| hydroxymethylfurfural (HMF) | O2+ NO+ | 2.5 2.5 | 126 126 | C6H6O3+ C6H6O3+ |
| Methional | O2+ | 2.5 | 104 | C4H8O5+ |
| Nonanal | NO+ | 2.7 | 141 | C9H17O+ |
| Octanal | NO+ | 3.0 | 127 | C8H15O+ |
| Pentanal | NO+ | 3.0 | 85 | C5H9O+ |
| phenylacetaldehyde | NO+ | 2.5 | 150 | C8H8O.NO+ |
| Pyrazine | NO+ | 2.8 | 80 | C4H4N2+ |
| tetramethylpyrazine | O2+ | 2.5 | 136 | C8H12N+ |
| trans-2-undecenal | H3O+ | 3.0 | 169 | C11H20O.H+ |
| Trimethylpyrazine | NO+ O2+ | 2.5 2.5 | 122 122 | C7H10N2+ C7H10N2+ |
| Volatile | Raw Seeds | pH 4 | pH 7 | pH 9 | 20% Glucose | 20% Fructose | 17% Whey Isolate | 17% Whey Concentrate |
|---|---|---|---|---|---|---|---|---|
| 1,4-butyrolactone | 3.13 e | 15.1 de | 17.0 de | 23.9 d | 88.1 b | 104 a | 52.4 c | 104 ab |
| 1-octanol | 1.64 d | 3.70 d | 4.46 d | 5.22 cd | 57.9 a | 55.3 a | 17.1 c | 41.6 b |
| 1-pentanol | 17.9 e | 76.2 d | 98.7 d | 119 d | 252 c | 310 b | 282 bc | 562 a |
| 2,3-diethyl-5-methylpyrazine | 0.60 e | 0.78 de | 1.33 cde | 1.31 cde | 2.10 c | 1.89 cd | 4.77 b | 7.89 a |
| dimethylpyrazine isomers | 4.88 f | 26.6 f | 37.4 ef | 68.2 de | 95.5 d | 193 b | 141 c | 333 a |
| 2-decenal | 1.39 bc | 1.72 bc | 1.03 c | 1.38 bc | 5.20 a | 3.80 ab | 5.78 a | 6.03 a |
| 2-heptenal | 0.86 e | 7.50 d | 8.26 d | 10.4 d | 19.3 c | 17.9 c | 25.7 b | 57.6 a |
| 2-isopropyl-3-methoxypyrazine | 3.27 cde | 1.84 e | 2.08 e | 2.22 de | 5.21 c | 4.87 cd | 8.16 b | 13.5 a |
| 2-methylpyrazine | 4.05 g | 22.3 fg | 43.0 ef | 53.6 de | 87.9 c | 124 b | 69.0 cd | 171 a |
| 2-pentylfuran | 2.28 d | 3.65 cd | 3.69 cd | 4.92 cd | 7.06 bc | 10.4 b | 9.06 b | 21.9 a |
| 3-methyl-2-ethylpyrazine isomers | 4.00 bc | 2.26 c | 2.55 c | 2.22 c | 5.17 bc | 6.46 b | 5.65 b | 11.2 a |
| hydroxymethylfurfural (HMF) | 1.98 d | 13.0 cd | 30.3 b | 18.8 bc | 21.6 bc | 22.4 bc | 22.6 bc | 56.0 a |
| Pinene isomers | 108 d | 191 c | 219 abc | 261 a | 242 ab | 264 a | 172 c | 204 bc |
| benzaldehyde | 2.49 c | 12.9 c | 13.7 c | 14.3 c | 21.4 c | 20.9 c | 73.7 b | 143 a |
| decanal | 2.04 c | 7.73 ab | 5.53 bc | 6.16 b | 8.46 ab | 6.94 b | 5.36 b | 11.5 a |
| furfural | 1.01 d | 11.4 d | 11.0 d | 12.4 cd | 333 a | 276 b | 19.8 cd | 41.2 c |
| hexanal | 4.54 e | 107 d | 103 d | 109 d | 161 bc | 127 cd | 189 b | 227 a |
| methional | 7.51 c | 19.2 c | 21.2 c | 20.7 c | 38.0 c | 60.4 c | 160 b | 653 a |
| nonanal | 2.67 f | 8.04 cde | 5.92 e | 6.20 de | 9.03 cd | 9.13 c | 11.8 b | 16.2 a |
| octanal | 1.96 d | 6.22 c | 5.91 cd | 5.66 cd | 20.1 a | 14.6 b | 8.51 c | 14.4 b |
| pentanal | 3.81 d | 3290 c | 3660 c | 4550 c | 10,140 b | 9760 b | 10,120 b | 19,300 a |
| phenylacetaldehyde | 3.37 d | 7.90 cd | 9.28 cd | 9.51 cd | 12.6 cd | 17.1 bc | 28.3 b | 38.4 a |
| pyrazine | 1.91 c | 2.38 c | 3.03 c | 3.61 c | 7.75 b | 6.99 b | 6.23 b | 14.4 a |
| tetramethylpyrazine | 6.52 e | 9.20 e | 11.8 de | 17.7 cd | 23.0 bc | 28.8 b | 27.4 b | 62.6 a |
| trans-2-undecenal | 2.34 e | 2.19 e | 2.22 e | 2.35 e | 28.4 a | 22.2 b | 8.17 d | 15.9 c |
| trimethylpyrazine | 1.90 e | 9.09 de | 12.1 de | 18.3 cd | 25.7 c | 39.4 b | 41.2 b | 101 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laemont, J.; Barringer, S. Effect of pH, Reducing Sugars, and Protein on Roasted Sunflower Seed Aroma Volatiles. Foods 2023, 12, 4155. https://doi.org/10.3390/foods12224155
Laemont J, Barringer S. Effect of pH, Reducing Sugars, and Protein on Roasted Sunflower Seed Aroma Volatiles. Foods. 2023; 12(22):4155. https://doi.org/10.3390/foods12224155
Chicago/Turabian StyleLaemont, Jessica, and Sheryl Barringer. 2023. "Effect of pH, Reducing Sugars, and Protein on Roasted Sunflower Seed Aroma Volatiles" Foods 12, no. 22: 4155. https://doi.org/10.3390/foods12224155
APA StyleLaemont, J., & Barringer, S. (2023). Effect of pH, Reducing Sugars, and Protein on Roasted Sunflower Seed Aroma Volatiles. Foods, 12(22), 4155. https://doi.org/10.3390/foods12224155







