Are Microplastics a Macro Issue? A Review on the Sources of Contamination, Analytical Challenges and Impact on Human Health of Microplastics in Food
Abstract
:1. Introduction
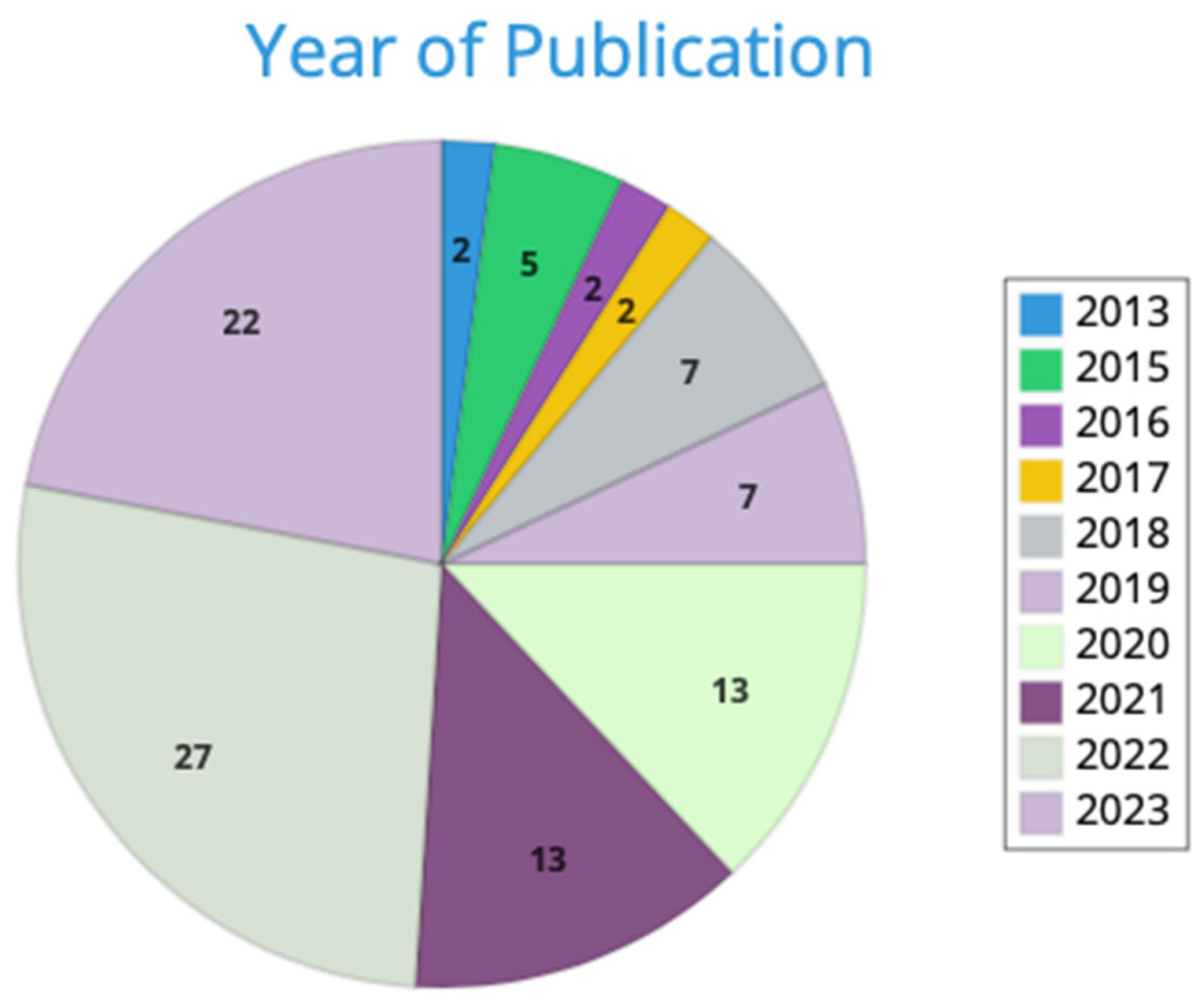
2. Materials and Methods
3. Results
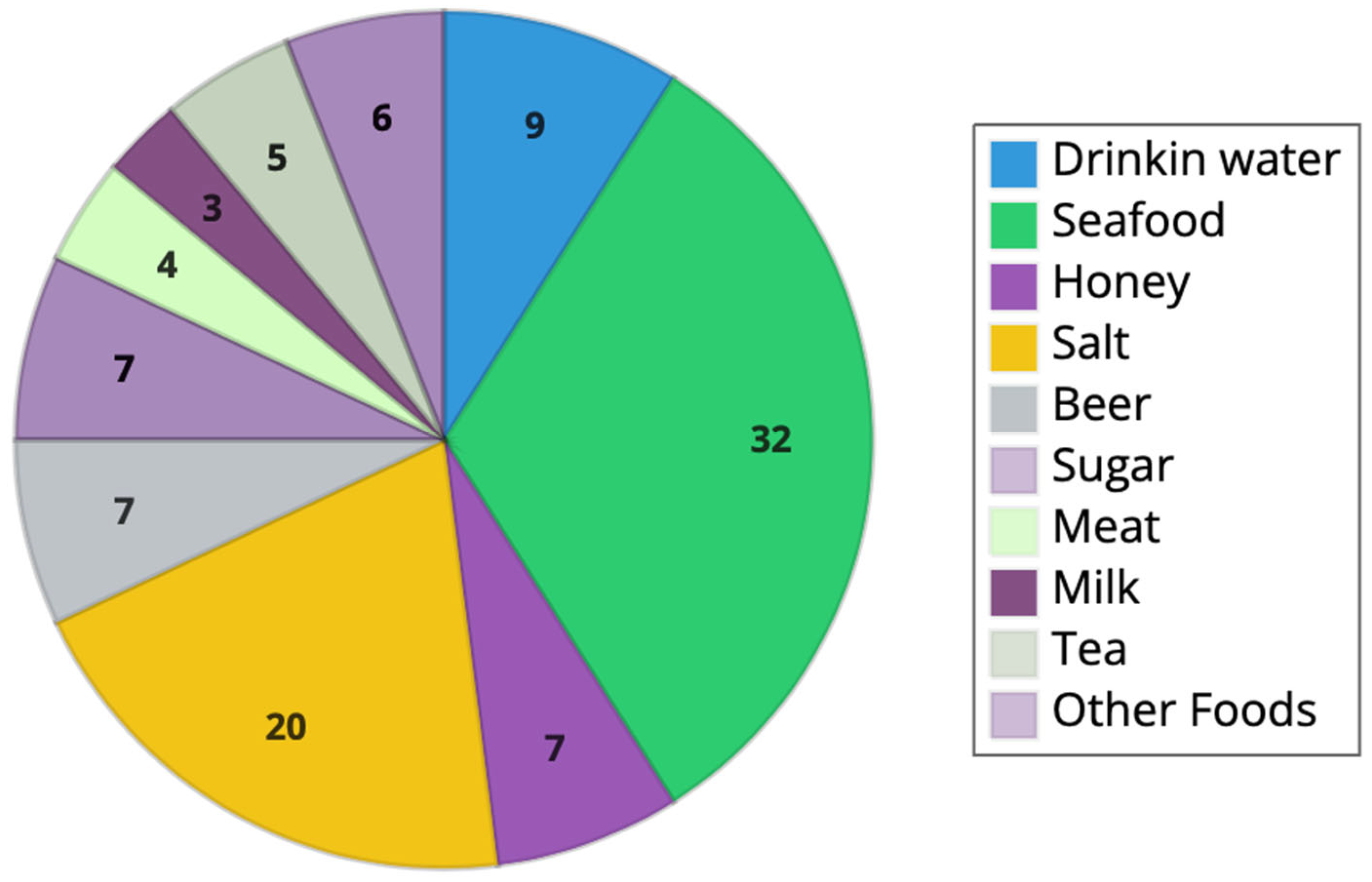
3.1. Main Sources of Microplastics in Food and Beverages
3.1.1. Mineral Bottled Water
3.1.2. Fish Products
3.1.3. Sea Salt
3.1.4. Honey
3.1.5. Beer
3.1.6. Sugar
3.1.7. Tea
3.1.8. Other Food Products
3.2. Analytical Challenges in Microplastic’s Determination in Food Samples
3.3. Microplastic’s Qualification: Techniques’ Limitations
3.4. Microplastic’s Impact on Human Health
4. Discussion
4.1. Sources of Microplastics in Food Products and Main Characteristics
4.2. Analytical Discrepancies in Food Sample Preparation
4.3. Microplastic’s Qualification: Techniques’ Limitations
4.4. Humans and Microplastics: Size and Impact on Humans
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to microplastics (<10 μm) associated to plastics bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar] [PubMed]
- Samandra, S.; Mescall, O.J.; Plaisted, K.; Symons, B.; Xie, S.; Ellis, A.V.; Clarke, B.O. Assessing exposure of the Australian population to microplastics through bottled water consumption. Sci. Total Environ. 2022, 837, 155329. [Google Scholar] [CrossRef] [PubMed]
- Weisser, J.; Beer, I.; Hufnagl, B.; Hofmann, T.; Lohninger, H.; Ivleva, N.P.; Glas, K. From the Well to the Bottle: Identifying Sources of Microplastics in Mineral Water. Water 2023, 12, 841. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspotter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Basantes, M.F.; Nacimba-Aguirre, D.; Conesa, J.A.; Fullana, A. Presence of microplastics in commercial canned tuna. Food Chem. 2022, 385, 132721. [Google Scholar] [CrossRef] [PubMed]
- Gündoğdu, S.; Kösker, A.R. Microplastic contamination in canned fish sold in Türkiye. PeerJ 2023, 11, e146627. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Almeida, C.M.R.; Guardiola, F.; Rodrigues, S.M.; Ramos, S. Optimization of an Analytical Protocol for the Extraction of Microplastics from Seafood Samples with Different Levels of Fat. Molecules 2022, 27, 5172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, Y.; Han, Y.; Zhou, W.; Shi, W.; Teng, S.; Ren, P.; Xiao, G.; Li, S.; Liu, G. Microplastics boost the accumulation of tetrabromobisphenol A in a commercial clam and elevate corresponding food safety risks. Chemosphere 2022, 292, 133499. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Dang, X.; Su, L.; Jabeen, K.; Wang, H.; Wang, Z. Effects of cooking methods on microplastics in dried shellfish. Sci. Total Environ. 2022, 837, 155787. [Google Scholar] [CrossRef]
