3.1. Extraction Yields and Total Phenolic Content of Skin and Seed Flours
The extraction yields (g of freeze-dried extract obtained per 100 g of flour used) were variable among the different samples (
Table 2) and ranged from 4.9% (Muscat blanc) to 15.6% (Pinot noir 1) for the skins and from 5.2% (Nebbiolo 2) to 17.5% (Pinot noir 1) for the seeds.
The extraction yields were, on average, lower than those reported by [
27] for white GP of four different cultivars. In our work, a less performing “green” solvent (hydroalcoholic solution) was used, aiming to obtain and characterize polyphenolic extracts intended for human consumption or use in the medical/cosmetic/pharmaceutical field.
As regards skins, the highest extraction yield was observed for Pinot noir 1, followed by Nebbiolo 1 > Arneis, Barbera, Cortese, Grignolino, Pinot noir 2 > Nebbiolo 2, Muscat blanc.
Regarding seeds, the highest extraction yield was observed for Pinot noir 1, followed by Muscat blanc, Cortese > Nebbiolo 1, Arneis, Pinot noir 2 > Grignolino, Barbera > Nebbiolo 2. The extraction yield from seeds was more influenced by the winemaking technique: higher in seeds derived from non-macerated pomace (UGP) and lower in seeds from long-macerated GP (i.e., Nebbiolo 2).
The total polyphenolic content (GAE) of the skin and seed flours before the extraction was calculated from the GAE content of the freeze-dried extracts (reported in
Table 3) according to the different extraction yields. The skin flours had GAE values ranging from 7.1 (Muscat blanc) to 26.2 (Pinot noir 1) mg/g d.w. of flour, while for the seed flours, the GAE values ranged from 12.6 (Nebbiolo 2) to 86.5 (Pinot noir 1) mg/g d.w. of flour. The polyphenolic content of the seeds was always higher than that of the respective skins.
The extraction yields from the seed flours were highly correlated with their total polyphenol content (r = 0.97), while for the skins, the correlation was lower (r = 0.81). Only about 65% of the variability in the extraction yields (R
2 value of the linear regression equation) was explained by the polyphenolic content, while the remaining 35% depended on the content of other molecules. From some skin flours (Pinot noir 2, Grignolino, Arneis, and Cortese), with a lower polyphenolic content and proportionally lower extraction yields than Pinot noir 1 (the richest in polyphenols), the obtained extracts were richer in total polyphenols than the extract from Pinot noir 1, which probably had a higher content of extractable compounds other than polyphenols (
Table 3). The lower correlation between GAE in the skin flours and the skin extracts could also be related to the presence of non-extractable polyphenols (NEPP) that remain in the residue after the extraction, permanently linked to dietary fiber. As we observed in a previous work [
28], the NEPP content was higher in the skins than in the seeds. The concentration of NEPP in the skins varied with the cultivar, while the provenience as UGP or FGP did not seem to have a discriminating effect; conversely, the NEPP content of the seeds was more homogeneous among the different samples.
3.2. Polyphenolic Content of Freeze-Dried Extracts
The total polyphenols content (GAE) varied from 127 to 298 mg/g d.w. for the skins and 242 to 540 mg/g d.w. for the seeds (
Table 3). The skin and seed extracts also differ in terms of total flavonoid content (108–322 mg/g d.w. in the skins and 303–754 mg/g d.w. in the seeds) and the condensed tannins content (59–143 mg/g d.w. in the skins and 158–414 mg/g d.w. in the seeds).
As regards the skin extracts, among white grape cultivars, Cortese and Arneis had a similar total polyphenols content (GAE), significantly higher than Muscat blanc; in addition, Cortese had a higher total flavonoids content than Arneis, linked to the higher condensed tannins content. Among red grape cultivars, the highest concentrations of total polyphenols, total flavonoids, and condensed tannins were observed for Pinot noir 2, followed by Grignolino.
As regards the seed extracts, significant differences in polyphenolic content were observed between white grape cultivars. Muscat blanc had the highest total polyphenols content (GAE), followed by Cortese and Arneis. On the other hand, Cortese extracts were the richest in condensed tannins and total flavonoids compared to the other two cultivars.
