A Novel Indicator Based on Polyacrylamide Hydrogel and Bromocresol Green for Monitoring the Total Volatile Basic Nitrogen of Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
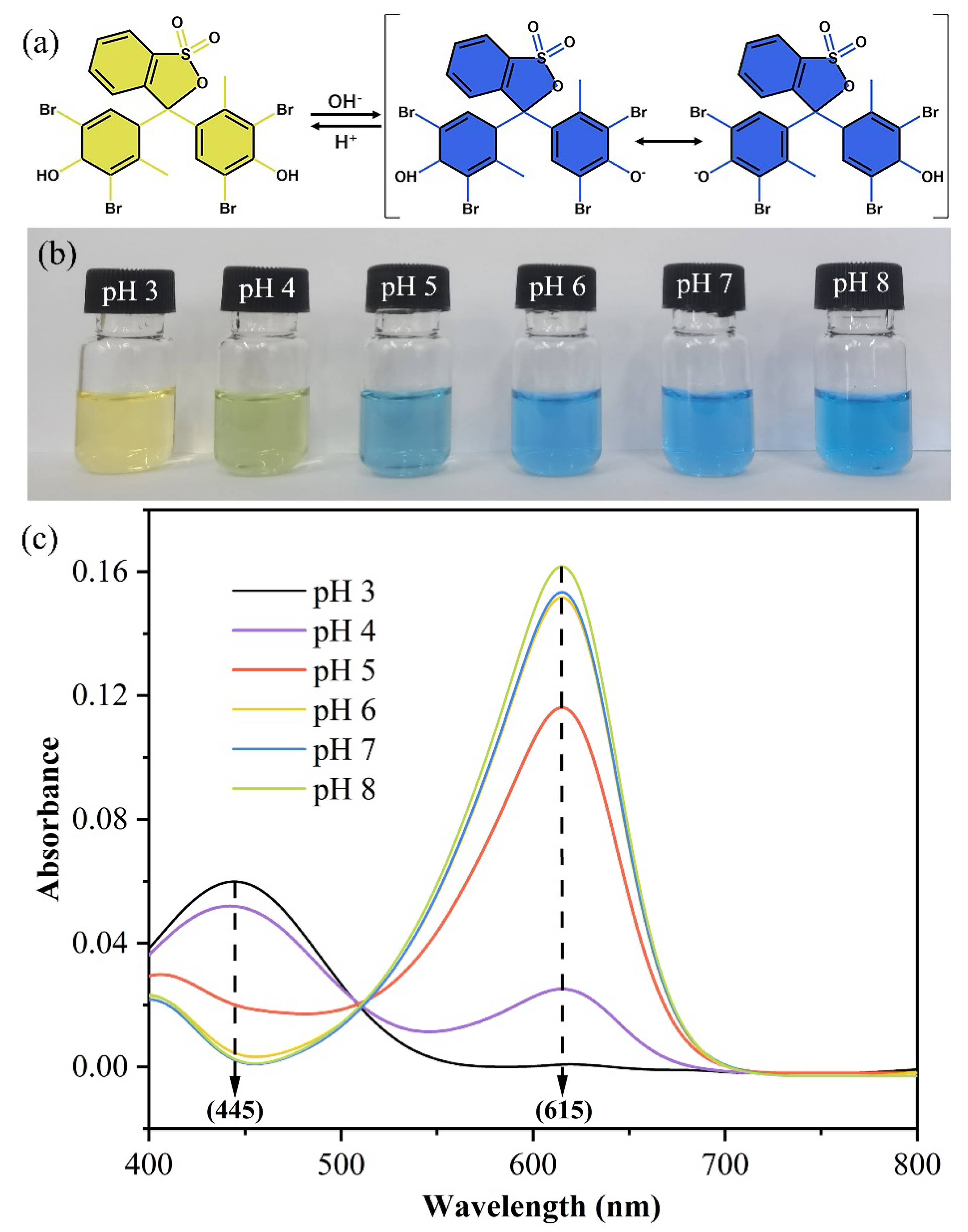
2.2. UV-Vis Spectra Characterization of BCG Solution
2.3. Preparation of the PAAm Hydrogel/BCG Indicator
2.4. Characterization and Properties
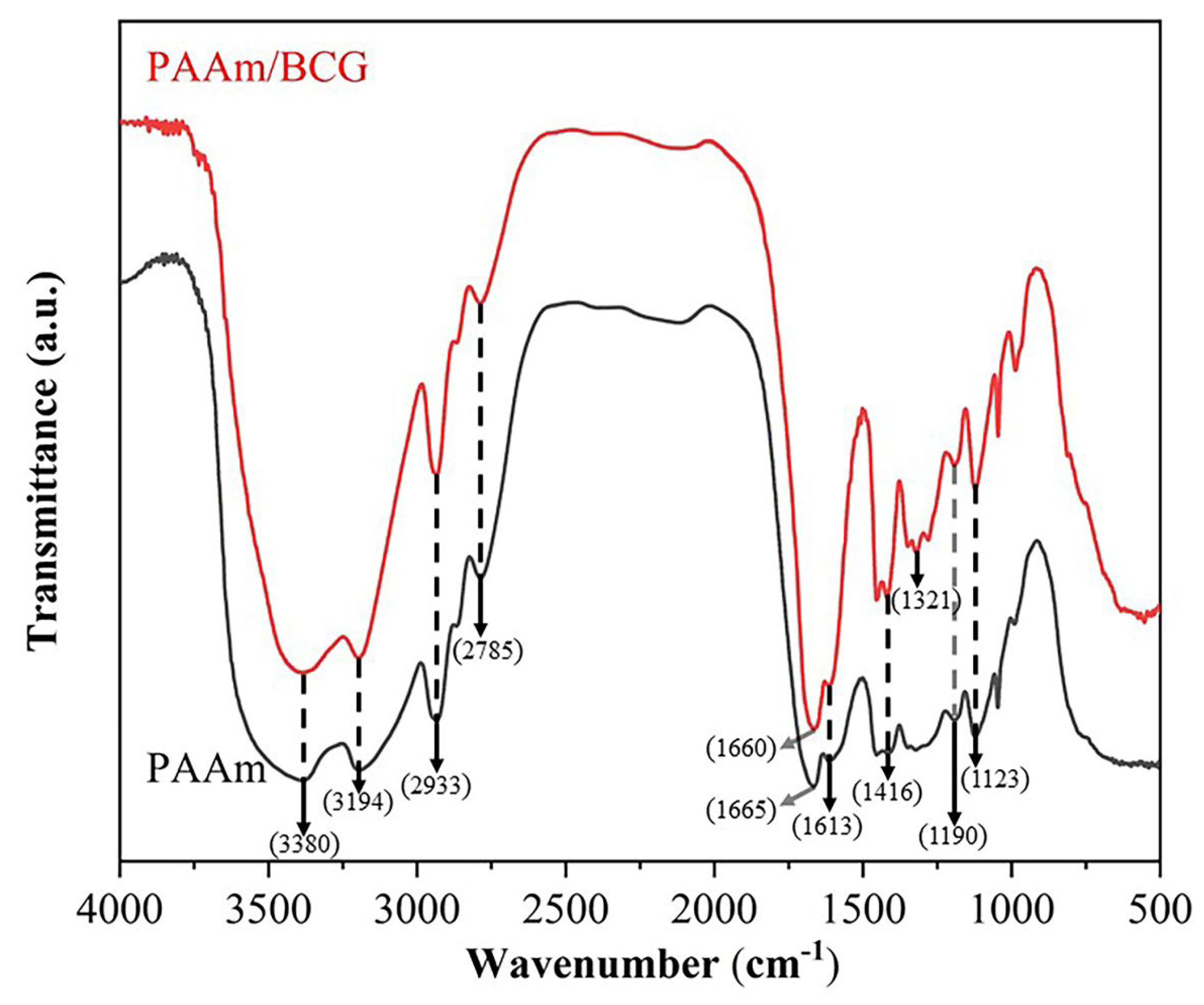
2.4.1. Fourier-Transform Infrared (FT-IR) Spectroscopy
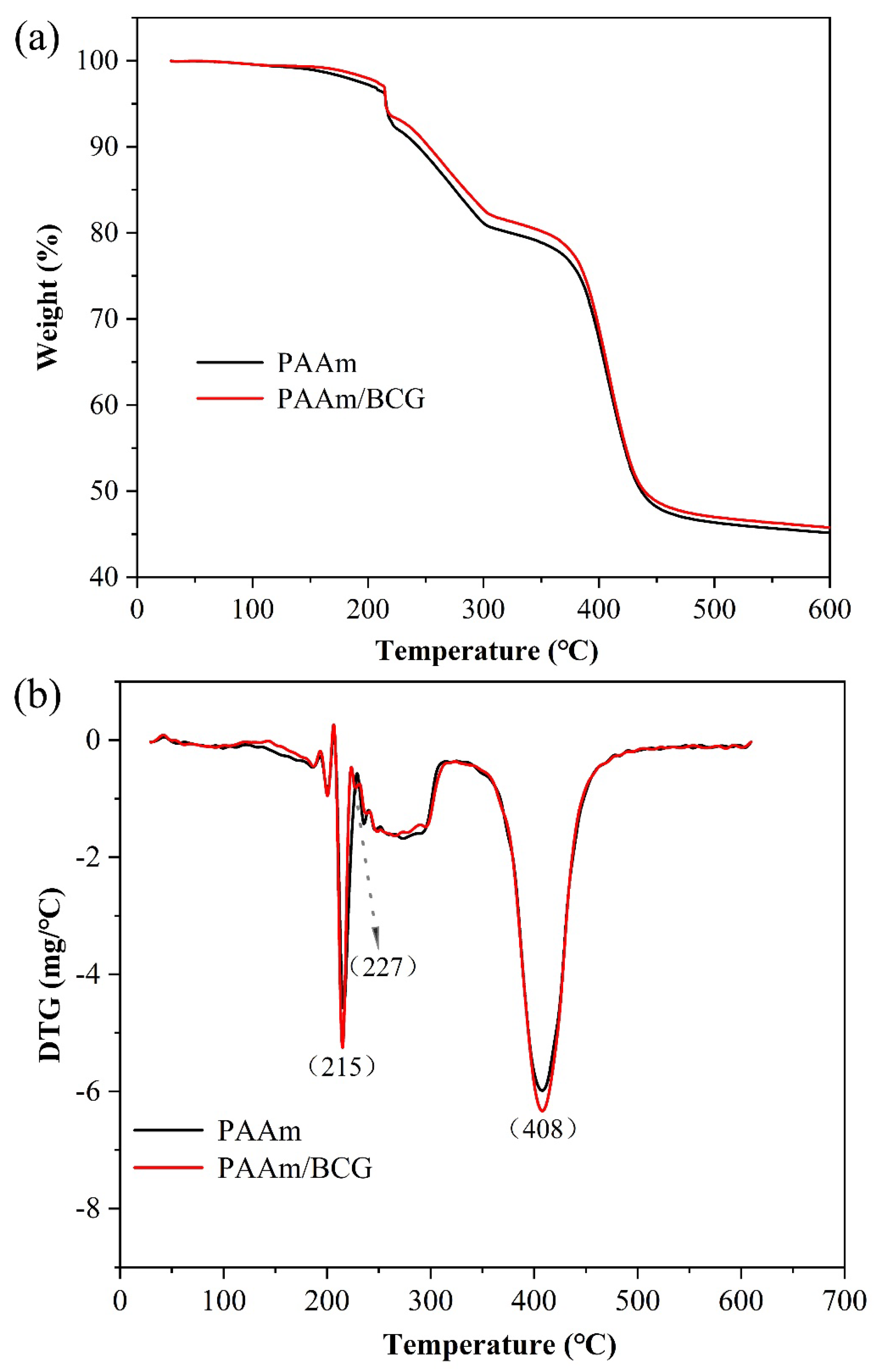
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Moisture Content (MC), Water Solubility (WS), and Water Absorption (WA)
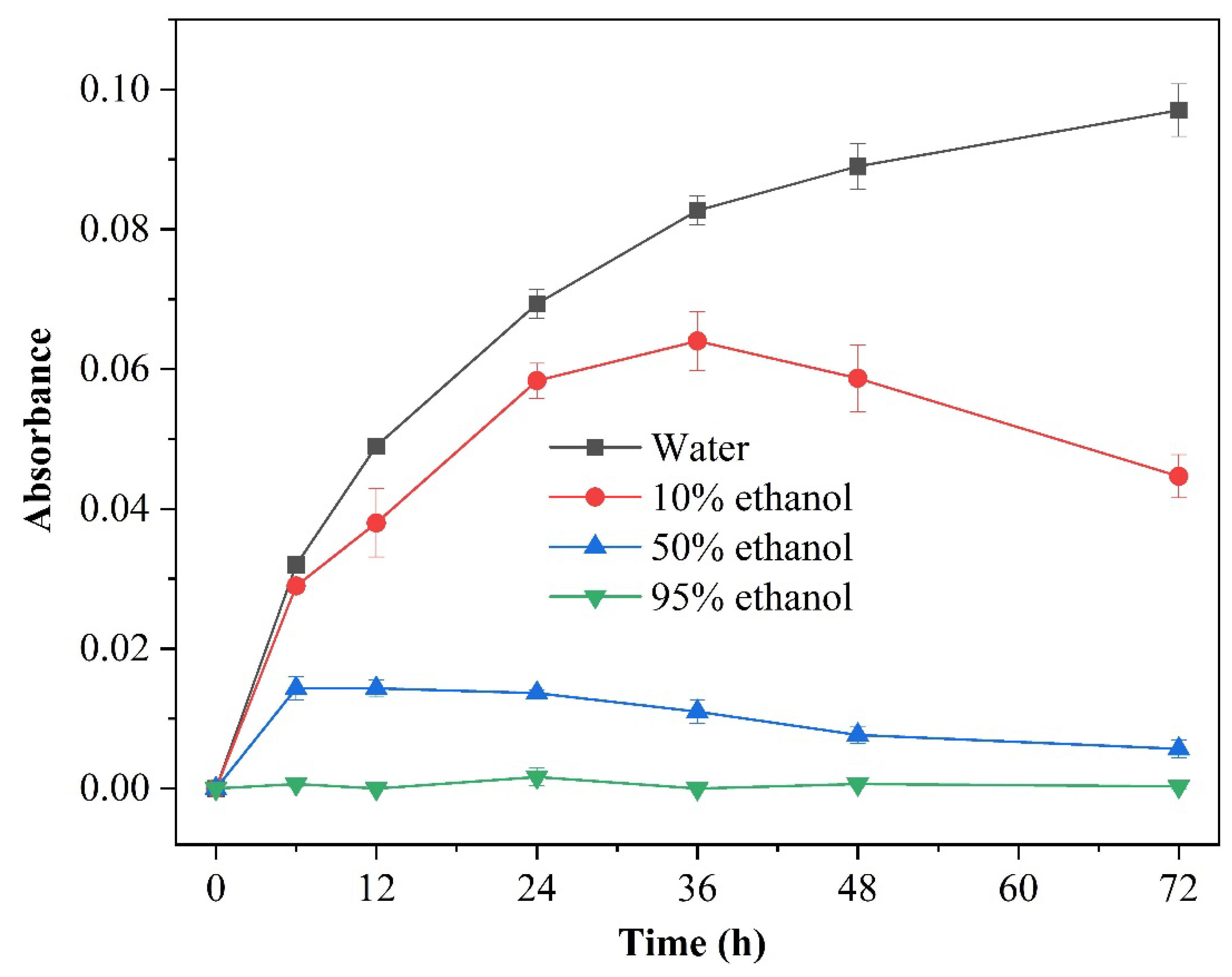
2.4.4. Release of BCG
2.5. Color Response Efficiency
2.6. Packaging Test
2.6.1. TVB-N Measurement
2.6.2. Determination of pH Values
2.7. Statistical Analysis
3. Results and Discussion
3.1. UV-Vis Spectra Analysis
3.2. Properties of the Indicator
3.2.1. FTIR Characterization
3.2.2. Thermal Stability
3.2.3. MC, WS, and WA Analysis
3.2.4. Release of BCG
3.3. Color Response Efficiency
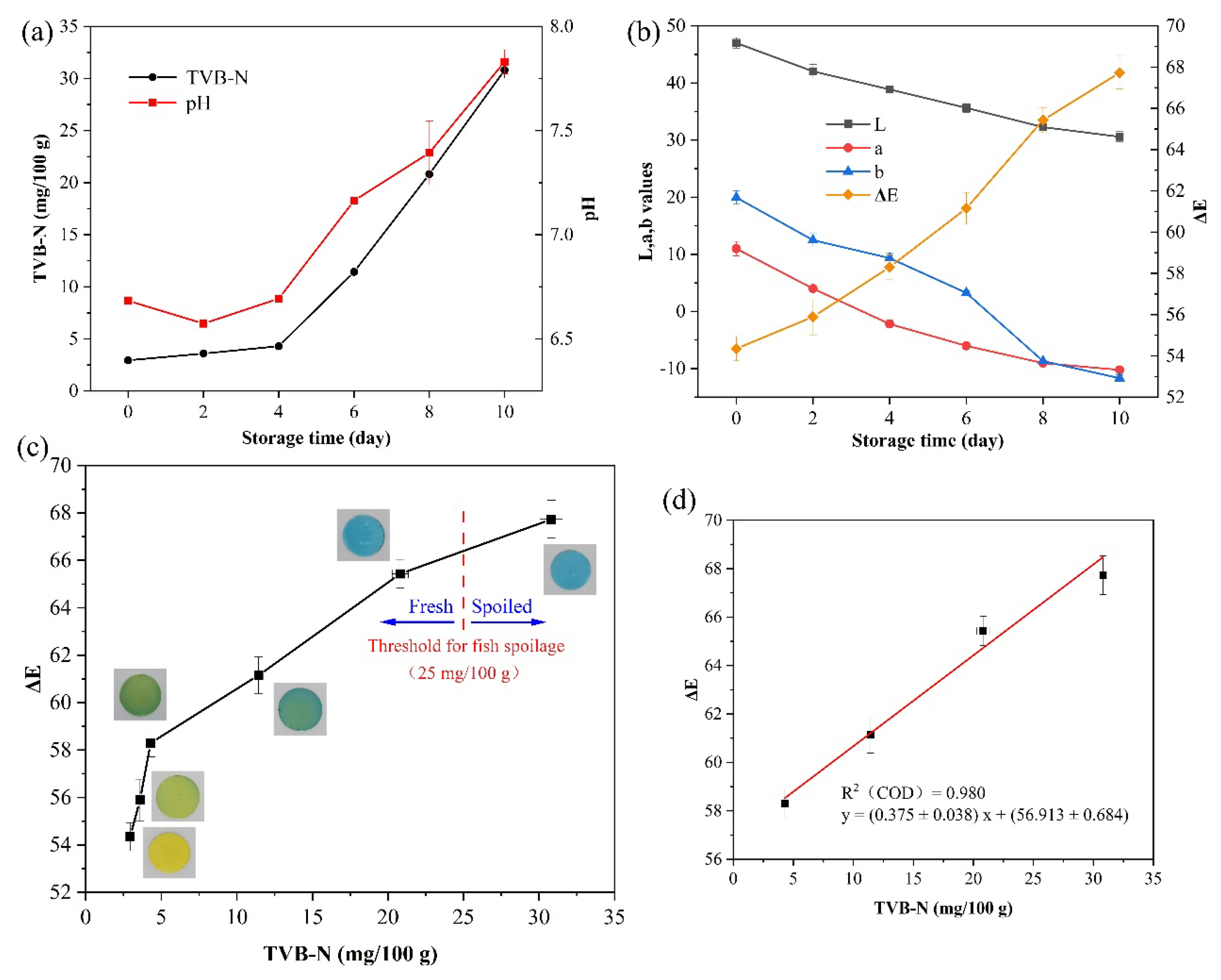
3.4. Packaging Test
3.4.1. Monitoring Freshness of Japanese Sea Bass (Lateolabrax japonicus)
3.4.2. Feasibility Analysis of Models and Data
3.5. The Advantages and Disadvantages of the PAAm Hydrogel/BCG Indicator
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuswandi, B. Freshness sensors for food packaging. In Reference Module in Food Science; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Quan, Z.; He, H.; Zhou, H.; Liang, Y.; Wang, S. Designing an intelligent nanofiber ratiometric fluorescent sensor sensitive to biogenic amines for detecting the freshness of shrimp and pork. Sens. Actuators B-Chem. 2021, 333, 129535. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Vicente Ros-Lis, J.; Vivancos, J.L.; Martinez-Manez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Sun, Y.; Sang, S.Y.; Jia, L.L.; Ou, C.R. Emerging Approach for Fish Freshness Evaluation: Principle, Application and Challenges. Foods 2022, 11, 1897. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent gelatin/oxidized chitin nanocrystals nanocomposite films containing black rice bran anthocyanins for fish freshness monitorings. Int. J. Biol. Macromol. 2019, 155, 1296–1306. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Di, L.A.; Shuai, D.A.; Ling, Z.A.; Kang, M.B.; Xl, A. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded Pickering emulsion for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar]
- Aghaei, Z.; Ghorani, B.; Emadzadeh, B.; Kadkhodaee, R.; Tucker, N. Protein-based halochromic electrospun nanosensor for monitoring trout fish freshness. Food Control 2020, 111, 107065. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Skinner, M.G.A. Water-based colourimetric optical indicators for the detection of carbon dioxide. Analyst 2010, 135, 1912–1917. [Google Scholar]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, L.; Geleta, G.S.; Ma, L.; Wang, Z. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for efficient removal of water-soluble dyes. Sci. Rep. 2017, 7, 7878. [Google Scholar] [CrossRef]
- Aghaei, Z.; Emadzadeh, B.; Ghorani, B.; Kadkhodaee, R. Cellulose acetate nanofibres containing alizarin as a halochromic sensor for the qualitative assessment of rainbow trout fish spoilage. Food Bioprocess Technol. 2018, 11, 1087–1095. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, D.W.; Ma, J.; Cheng, J.; Wang, Z.B.; Tang, Z. A volatile basic nitrogens-responsive tag based on aggregation-induced emission luminogen for real-time monitoring and in situ visualization of salmon freshness. Anal. Chim. Acta 2022, 1221, 340122. [Google Scholar] [CrossRef]
- He, H.; Song, Y.; Li, M.; Zhang, H.; Li, J.; Huang, H.; Li, Y. A novel anthocyanin electrospun film by caffeic acid co-pigmentation for real-time fish freshness monitoring. Anal. Methods 2023, 15, 228–239. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhu, L.; Tang, Y.; Gao, X.; Tang, L.; Li, J. Dual-mode smart packaging based on tetraphenylethylene-functionalized polyaniline sensing label for monitoring the freshness of fish. Sens. Actuators B-Chem. 2020, 323, 128694. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.W. Melanin-mediated synthesis of silver nanoparticle and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Ezati, P.; Priyadarshi, R.; Bang, Y.J.; Rhim, J.W. CMC and CNF-based intelligent pH-responsive color indicator films integrated with shikonin to monitor fish freshness. Food Control 2021, 126, 108046. [Google Scholar] [CrossRef]
- Razavi, R.; Molaei, R.; Moradi, M.; Tajik, H.; Ezati, P.; Shafipour Yordshahi, A. Biosynthesis of metallic nanoparticles using mulberry fruit (Morus alba L.) extract for the preparation of antimicrobial nanocellulose film. Appl. Nanosci. 2019, 10, 465–476. [Google Scholar] [CrossRef]