- Leung, M.M.; Ho, Y.-W.; Lee, C.-H.; Wang, Y.; Hu, M.; Hin Kwok, K.W.; Chua, S.-L.; Fang, J.K. Improved Raman spectroscopy-based approach to assess microplastics in seafood. Environ. Pollut. 2021, 289, 117648. [Google Scholar] [CrossRef]
- Bitencourt, G.R.; Mello, P.A.; Flores, E.M.M.; Pirola, C.; Carnaroglio, D.; Bizzi, C.A. Determination of microplastic content in seafood: An integrated approach combined with the determination of elemental contaminants. Sci. Total Environ. 2020, 749, 142301. [Google Scholar] [CrossRef] [PubMed]
- Corami, F.; Rosso, B.; Roman, M.; Picone, M.; Gambaro, A.; Barbante, C. Evidence of small microplastics (<100 μm) ingestion by Pacific oysters (Crassostrea gigas): A novel method of extraction, purification, and analysis using Micro-FTIR. Mar. Pollut. Bull. 2020, 160, 111606. [Google Scholar] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Alma, A.M.; de Groot, G.S.; Buteler, M. Microplastics incorporated by honeybees from food are transferred to honey; wax and larvae. Environ. Pollut. 2023, 320, 121078. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey; Beer; Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Di Fiore, C.; Sammartino, M.P.; Giannattasio, C.; Avino, P.; Visco, G. Microplastic contamination in commercial salt: An issue for their sampling and quantification. Food Chem. 2023, 404, 134682. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Pirsaheb, M.; Amin, A.; Kianpour, S.; Hossini, H. Microplastic pollution in table salt and sugar: Occurrence, qualification and quantification and risk assessment. J. Food Compos. Anal. 2023, 119, 105261. [Google Scholar] [CrossRef]
- Nakat, Z.; Dgheim, N.; Ballaout, J.; Bou-Mitri, C. Occurrence and exposure to microplastics in salt for human consumption, present on the Lebanese market. Food Control 2023, 145, 109414. [Google Scholar] [CrossRef]
- Manimozhi, N.; Ravi, V.; Sudhan, C.; Manimekalai, D.; Shalini, R.; Abarna, K.M. Spatiotemporal occurrence, distribution, and characterization of microplastics in salt pans of the coastal region of the Gulf of Mannar, southeast coast of India. Reg. Stud. Mar. Sci. 2022, 53, 102350. [Google Scholar] [CrossRef]
- Kapukotuwa, R.W.M.G.K.; Jayasena, N.; Weerakoon, K.C.; Abayasekara, C.L.; Rajakaruna, R.S. High levels of microplastics in commercial salt and industrial salterns in Sri Lanka. Mar. Pollut. Bull. 2022, 174, 113239. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.A.M.; Uddin, A.; Bhuiya, A.; Rahman, S.M.A.; Kibria, G. Occurrence, spatial distribution, and characterization of microplastic particles in the salt pans from the Southeastern part of the Bay of Bengal. Reg. Stud. Mar. Sci. 2023, 61, 102846. [Google Scholar] [CrossRef]