The polyphenolic content of Pinot noir seeds extract (as both UGP and FGP) fell within the concentration range of white grape cultivars. Considering the other red grape cultivars, Pinot noir was followed by Nebbiolo 1 > Grignolino > Barbera > Nebbiolo 2.
The richness in polyphenols of Pinot noir seeds is consistent with the data reported in the literature. The seed extracts from UGP (white cultivars and unfermented Pinot noir 1) were all significantly richer in polyphenolic compounds than those from FGP (excluding Pinot noir 2).
3.2.1. Condensed Tannins Composition
The qualitative composition of condensed tannins in the extracts is reported in
Table 3. The mDP varied from 4.8 (Pinot noir 1) to 7.9 (Cortese) in the skin extracts and from 3.4 (Pinot noir 1) to 6.6 (Cortese) in the seed extracts, with statistically significant differences between cultivars.
These mDP values are similar [
29,
30] or lower than those reported in the literature for skins and seeds from unfermented grapes ([
31,
32] for Pinot noir; [
25,
33] for red grape cultivars; [
34] for white and red grape cultivars). The mDP values were also similar to or higher than those we observed in previous works [
21,
33] for skins and seeds from GP of the same red grape cultivars and similar to the values reported for GP by [
27].
As regards the condensed tannins profile, (
−)-epicatechin (EC) was the most abundant extension subunit both in the skins (from 46.0 to 65.0%) and in the seeds (from 52.1 to 63.0%). In most skin samples, the second most abundant extension subunit was (+)-catechin (C), followed by (
−)-epicatechin-3-O-gallate (ECG), except for Arneis, Cortese, and Grignolino where ECG was more abundant than C. Conversely, ECG prevailed on C for all seed samples. Furthermore, (
−)-epigallocatechin (EGC) was the lowest percentage subunit (from 0.8 to 5.5%) and was present only as an extension subunit in the skins. Among the terminal subunits, C prevailed over EC in the skin tannins and in most of the seed tannins (only in three cases did EC slightly exceed C). As regards the prevalence of EC both as an overall subunit and as an extension subunit and the prevalence of C as a terminal subunit, our data are consistent with those reported by [
27] for condensed tannins extracted from GP.
The subunit profiles of the condensed tannins extracted from GP resulted differently from those reported for skins and seeds from whole fresh grapes. In the skins, EC (dominant subunit) is followed by EGC [
25,
31,
34], while in the seeds, EC is followed by C or ECG, depending on the cultivar. An important percentage loss of EGC was observed in the fermented GP of some red cultivars compared to the corresponding fresh grapes [
33], possibly due to its selective extraction during the fermentative maceration.
As regards the galloylation degree of condensed tannins (%ECG), the literature reports a marked difference between skins and seeds from whole fresh grapes. Seed tannins generally have a higher galloylation degree than skin tannins [
31]. In the present work on GP, except for Muscat blanc, the differences in %ECG between seeds and skins were modest, and, in some cases, the skin tannins had, on average, higher %ECG than the seed tannins. These data are consistent with the results of our previous work focused on red grape cultivars [
33]. For whole fresh grapes, the galloylated forms in seed tannins were 4.3 to 6 times higher than in skin tannins, but they dropped to 0.7–2.1 times for the respective fermented GP.
During the fermentative maceration and the subsequent pressing, a part of the tannins extracted from the seeds, rich in galloylated subunits, are probably selectively adsorbed on the skins. To our knowledge, this selective adsorption was never reported in the bibliography. In a previous work [
35], we observed a higher adsorption of the trihydroxylated forms of anthocyanins compared to the dihydroxylated ones on the yeast’s cell walls during alcoholic fermentation. We already reported on the higher percentage losses during the fermentative maceration of the EGC subunits of skin tannins (trihydroxylated on the B ring) compared to C and EC (dihydroxylated). As regards the extracts from UGP, considering that it was not subjected to fermentative maceration but only to pressing, we could have expected the prevalence of the galloylated forms of tannins in the seeds compared to the skins, which, on the contrary, was observed only for Muscat blanc. However, it cannot be excluded that extraction/adsorption phenomena may already occur during crushing and pressing and during storage of GP before drying. In a previous work on the high-temperature drying of GP [
19], we observed an increase in the polyphenolic content of the skins at the expense of the seeds.