- Ezati, P.; Bang, Y.J.; Rhim, J.W. Preparation of a shikonin-based pH-sensitive color indicator for monitoring the freshness of fish and pork. Food Chem. 2020, 337, 127995. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Firoozbakht, F. Determination of the acidity constants of neutral red and bromocresol green by solution scanometric method and comparison with spectrophotometric results. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 13–20. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Chen, K.; Wang, J.; Wang, Y.; Tang, Y.; Gao, X. An on-package colorimetric sensing label based on a Sol-gel matrix for fish freshness monitoring. Food Chem. 2020, 307, 125580. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhao, L.; Wang, J.; Wang, S.; Liu, Y.; Liu, X. High-strength and high-toughness sodium alginate/polyacrylamide double physically crosslinked network hydrogel with superior self-healing and self-recovery properties prepared by a one-pot method. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 589, 124402. [Google Scholar] [CrossRef]
- Yuan, N.; Xu, L.; Wang, H.; Fu, Y.; Zhang, Z.; Liu, L. Dual physically cross-linked double network hydrogels with high mechanical strength, fatigue resistance, notch-insensitivity, and self-healing properties. Soft Matter 2016, 8, 34034–34044. [Google Scholar] [CrossRef]
- El-din, H.M.N.; El-Nagga, A.W.M.; Ali, F.I. Miscibility of poly(vinyl alcohol)/polyacrylamide blends before and after gamma irradiation. Polym. Int. 2003, 52, 225–234. [Google Scholar] [CrossRef]
- Wang, X.; Hu, D.; Yang, J. Synthesis of PAM/TiO2 composite microspheres with hierarchical surface morphologies. Chem. Mater. 2007, 19, 2610–2621. [Google Scholar] [CrossRef]