- Thiele, C.J.; Grange, L.J.; Haggett, E.; Hudson, M.D.; Hudson, P.; Russell, A.E.; Zapata-Restrepo, L.M. Microplastics in European sea salts—An example of exposure through consumer choice and of interstudy methodological discrepancies. Ecotoxicol. Environ. Saf. 2023, 255, 114782. [Google Scholar] [CrossRef] [PubMed]
- Kuttykattil, A.; Raju, S.; Vanka, K.S.; Bhagwat, G.; Carbery, M.; Vincent, S.G.T.; Raja, S.; Palanisami, T. Consuming microplastics? Investigation of commercial salts as a source of microplastics (MPs) in diet. Int. J. Environ. Res. Public Health 2023, 30, 930–942. [Google Scholar] [CrossRef]
- Kim, J.; Pham, D.T.; Park, H.-J.; Chae, M.; Lee, S.-H.; Hong, S.; Kim, J.-Y.; Jung, J.; Lee, B.T.; Kwon, J.-H. Development and validation of analytical methods for detecting and identifying microplastics in salts, soy sauce, and salted pollock roe. J. Food Compos. Anal. 2022, 114, 104856. [Google Scholar] [CrossRef]
- Sivagami, M.; Selvambigai, M.; Devan, U.; Velangani, A.A.J.; Karmegam, N.; Biruntha, M.; Arun, A.; Kim, W.; Govarthanan, M.; Kumar, P. Extraction of microplastics from commonly used sea salts in India and their toxicological evaluation. Chemosphere 2018, 263, 128181. [Google Scholar] [CrossRef]
- Sathish, N.M.; Jeyasanta, I.; Patterson, J. Microplastics in Salt of Tuticorin, Southeast Coast of India. Arch. Environ. Contam. Toxicol. 2020, 79, 111–121. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2019, 7, 8620. [Google Scholar] [CrossRef]
- Habschied, K.; Galic, V.; Mastanjevic, K.; Šibalic, M.; Krstanovic, V. A Survey on Detection of Plastic-Related Chemicals in Beer Packaged in PET Using FT-IR Technology. Beverages 2023, 8, 73. [Google Scholar] [CrossRef]
- Afrin, S.; Rahman, M.; Hossain, N.; Uddin, K.; Malafaia, G. Are there plastic particles in my sugar? A pioneering study on the characterization of microplastics in commercial sugars and risk assessment. Sci. Total Environ. 2022, 837, 155849. [Google Scholar] [CrossRef]
- Afrin, S.; Rahman, M.; Siddique, M.; Uddin, K.; Malafaia, G. Is there tea complemented with the appealing flavor of microplastics? A pioneering study on plastic pollution in commercially available tea bags in Bangladesh. Sci. Total Environ. 2022, 837, 155833. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.Z.; Al Kindi, R.; Al Salem, F.; Kittaneh, W.F.; Poulose, V.; Iftikhar, S.H.; Mourad, A.I.; Thiemann, T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. Int. J. Environ. Res. Public Health 2022, 19, 13442. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A.; et al. Detection and characterization of small-sized microplastics (≥5 μm) in milk products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Humpf, H.; Fürst, P. Determination of particle abrasion through milling with five different salt grinders—A preliminary study by micro-Raman spectroscopy with efforts towards improved quality control of the analytical methods. Food Addit. Contam. 