3.2.2. Monomeric and Oligomeric Flavan-3-ols
Table 4 reports the content of the flavan-3-ols identified and quantified in the extracts: (+)-catechin (C), (−)-epicatechin (EC), and (−)-epicatechin-3-O-gallate (ECG) among monomers, procyanidin B1, B2, and B3 among dimers, and trimer C1.
Monomers were the most abundant molecules, mostly in seed extracts (22–319 μmol/g d.w. for seeds, 4.8–24.8 μmol/g d.w. for skins). Significant differences were observed between the extracts of the different cultivars: Pinot noir’s skins and seeds (as both UGP and FGP) were the richest in monomeric flavan-3-ols. C was the most abundant molecule in most of the skin and seed samples. Its concentration always exceeded that of EC, except for Grignolino (skins and seeds) and Nebbiolo (skins). The predominance of C on EC was observed in some white GP (Macabeu and Parellada), while in others (Chardonnay and Premsal blanc), C and EC were at similar concentrations [
27]. C prevailed over EC in both skins and seeds of Greek red and white grapes [
36,
37], and, apart from one case (Shiraz seeds), in all skins and seeds of red and white grapes studied by [
38]. ECG was present in all seed extracts, while small amounts were quantified in six out of nine skin samples. In the literature, ECG was identified only in grape seeds [
39], and its presence in GP skins could depend on adsorption phenomena occurring during winemaking or the subsequent stages of GP processing.
Like monomers, oligomeric flavanols were more abundant in seeds than in skins. As regards skins, dimer B1 was predominant in seven out of nine extracts, and dimer B2 in Pinot noir (as both UGP and FGP). Dimer B3 was overall the least important. It was identified and quantified in only four out of nine extracts. As regards seeds, a higher variability of composition was observed between the different cultivars, with a variable prevalence of one of the four analyzed oligomers.
The varietal variability of oligomeric flavanols is confirmed by the literature; in general, the prevalence of procyanidin B1 or procyanidin B2 is reported depending on the cultivar [
27]. Only [
38] observed the prevalence of dimer B3 in the skins of some red and white cultivars grown in warm climates.
3.2.3. Flavonols
Table 5 reports the contents of the main flavonols identified and quantified in the skin extracts: quercetin, kaempferol, and their respective glucoside and glucuronide forms. Moreover, myricetin-3-O-glucoside was identified in the skins of Grignolino, Barbera, Pinot noir 1, and Nebbiolo 1, and free myricetin only in Nebbiolo 1 (data not reported). The presence of aglycon myricetin and its glucoside form only in some red GP agrees with [
38], who identified 3-O-glycosides (glucuronide and glucoside) forms of myricetin only in the skins of red grapes. The authors of [
40,
41] identified in white grape skins only 3-O-glycosides based on kaempferol, quercetin, and isorhamnetin flavonoid structures.
Flavonols are absent in seeds: these compounds accumulate only in the grape skins with the role of protecting berries from light, particularly from UVB. Only the 3-O-glycosides forms of flavonols (3-O-glucosides, 3-O-galactosides, and 3-O-glucuronides) were identified in grapes. The presence of quercetin, kaempferol, and myricetin as aglycones in GP skins is due to the enzymatic hydrolysis of the 3-O-glycosides forms during the winemaking process [
42]. Therefore, the aglycons quercetin and kaempferol were more abundant in the skins from FGP, subjected to macerations, while the 3-O-glycosides forms prevailed in the skins from UGP, separated from the must immediately after pressing.
Given the differences between UGP and FGP in the content of free and glycosylated forms of flavonols, the skins of the different cultivars were compared for the total flavonols content (sum of the single compounds), expressed in μmol/g d.w. (
Table 5). Statistically significant differences were observed between all cultivars: the highest concentrations were observed for Arneis skins, followed by Muscat blanc > Barbera > Nebbiolo 1 > Pinot noir 2. The presence of flavonol makes the skins fraction of GP interesting (particularly in the food sector) due to their important bioactive properties (antiradical, anti-inflammatory, antioxidant, antiviral, antimicrobial, anticancer) [
43].