- Liang, W.; Luo, Z.; Zhou, L. Preparation and characterization of the n-HA/PVA/CS porous composite hydrogel. Chin. J. Chem. Eng. 2020, 28, 598–602. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Wongmaneepratip, W.; He, Y.; Zhao, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. 2021, 354, 129581. [Google Scholar] [CrossRef]
- Guiga, W.; Swesi, Y.; Galland, S.; Peyrol, E.; Degraeve, P.; Sebti, I. Innovative multilayer antimicrobial films made with Nisaplin or nisin and cellulosic ethers: Physico-chemical characterization, bioactivity and nisin desorption kinetics. Innov. Food Sci. Emerg. Technol. 2010, 11, 352–360. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Fabrication of cellulose nanofiber-based functional color indicator film incorporated with shikonin extracted from Lithospermum erythrorhizon root. Food Hydrocoll. 2021, 114, 106566. [Google Scholar] [CrossRef]
- Wei, Y.C.; Cheng, C.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocoll. 2017, 69, 491–502. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Kim, H.C.; Kafy, A.; Kim, J.W.; Kim, J. Calcinated tea and cellulose composite films and its dielectric and lead adsorption properties. Carbohydr. Polym. 2017, 171, 183–192. [Google Scholar] [CrossRef]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef]
- Wells, N.; Yusufu, D.; Mills, A. Colourimetric plastic film indicator for the detection of the volatile basic nitrogen compounds associated with fish spoilage. Talanta 2019, 194, 830–836. [Google Scholar] [CrossRef]
- Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Sun, Z.; Li, Z. Amine-responsive bilayer films with improved illumination stability and electrochemical writing property for visual monitoring of meat spoilage. Sens. Actuators B-Chem. 2020, 302, 127130. [Google Scholar] [CrossRef]
- Lou, X.; Zhai, D.; Yang, H. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 2021, 123, 107697. [Google Scholar] [CrossRef]
- Liu, Y.; Kai, Y.; Yang, H. Biodegradable fish gelatin/chitosan-based active films alter chill-stored golden pomfret (Trachinotus blochii) metabolites mainly through modulating four metabolic pathways. Food Packag. Shelf Life 2023, 36, 101046. [Google Scholar] [CrossRef]
- He, Y.; Xie, Z.; Xu, Y.; Guo, C.; Zhao, X.; Yang, H. Effect of slightly acid electrolysed water ice on metabolite and volatilome profile of shrimp (Penaeus vannamei) during cold storage. Food Control 2023, 145, 109421. [Google Scholar] [CrossRef]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of Fish Gelatin and Tea Polyphenol Coating on the Spoilage and Degradation of Myofibril in Fish Fillet During Cold Storage. Food Bioprocess Technol. 2017, 10, 89–102. [Google Scholar] [CrossRef]
- Feng, X.; Bansal, N.; Yang, H. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef]
- Lee, E.J.; Shin, H.S. Development of a freshness indicator for monitoring the quality of beef during storage. Food Sci. Biotechnol. 2019, 28, 1899–1906. [Google Scholar] [CrossRef]
- Nowzari, F.; Shabanpour, B.; Ojagh, S.M. Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Nondestructive measurement of total volatile basic nitrogen (TVB-N) in pork meat by integrating near infrared spectroscopy, computer vision and electronic nose techniques. Food Chem. 2014, 145, 228–236. [Google Scholar] [CrossRef]