2020, 37, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Brachner, A.; Fragouli, D.; Duarte, I.F.; Farias, P.M.A.; Dembski, S.; Ghosh, M.; Barisic, I.; Zdzieblo, D.; Vanoirbeek, J.; Schwabl, P.; et al. Assessment of human health risks posed by nano-and microplastics is currently not feasible. Int. J. Environ. Res. Public Health 2020, 17, 8832. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Kocareva, J.; Noack, D.; Kuballa, T. Microplastic identification in German beer—An artefact of laboratory contamination? Dtsch. Lebensm.-Rundsch. 2015, 111, 437–440. [Google Scholar]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine mammals and microplastics: A systematic review and call for standardisation. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef]
- Singh, T. Generation of microplastics from the opening and closing of disposable plastic water bottles. J. Water Health 2021, 19, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Castelvetro, V.; Corti, A.; Bianchi, S.; Giacomelli, G.; Manariti, A.; Vinciguerra, V. Microplastics in fish meal: Contamination level analyzed by polymer type; including polyester (PET); polyolefins; and polystyrene. Environ. Pollut. 2020, 273, 115792. [Google Scholar] [CrossRef] [PubMed]
- Di Giacinto, F.; Di Renzo, L.; Mascilongo, G.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Bogdanovic, T.; Petricevic, S.; Listes, E.; Brkljaca, M.; et al. Detection of microplastics; polymers and additives in edible muscle of swordfish (Xiphias gladius) and bluefin tuna (Thunnus thynnus) caught in the Mediterranean Sea. J. Sea Res. 2023, 192, 102359. [Google Scholar] [CrossRef]
- Kandeyaya, K.; Ranatunga, S.; Ranatunga, R. Occurrence of microplastics in some commercially important seafood varieties from Negombo, Sri Lanka. Reg. Stud. Mar. Sci. 2023, 62, 102958. [Google Scholar] [CrossRef]
- Anandhan, K.; Tharini, K.; Hamid Thangal, S.; Yogeshwaran, A.; Muralisankar, T. Occurrence of Microplastics in the Gastrointestinal Tracts of Edible Fishes from South Indian Rivers. Bull. Environ. Contam. Toxicol. 2022, 109, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Dambrosio, A.; Cometa, S.; Capuozzo, F.; Ceci, E.; Derosa, M.; Quaglia, N.C. Occurrence and characterization od microplastics in commercial mussels (Mytilus galloprovincialis) from Apulia region (Italy). Foods 2023, 12, 1495. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon; Linnaeus 1758) from coastal waters of the southern North Sea and channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Martinez, I.E. Consumption of commercially sold dried fish snack “Charales” contaminated with microplastics in Mexico. Environ. Pollut. 2023, 332, 121961. [Google Scholar] [CrossRef] [PubMed]