3.2.4. Phenolic Acids
Table 5 reports the content of gallic acid and hydroxycinnamyltartaric acid (HCTA) in the extracts.
Gallic acid was present in the skin and seed extracts at higher concentrations for seeds (except Grignolino and Nebbiolo 2). Muscat blanc seeds were those with the highest gallic acid content.
Modest concentrations of HCTAs, particularly trans-caftaric acid, normally the most abundant HCTA in grapes and wines [
44], were detected, together with traces of other HCTAs. These acids are found mainly in grape juice but also in the skins, where the concentration ratios between some of the HCTAs have been used as varietal indices [
45]. The total HCTA content in GP skins ranged from 0 (Pinot noir 1) to 5.3 µmol/g (Barbera); statistically significant differences linked to the cultivar and the kind of GP were observed. In addition, ref. [
44] found significant differences between cultivars in the HCTA content of grape skins; since these differences varied with the vintage, the authors hypothesized the existence of a combined effect linked to the degree of ripeness of the grapes. Finally, t-caftaric acid was also identified in some seed samples (possible adsorption on the seed’s surface). Similarly, ref. [
44] reported the presence of t-caftaric and t-cutaric acid in GP seeds.
3.3. DPPH Index
The antiradical capacity was significantly higher for seeds than skins (
Table 3). However, this difference did not depend only on the higher polyphenolic content of the seeds, as can be seen from the comparison between the average values of the angular coefficients of the lines passing through the origin, which model the relationship between DPPH
AAE and GAE, respectively, equal to 0.0129 for the skins and 0.0172 for the seeds. At equal GAE values, the seeds had a higher antiradical capacity (DPPH) than the skins. This result could depend on the fact that condensed tannins, proportionally more abundant in seeds than in skins, have a higher antiradical activity than other classes of polyphenols [
46].
Correlations between the DPPH Index and the Main Polyphenolic Compounds
Table 6 shows the correlation matrices between the DPPH
AAE index and the main parameters describing the polyphenolic profile of the extracts (as classes of polyphenolic compounds: total polyphenols, total flavonoids, condensed tannins, total monomeric and total dimeric flavan-3ols, total HCTA and total flavonols; as single compounds: gallic acid and trimer C1).
The correlation between DPPH and the main polyphenolic indices (total polyphenols, condensed tannins, and total flavonoids) was overall higher for seeds than for skins. This probably depends on the fact that seed polyphenols are mainly composed of flavan-3-ols (monomers and polymers), while skin polyphenols also include HCTA and flavonols, and this confirms the existence of a structure–activity relationship of the polyphenolic constituents in the different samples [
47].
Overall, the highest correlations concerned the DPPH parameter and the GAE index. As regards polyphenolic compounds, a high correlation was observed between monomeric flavan-3-ols and dimeric flavan-3-ols in both the skin and seed extracts, while condensed tannins were correlated to monomeric and dimeric flavan-3-ols only in seed extracts. Finally, in the skin extracts, the HCTAs were correlated with gallic acid, while total flavanols were not correlated with any of the other parameters.
The relationship (univariate linear regression) between the DPPH parameter and each of the main polyphenolic compounds was also studied.
Table 7 shows the regression lines between DPPH (variable Y) and each class of compound considered (variable X) separately for skin and seed extracts. The regression lines between DPPH and GAE were those with the best modeling capacity (highest
R2).