- Zeng, F.; Ye, Y.; Liu, J.; Fei, P. Intelligent pH indicator composite film based on pectin/chitosan incorporated with black rice anthocyanins for meat freshness monitoring. Food Chem. X 2023, 17, 100531. [Google Scholar] [CrossRef]
- Li, B.; Bao, Y.; Li, J.; Bi, J.; Chen, Q.; Cui, H.; Wang, Y.; Tian, J.; Shu, C.; Wang, Y.; et al. A sub-freshness monitoring chitosan/starch-based colorimetric film for improving color recognition accuracy via controlling the pH value of the film-forming solution. Food Chem. 2022, 388, 132975. [Google Scholar] [CrossRef]
- Chen, X.; Ji, W.; Nan, X.; Wang, H.; Li, J.; Dong, L.; Sheng, G.; Zhou, Q. Preparation and Characterization of Aronia melanocarpa/Gellan Gum/Pea Protein/Chitosan Bilayer Films. Foods 2022, 11, 2835. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Z.; Yang, M.; Lu, X.; Fu, L.; Jiang, G. Preparation and characterization of sodium cellulose sulfate/chitosan composite films loaded with curcumin for monitoring pork freshness. Curr. Res. Food Sci. 2022, 5, 1475–1483. [Google Scholar] [CrossRef]
- Wu, H.; Jiao, C.; Li, S.; Li, Q.; Zhang, Z.; Zhou, M.; Yuan, X. A Facile Strategy for Development of pH-Sensing Indicator Films Based on Red Cabbage Puree and Polyvinyl Alcohol for Monitoring Fish Freshness. Foods 2022, 11, 3371. [Google Scholar] [CrossRef]
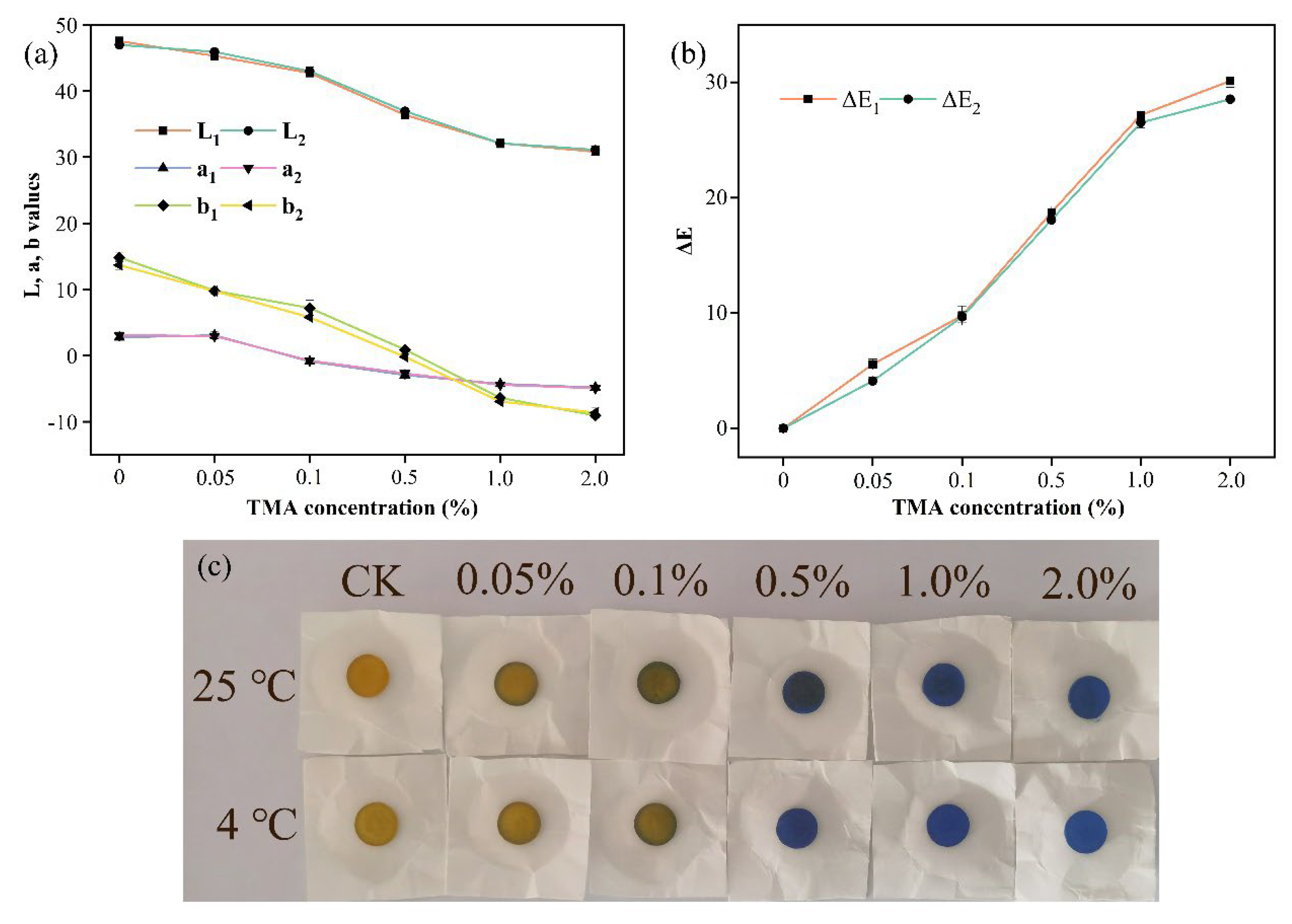
| Samples | MC (%) | WS (%) | WA (%) |
|---|---|---|---|
| PAAm hydrogel | 50.26 ± 0.44 a | 14.26 ± 0.43 a | 132.38 ± 9.22 a |
| PAAm hydrogel/BCG | 43.45 ± 1.08 b | 9.6 ± 2.8 b | 103.02 ± 13.21 b |
| pH | |||||||
|---|---|---|---|---|---|---|---|