- Rukmangada, R.; Naidu, B.C.; Nayak, B.B.; Balange, A.; Chouksey, M.K.; Xavier, K.A.M. Microplastic contamination in salted and sun-dried fish and implications for food security—A study on the effect of location, style and constituents of dried fish on microplastics load. Mar. Pollut. Bull. 2023, 191, 114909. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Pham, D.T.; Kim, J.; Lee, S.; Kim, J.; Kim, D.; Hong, S.; Jung, J.; Kwon, J. Analysis of microplastics in various foods and assessment of aggregate human exposure via food consumption in Korea. Environ. Pollut. 2023, 322, 121153. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, L.; Dai, J.; Wang, G. A microscopic survey on microplastics in beverages: The case of beer, mineral water and tea. Analyst 2022, 6, 1099–1105. [Google Scholar] [CrossRef]
- Hernandez, L.; Xu, E.; Larsson, H.; Tahara, R.; Maisuria, V.; Tufenkj, N. Plastic teabags release billion of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Bilal, M.; Taj, M.; Hassan, H.U.; Yaqub, A.; Shah, M.I.A.; Sohail, M.; Rafiq, N.; Atique, U.; Abbas, M.; Sultana, S.; et al. First report on microplastics quantification in poultry chicken and potential human health risks in pakistan. Toxics 2023, 11, 612. [Google Scholar] [CrossRef]
- Prihandari, R.; Karnpanit, W.; Kittibunchakul, S.; Kemsawasd, V. Development of Optimal Digesting Conditions for Microplastic Analysis in Dried Seaweed Gracilaria fisheri. Foods 2021, 10, 2118. [Google Scholar] [CrossRef]
- Ribeiro, F.; Okoffo, E.; O’Brien, E.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef]
- Squillante, J.; Scivicco, M.; Ariano, A.; Nolasco, A.; Esposito, F.; Cacciola, N.A.; Severino, L.; Cirillo, T. Occurrence of phthalate esters and preliminary data on microplastics in fish from the Tyrrhenian Sea (Italy) and impact on human health. Environ. Pollut. 2023, 316, 120664. [Google Scholar] [CrossRef]
- Taghipour, H.; Ghayebzadeh, M.; Mousavi, S.M.S.; Sharifi, H.; Payandeh, A. Incidence and exposure to microplastics in table salt present in the Iran market. Toxicol. Rep. 2023, 11, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Canha, N.; Jafarova, M.; Grifoni, L.; Gamelas, C.A.; Alves, L.C.; Almeida, S.M.; Loppi, S. Microplastic contamination of lettuces grown in urban vegetable gardens in Lisbon (Portugal). Sci. Rep. 2023, 13, 14278. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Perez-Guevara, F.; Elizalde-Martinez, I.; Kutralam-Muniasamy, G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks Future research and environmental considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef] [PubMed]