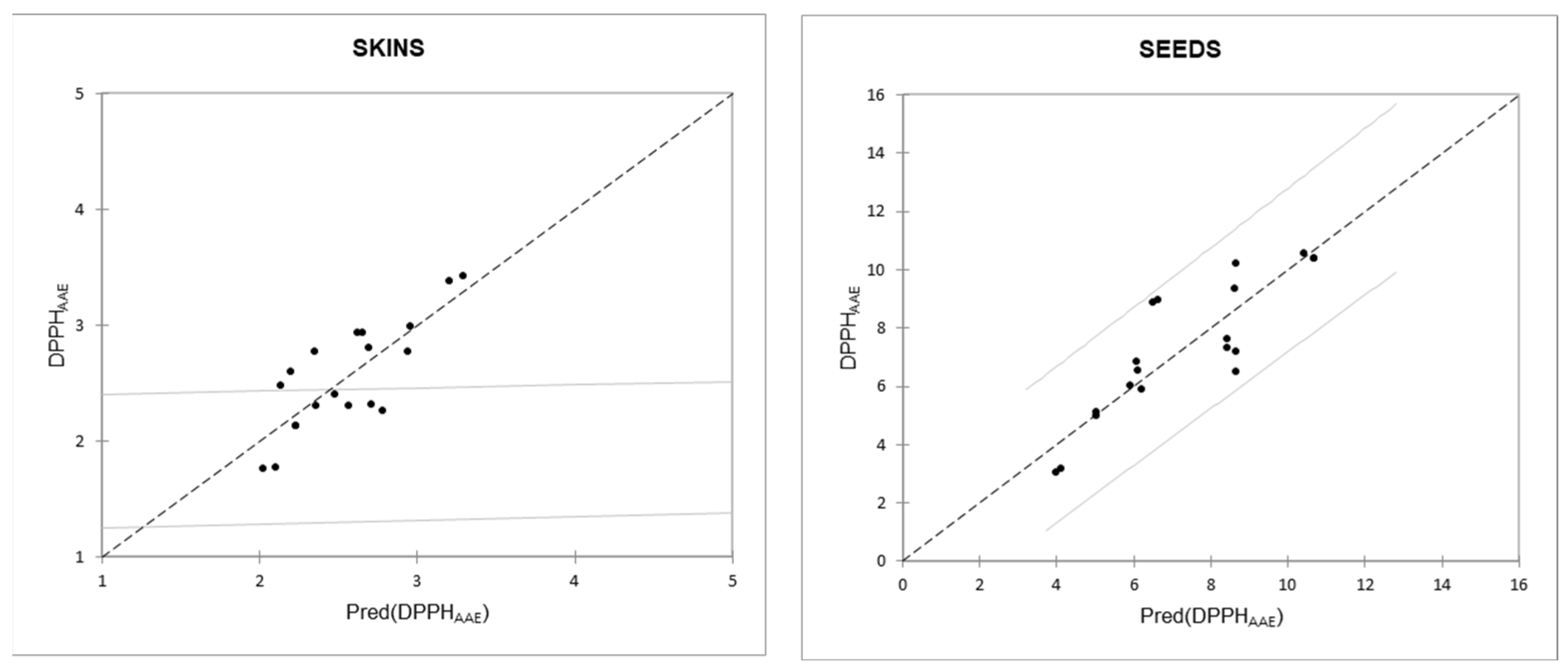
Considering all classes of polyphenolic compounds, the multiple linear regression lines that best model the relationship with the DPPH parameter (variable Y) are those described by the variables “condensed tannins” and “total flavonols” for the skin extracts and those described by the variables “condensed tannins” and “gallic acid” for the seed extracts (
Figure 1); the relationship between DPPH and GAE has R
2 values close to those of the two aforementioned regression lines (
Table 7).
3.4. Fractionation of Freeze-Dried Extracts
The fractionation was aimed at separating oligomeric and polymeric flavan-3-ols (proanthocyanidins) according to their molecular weight: monomers (Fraction 2A), oligomers (Fraction 2B), and polymers (Fraction 3). Fraction 1 was not considered since it did not contain flavonoids.
The distribution of total polyphenols (GAE) in the different fractions is reported in
Figure 2 (skin extracts) and
Figure 3 (seed extracts).