| Control | 3 | 4 | 5 | 6 | 7 | 8 | |
| L | 96.52 | 36.32 ± 1.46 c | 35.69 ± 0.82 bc | 33.37 ± 0.24 ab | 31.57 ± 2.47 a | 31.48 ± 0.47 a | 31.23 ± 0.28 a |
| a | −0.24 | 1.42 ± 0.10 e | −0.48 ± 0.19 d | −3.46 ± 0.31 a | −2.38 ± 0.17 b | −2.01 ± 0.29 bc | −1.73 ± 0.11 c |
| b | 0.46 | 7.27 ± 1.07 c | 6.75 ± 0.39 c | −0.61 ± 0.38 b | −1.06 ± 0.64 b | −4.81 ± 0.69 a | −5.59 ± 0.42 a |
| ΔE | 0 | 60.59 ± 1.56 a | 61.13 ± 0.85 a | 63.21 ± 0.24 ab | 64.98 ± 2.4 b | 65.25 ± 0.44 b | 65.56 ± 0.2 b |
| Color changes |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Tang, H.; Cai, K.; Liang, R.; Tong, L.; Ou, C. A Novel Indicator Based on Polyacrylamide Hydrogel and Bromocresol Green for Monitoring the Total Volatile Basic Nitrogen of Fish. Foods 2023, 12, 3964. https://doi.org/10.3390/foods12213964
Zhang Z, Tang H, Cai K, Liang R, Tong L, Ou C. A Novel Indicator Based on Polyacrylamide Hydrogel and Bromocresol Green for Monitoring the Total Volatile Basic Nitrogen of Fish. Foods. 2023; 12(21):3964. https://doi.org/10.3390/foods12213964
Chicago/Turabian StyleZhang, Zhepeng, Haiqing Tang, Keyan Cai, Ruiping Liang, Li Tong, and Changrong Ou. 2023. "A Novel Indicator Based on Polyacrylamide Hydrogel and Bromocresol Green for Monitoring the Total Volatile Basic Nitrogen of Fish" Foods 12, no. 21: 3964. https://doi.org/10.3390/foods12213964
APA StyleZhang, Z., Tang, H., Cai, K., Liang, R., Tong, L., & Ou, C. (2023). A Novel Indicator Based on Polyacrylamide Hydrogel and Bromocresol Green for Monitoring the Total Volatile Basic Nitrogen of Fish. Foods, 12(21), 3964. https://doi.org/10.3390/foods12213964