- Köktürk, M.; Betül Özgeriş, F.; Atamanalp, M.; Uçar, A.; Özdemir, S.; Parlak, V.; Avni Duyar, H.; Alak, G. Microplastic-induced oxidative stress response in turbot and potential intake by humans. Drug Chem. Toxicol. 2023. [Google Scholar] [CrossRef]
- WHO. Dietary and Inhalation Exposure to Nano- and Microplastic Particles and Potential Implications for Human Health; Licence: CC BY- NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Agarwal, R.; Roy, K. Intracellular delivery of polymeric nanocarriers: A matter of size, shape, charge, elasticity and surface composition. Ther. Deliv. 2013, 4, 705–723. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Lou, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges abd prospects. Trend. Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Ren, T.; Wang, J.; Shan, J. Microplastics contamination in food and beverages: Direct expoaure to humans. J. Food Sci. 2021, 86, 2816–2837. [Google Scholar]
- Eerkes-Medrano, D.; Leslie, H.A.; Quinn, B. Microplastics in drinking water: A review and assessment. Environ. Sci. Health 2019, 7, 69–75. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, Q.; Wei, X.; Chen, C.; Ma, J.; Crittenden, J.C.; Liu, B. Tracing microplastics in rural drinking water in Chongqing, China: Their presence and pathways from source to tap. J. Hazard. Mater. 2023, 459, 132206. [Google Scholar] [CrossRef] [PubMed]
- Nur, A.U.; Hossain, M.B.; Banik, P.; Choudhury, T.R.; Liba, S.I.; Umamaheswari, S.; Albeshr, M.F.; Senapathi, V.; Arai, T.; Yu, J. Microplastic contamination in processed and unprocessed sea salts from a developing country and potential risk assessment. Chemosphere 2022, 308, 136395. [Google Scholar] [CrossRef]
- Lopez-Rosales, A.; Andrade, J.M.; Lopez-Mahía, P.; Muniategui-Lorenzo, S. Development of an analytical procedure to analyze microplastics in edible macroalgae using an enzymatic-oxidative digestion. Mar. Pollut. Bull. 2022, 183, 114061. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Hollman, P.; Peters, R. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 49, 8947. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Bastyans, S.; Jackson, S.; Fejer, G. Micro and nano-plastics, a threat to human health? Emerg. Top Life Sci. 2022, 6, 411–422. [Google Scholar]
- Visalli, G.; Facciolà, A.; Pruiti Ciarello, M.; De Marco, G.; Maisano, M.; Di Pietro, A. Acute and Sub-Chronic Effects of Microplastics (3 and 10 μm) on the Human Intestinal Cells HT-29. Int. J. Environ. Res. Public Health 2021, 18, 5833. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics and human health: Integrating pharmacokinetics. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1489–1511. [Google Scholar] [CrossRef]
- Ramsperger, A.F.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef]

| Concept #1 | Concept #2 | Concept #3 | Concept #4 |
|---|---|---|---|