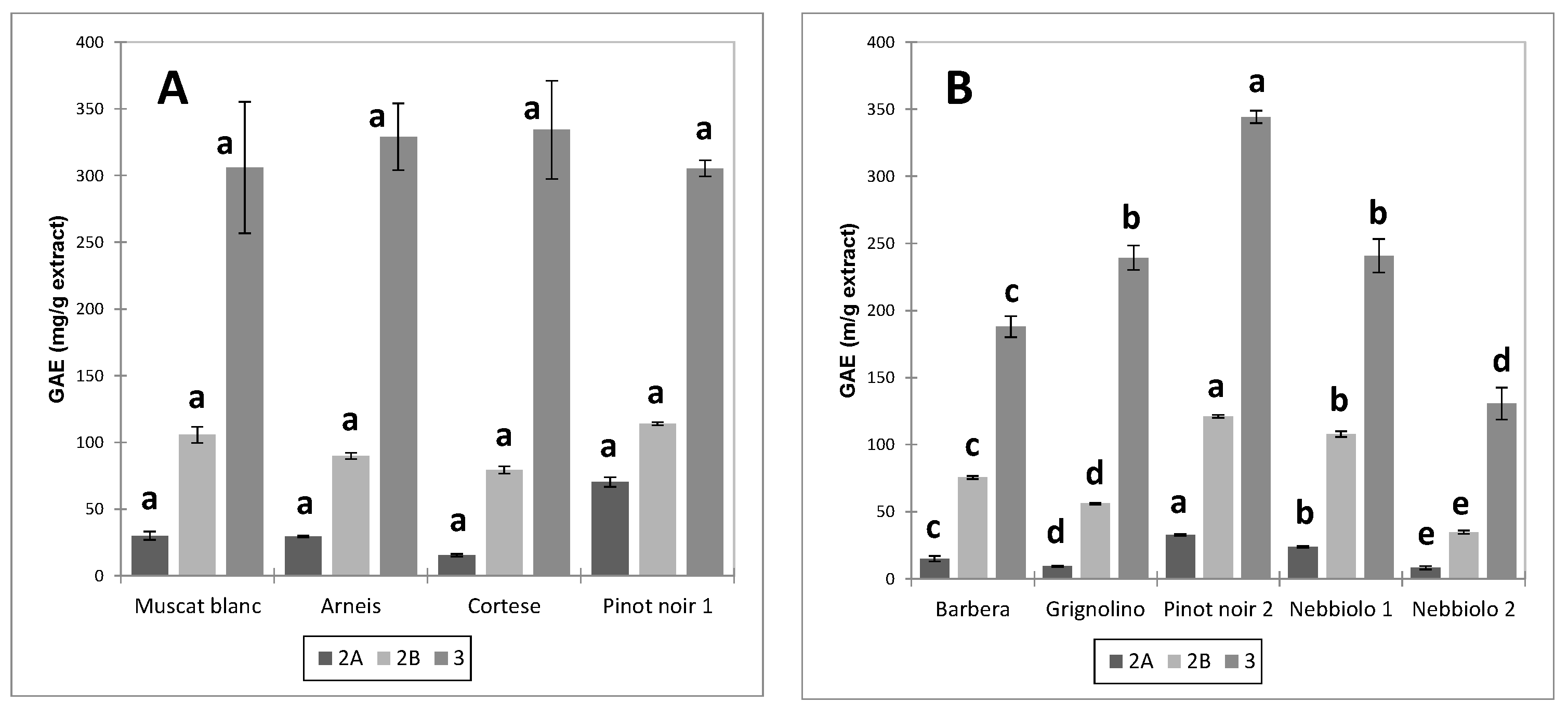
Fraction 3 was the richest in GAE, followed by fraction 2B and fraction 2A. The order was unchanged for all extracts. These data are consistent with the findings of [
25] for wine and grape skins and seeds. The total polyphenols content of the seed extracts exceeded that of the skin extracts for all fractions. Significant differences were observed due to the cultivar and the winemaking technique (UGP or FGP).
As regards unfermented pomace (UGP), the extracts from Cortese and Pinot noir skins had the highest content of total polyphenols (GAE) in fraction 3 compared to Arneis and Muscat blanc. In proportion to the other cultivars, the total polyphenol content in fraction 2 of Pinot noir (PN1) was lower. Among the fermented pomace (FGP), the highest content of polyphenolic compounds in the three different fractions was observed for Pinot noir (PN2), followed by Grignolino, Nebbiolo (Ne2 and Ne1), and finally by Barbera, with statistically significant differences between all cultivars for fractions 2 and 3. In this case, no variations were observed in the proportions of the polyphenolic content between fractions, with the exception of fraction 2A, the least abundant.
Conversely, no statistically significant differences were detected between the fractions of seed extracts from UGP. As regards FGP seeds, the polyphenolic content of fraction 3 dropped significantly in the order Pn2 > Ne1 = Gr > Ba > Ne2.
In the case of skins, it is interesting to notice how the polyphenolic profile of the three fractions was superimposable for the two Nebbiolo samples derived from macerations of different duration (
Figure 2b), while this did not happen for the respective seeds (
Figure 3b), where the polyphenolic concentration of the three fractions was lower for Nebbiolo 2 (subjected to long maceration) than for Nebbiolo 1 (short maceration). This evidence confirms that with the prolongation of fermentative maceration, the extraction mainly concerns the polyphenolic component of the seeds (delayed extraction due to the presence of ethanol), while in the case of the skins, the losses in polyphenolic compounds due to extraction are counteracted by adsorption phenomena.
3.4.1. Oligomeric and Polymeric Flavan-3-ols in the Skin Extracts
Table 8 reports the content of total polyphenols and condensed tannins in fractions 2B and 3 of the skin extracts.
The highest recoveries of condensed tannins were observed in fraction 3 (between 31.3 and 67.0% of the condensed tannins content in the total extracts), while in fraction 2B, the recoveries were more modest (4.6–11.0%). The concentrations of total polyphenols (GAE) and condensed tannins in fractions 3 and 2B were proportional to the respective concentrations in the total extracts (values of the correlation coefficients between the total extract and fractions 3 and 2B, respectively, equal to 0.934 and 0.903 for the GAE index and 0.937 and 0.921 for condensed tannins).
The fractionation separated the condensed tannins according to the different sizes. Fraction 2B contained oligomeric proanthocyanins with mDP values ranging from 2.2 to 3.0, while fraction 3 contained polymeric proanthocyanins with mDP values ranging from 7.3 to 10.4 (1.6 to 3.3 units higher than those observed in the total extracts).
The percentage weights of the different subunits (total, terminal, and extension subunits) that make up the polymeric proanthocyanins of fraction 3 were very similar to those observed for the total extracts (highly significant correlations and r values between 0.709 and 0.950). The same was not observed for the percentage weights of oligomeric proanthocyanins (fraction 2B), for which the correlation coefficients were lower and variable among the different subunits (r values ranging from −0.196 to 0.925).
The main differences in the subunit profiles between polymeric (fraction 3) and oligomeric (fraction 2B) proanthocyanins concerned the percentage weight of the terminal units, which was higher in fraction 2B (tannins with lower mDP). Overall, apart from some exceptions, the percentage weight of the C subunit was, on average, lower for polymeric proanthocyanins (fraction 3) than for oligomeric ones (fraction 2B); conversely, the percentage weight of the EC and G subunits was, on average, higher in fraction 3 than in fraction 2B.
3.4.2. Oligomeric and Polymeric Flavan-3-ols in the Seed Extracts
Table 9 reports the content of total polyphenols and condensed tannins in fractions 2B and 3 of the seed extracts.
In addition, in the case of seed extracts, polymeric proanthocyanidins (fraction 3) were more abundant (from 35 to 52% of the condensed tannins content in the total extracts) than oligomeric ones (fraction 2B, from 10.6 to 24.1% of the condensed tannins content in the total extracts). The mDP of oligomeric proanthocyanins (2B) varied between 2.9 and 3.5 units, and these values were similar and homogeneous among the different cultivars, as already observed for the skin extracts. The polymeric proanthocyanins (fraction 3) had mDP values ranging from 6.7 to 10.4 units (2.8 to 4.8 units higher than those of the total extracts).
Compared to the total extract, the subunit composition of oligomeric and polymeric proanthocyanins in the two fractions changed. In fraction 2B, the percentage weight of C increased, while the opposite was observed for fraction 3. As already observed in other works [
21,
48], the condensed tannins with a lower mDP were richer in C than those with a higher mDP. In both fractions (2B and 3), the percentage weight of the galloylated forms decreased compared to the total extract.
In fraction 2A of the skin extracts, the B2 dimer was detected in trace amounts, while in fraction 2A of the seed extracts, the B1, B2, and B3 dimers and the C1 trimer were detected in quantifiable concentrations, only for some cultivars (data not reported).