| Microplastic * plastic particle * microplastic particle * | Analytical determination * Size Sample treatment * | Human health Negative effect * Adverse effect * | Food Food matrices Beverages |
| OR | OR | OR | OR |
| AND | |||
| Digestion Agent | Time (h) | Temperature (°C) | Digestion Efficiency | Ref. |
|---|---|---|---|---|
| Edible fish tissues | ||||
| KOH (10%) + H2O2 (30%) | 42 | N/A | 97.4 ± 0.5% | [10] |
| KOH (10%) | 24 | 60 | N/A | [59] |
| 36 | 60 | N/A | [51] | |
| HNO3 (6.3%) | Heating time of 10 min. | Up to 200 | N/A | [11] |
| Holding time of 10 min. | 200 | |||
| HNO3 (69%) | 72 | 60 | 98.55 ± 0.37% | [52] |
| H2O2 (30%) | 24 | 30 | N/A | [60] |
| 48 | 65 | N/A | [44] | |
| 24–48 | R.T. | |||
| 24 | 65 | N/A | [48] | |
| 24–48 | R.T. | |||
| 72 | 45 | N/A | [12] | |
| 72 | 60 | 74.73 ± 0.77% | [52] | |
| enzymes +H2O2 (30%) | 36 | 60 | 99–100% | [56] |
| NaOH (10%) | 72 | 60 | 63.86 ± 1.47% | [52] |
| Sodium dodecyl sulfate (10%) | 72 | 60 | 40.30 ± 2.70% | [52] |
| Trypsin (5%) | 72 | 40 | 57.60 ± 7.85% | [52] |
| Meat | ||||
| KOH (10%) | 10 | 75 | N/A | [32] |
| 36 | 55 | [57] | ||
| Sea Salt | ||||
| Not digested (dissolved into distilled water) | N/A | N/A | N/A | [17,18,19,28] |
| Fenton’s reagent | 30 to 60 min | R.T and 75 | N/A | [21] |
| H2O2 (30%) | 24 | 65 | N/A | [20,24] |
| 24 | 60 | [22] | ||
| 24 | 50 | [61] | ||
| 48 | 40 | [23] | ||
| KOH (30%) | N/A | N/A | N/A | [25] |
| KOH (30%) + H2O2 (35%) | N/A | N/A | N/A | [25] |
| Sugar | ||||
| Not digested (dissolved into distilled water) | N/A | N/A | N/A | [18] |
| Beer | ||||
| H2O2 | 72 | N/A | N/A | [16] |
| direct filtration | N/A | N/A | N/A | [29] |
| Honey | ||||
| H2O2 | 72 | N/A | N/A | [16] |
| Milk | ||||
| KOH | 48–72 | N/A | N/A | [34] |
| Lettuce | ||||
| H2O2 | N/A | N/A | N/A | [62] |
| Technique | Size Limitation of the Technique | Microplastics Size Range | Particle Type | Ref. |
|---|---|---|---|---|
| SEM-EDX | N/A | 1.28–4.2 μm | N/A | [1] |
| LDIR | 20–500 μm | 6–480 μm | PET, PP, PA, PE | [2] |
| μ-FTIR | >20 μm | 11–530 μm | PE | [3] |
| μ-ATR-FTIR | >7 μm | 11–50 μm | ||
| μ-Raman | >0.45 μm | ~5 μm | PET | [4] |
| μ-FTIR | >20 μm | 5–4659 μm | PET, PS, Nylon | [5] |
| μ-Raman | >0.45 μm | 0.06–5.89 mm | PO | [6] |
| μ-FTIR | >20 μm | N/A | LDPE, PET, PE, PS, AC | [7] |
| μ-FTIR | >20 μm | 0.005–5 mm | PET, Rayon, PES, Nylon, PP, CP, PE | [9] |
| μ-Raman | >0.45 μm | 412–648 μm | PP, PE, PS, PET | [10] |
| μ-Raman | >0.45 μm | 190–3800 μm | PP, PET | [13] |
| μ-FTIR | >20 μm | 2.48–6742.48 mm | PE, PP, PAM | [16] |
| μ-ATR-FTIR μ-Raman | >7 μm >0.45 μm | 0–1000 μm | PP, PA, PE | [17] |
| μ-ATR-FTIR | >7 μm | 45 μm–4.3 mm | PE, PP | [18] |
| μ-ATR-FTIR | >20 μm | N/A | PP, PE, PES | [19] |
| μ-FTIR | >20 μm | 0.39–7.02 mm | PE, PP | [20] |