As regards monomer flavan-3-ols, they were identified in fraction 2A in the skins and, only for some cultivars, were detected in trace amounts in fraction 2B. In the seeds, where their concentration is higher, they were predominantly present in fraction 2A and at very low concentrations (0–1 mg/g dw as catechin) also in fraction 2B. The monomer flavan-3-ols content in fractions 2A is reported in
Table 10. For both skins and seeds, the content of (+)-catechin and (−)-epicatechin in fraction 2A was significantly correlated to the content of the same molecules in the respective extracts. The correlation coefficients (Pearson’s r) are, respectively, equal to 0.95 and 0.89 for C and EC in the skins and 0.98 and 0.91 for C and EC in the seeds.
3.5. PCA Analysis
The data related to the polyphenolic composition of skin and seed extracts were finally subjected to PCA.
Figure 4 shows the loadings (variables) and scores (samples) in the space defined by the first two Principal Components, which together describe 73% of the total data variability.
The first Component discriminates the seed extracts from the skin extracts. Compared to skins, seeds were characterized by higher polyphenolic content (GAE, total flavonoids, condensed tannins, flavonols, gallic acid, oligomeric compounds), lower HCTA content, and the absence of flavonols. The highest variability was observed for the seed extracts. The seeds from UGP (Mb, Co, Ar, and Pn1) were, on average, richer in polyphenolic compounds than those from FGP, with the exception of Pinot noir 2. A higher uniformity was observed for the skin extracts. The second Component discriminates the extracts according to the composition of condensed tannins: in particular, the skin and seed tannins of Grignolino and Cortese were richer in galloylated units.
The PCA was repeated separately for skin and seed extracts (
Figure 5).
Figure 5A shows the distribution of the skin samples. The skins of Pinot noir 2 (FGP), Grignolino (FGP), and Cortese (UGP) were the richest in total polyphenolic compounds (GAE, total flavonoids, condensed tannins) and had the highest anti-radical activity (DPPH).
The origin of GP (FGP or UGP, white or red cultivars) was not a discriminating factor for skin extracts as observed for seed extracts. Unlike what was expected and what was observed for skin flours, the extracts from unfermented Pinot noir skins (PN1, UGP) were poorer in polyphenolic compounds than those from fermented skins (PN2, FGP) (differences in extraction yields, paragraph 3.1). Furthermore, Nebbiolo 2 skins, macerated for a longer duration, were richer in polyphenols than Nebbiolo 1 skins, macerated for shorter times (adsorption on the skins of the polyphenols solubilized in the must—wine).
It is also interesting to notice how the antiradical activity (DPPH parameter) was positively associated with the total content of polyphenolic compounds (total flavonoids, GAE, condensed tannins) while it was independent or negatively correlated with the content of single classes of polyphenolic compounds, respectively, monomeric and dimeric flavanols, and flavonols. This result confirms what was reported by [
47], who observed the presence of correlations between the total polyphenols content (GAE) and the anti-radical activity determined with the DPPH test for GP seed extracts, particularly rich in condensed tannins, unlike onion skin extracts, richer in flavonols, which were more reactive towards the hydroxyl radical. Other authors observed that the antioxidant activity of the extracts was positively correlated with the mDP of condensed tannins up to mDP 10, while it dropped and remained at a lower level with mDP > 10 [
49]. In our case, we observed that for the seeds, in which the percentage weight of condensed tannins in the total polyphenolic content was higher than for the skins, each concentration unit of total polyphenols (GAE) corresponds to a higher value of antiradical activity (DPPH).
As regards seeds (
Figure 5B), the main differences in concentration (first Principal Component) were linked to the cultivar (Pinot noir was the richest) and also to the winemaking technique: UGPs were richer than FGPs. In the case of Nebbiolo, the polyphenolic content dropped in the seeds subjected to longer macerations (lower in Nebbiolo 2 than in Nebbiolo 1). As observed for the skins, the DPPH parameter was positively associated with the overall polyphenolic content (GAE, total flavonoids, condensed tannins) but not with the content of the individual classes of flavonols (monomers, dimers, and trimers).