| μ-ATR-FTIR | >7 μm | 65–2500 μm | LDPE, HDPE | [21] |
| μ-ATR-FTIR | >7 μm | 300–5000 μm | PP, PE, PET, PS | [22] |
| μ-Raman | >0.45 μm | 5 to >1000 μm | Rayon, PP, PES, PE | [23] |
| μ-ATR-FTIR | >7 μm | 23.2 μm–3.9 mm | PA, PU | [24] |
| μ-FTIR | >20 μm | 45–100 μm | HDPE, PP, PET, PS, PA | [25] |
| μ-FTIR | >20 μm | 3.8 μm–5.2 mm | CP, PS, PA, PAR | [26] |
| SEM-EDX | N/A | 100–500 μm | PE | [27] |
| μ-FTIR | >20 μm | 30 μm–3.5 mm | PET, PP, PE | [28] |
| μ-FTIR | >20 μm | N/A | PET | [29] |
| μ-FTIR | >20 μm | <300 μm | PVC, PET, PTFE, HDPE, Nylon, ABS | [30] |
| μ-FTIR | >20 μm | 15.6–1151.1 μm | PE | [32] |
| μ-FTIR | >20 μm | 5–20 μm | PS, PP, PE | [34] |
| μ-Raman | >0.45 μm | <5 μm | PET | [35] |
| μ-ATR-FTIR | >7 μm | N/A | PES, PET, PS | [42] |
| μ-Raman | >0.45 μm | 1.2–10 μm | PE, PET, PC | [43] |
| μ-FTIR | >20 μm | 0.06–0.11 mm | PE, PP, PS, Nylon | [44] |
| μ-FTIR | >20 μm | 0.2–22 mm | PE, PP | [46] |
| μ-Raman | >0.45 μm | 15–1175 μm | PS | [47] |
| SEM/EDX | N/A | 100–1000 μm | PET, PE | [50] |
| μ-Raman | >0.45 μm | <300 μm | PE, PP, PET | [54] |
| μ-FTIR | >20 μm | 520 nm–270 μm | Nylon, PET | [56] |
| μ-FTIR | >20 μm | 453–3885 μm | Nylon, PP, HDPE | [60] |
| μ-Raman | >0.45 μm | N/A | PE, PP, PS, PVC, PET | [58] |
| μ-Raman | >0.45 μm | 0.1–3 mm | PA, PET, PAS | [63] |
| μ-ATR-FTIR | >7 μm | 24–1670 μm | PES, ABS, EPM, nylon-6, CP, and viscose | [51] |
| μ-FTIR | >20 μm | <100 μm to 5000 μm | PP, PE | [52] |
| μ-FTIR | >20 μm | 50–500 μm | PVC, LDPE, PS, PP | [57] |
| μ-FTIR | >20 μm | 20–150 μm | PE, PP, PS | [61] |
| μ-Raman | >0.45 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fiore, C.; Carriera, F.; Russo, M.V.; Avino, P. Are Microplastics a Macro Issue? A Review on the Sources of Contamination, Analytical Challenges and Impact on Human Health of Microplastics in Food. Foods 2023, 12, 3915. https://doi.org/10.3390/foods12213915
Di Fiore C, Carriera F, Russo MV, Avino P. Are Microplastics a Macro Issue? A Review on the Sources of Contamination, Analytical Challenges and Impact on Human Health of Microplastics in Food. Foods. 2023; 12(21):3915. https://doi.org/10.3390/foods12213915
Chicago/Turabian StyleDi Fiore, Cristina, Fabiana Carriera, Mario Vincenzo Russo, and Pasquale Avino. 2023. "Are Microplastics a Macro Issue? A Review on the Sources of Contamination, Analytical Challenges and Impact on Human Health of Microplastics in Food" Foods 12, no. 21: 3915. https://doi.org/10.3390/foods12213915
APA StyleDi Fiore, C., Carriera, F., Russo, M. V., & Avino, P. (2023). Are Microplastics a Macro Issue? A Review on the Sources of Contamination, Analytical Challenges and Impact on Human Health of Microplastics in Food. Foods, 12(21), 3915. https://doi.org/10.3390/foods12213915







