Determination of Modified QuEChERS Method for Chlorothalonil Analysis in Agricultural Products Using Gas Chromatography–Mass Spectrometry (GC-MS/MS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Selection and Collection
2.3. GC-MS/MS Analytical Conditions
2.4. Sample Preparation
2.5. Method Validation
3. Results and Discussion
3.1. Optimization of Analytical Conditions in GC-MS/MS
3.2. Optimization of Sample Preparation
3.2.1. Selection of Extraction
3.2.2. Selection of Clean-Up Procedure
3.3. Method Validation
3.3.1. Selectivity, Linearity, Matrix Effect, Limit of Detection, Limit of Quantification
3.3.2. Accuracy and Precision
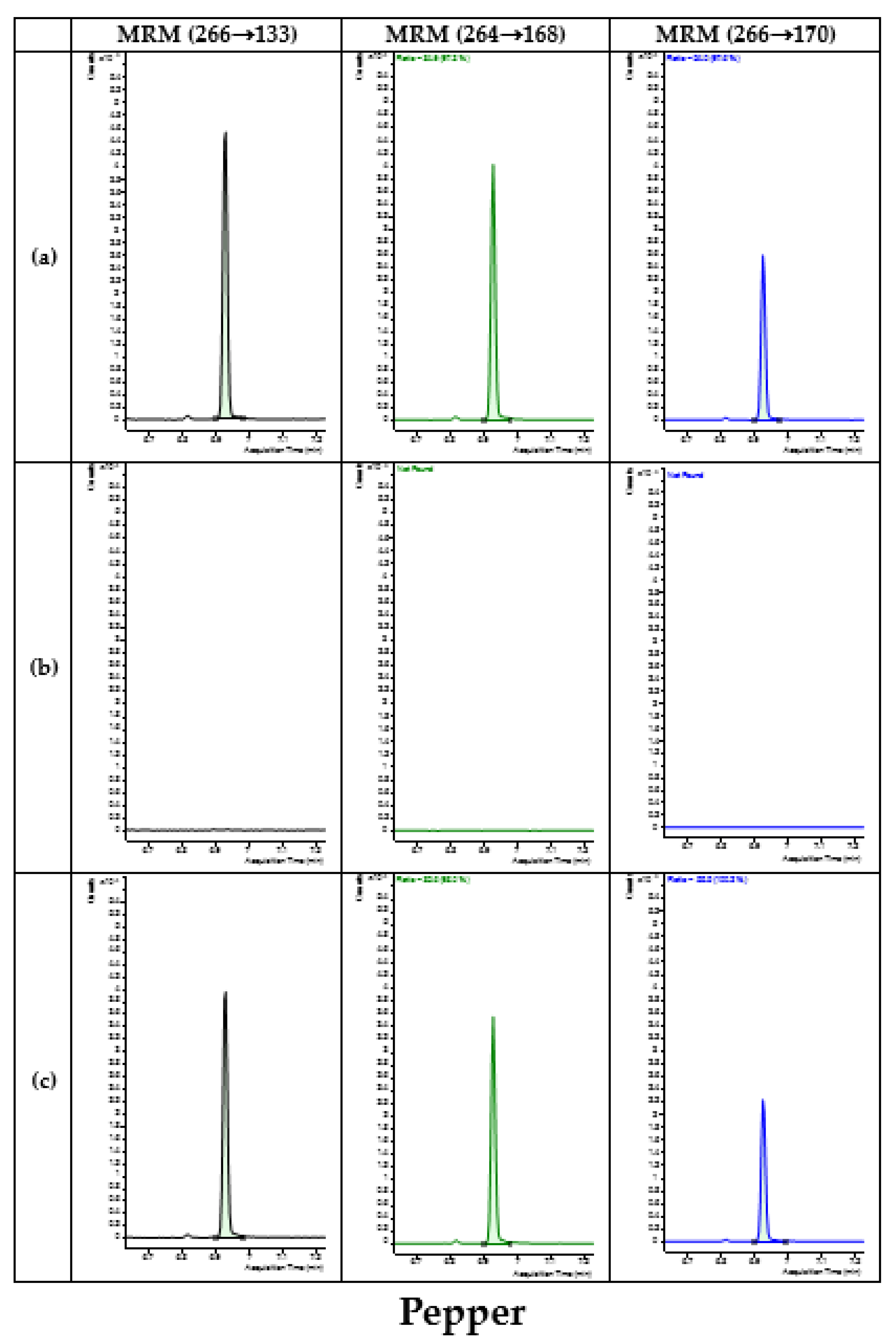
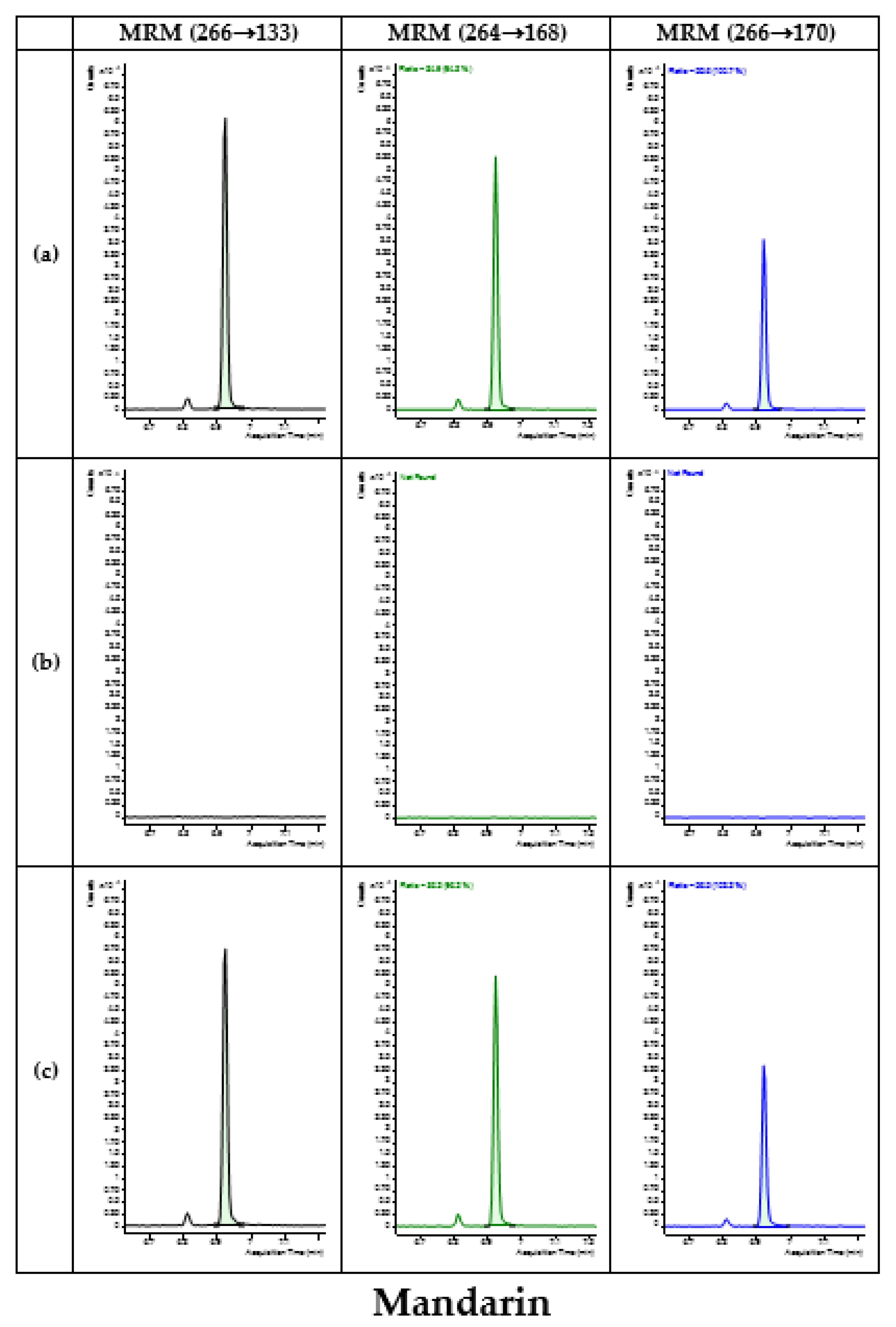
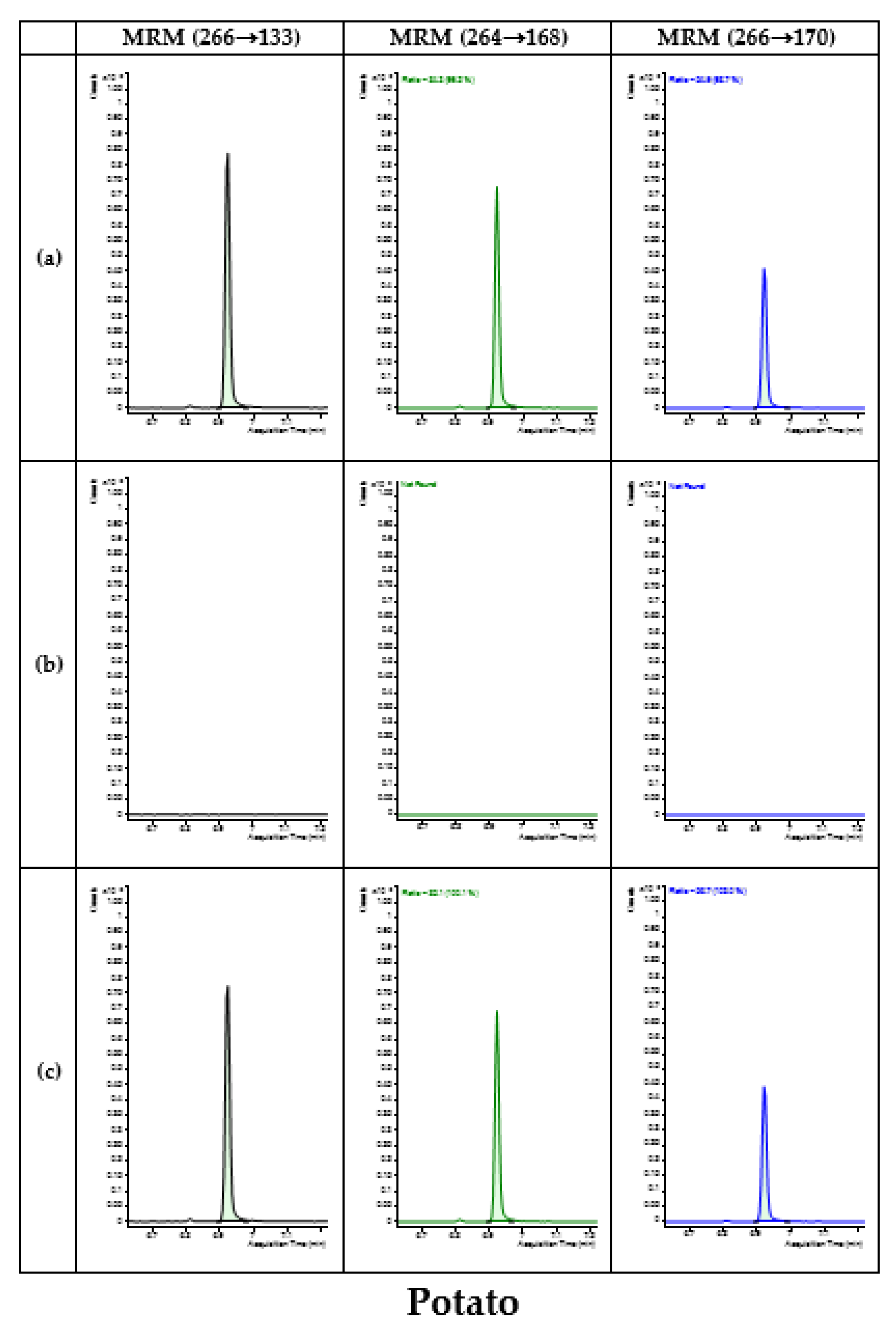
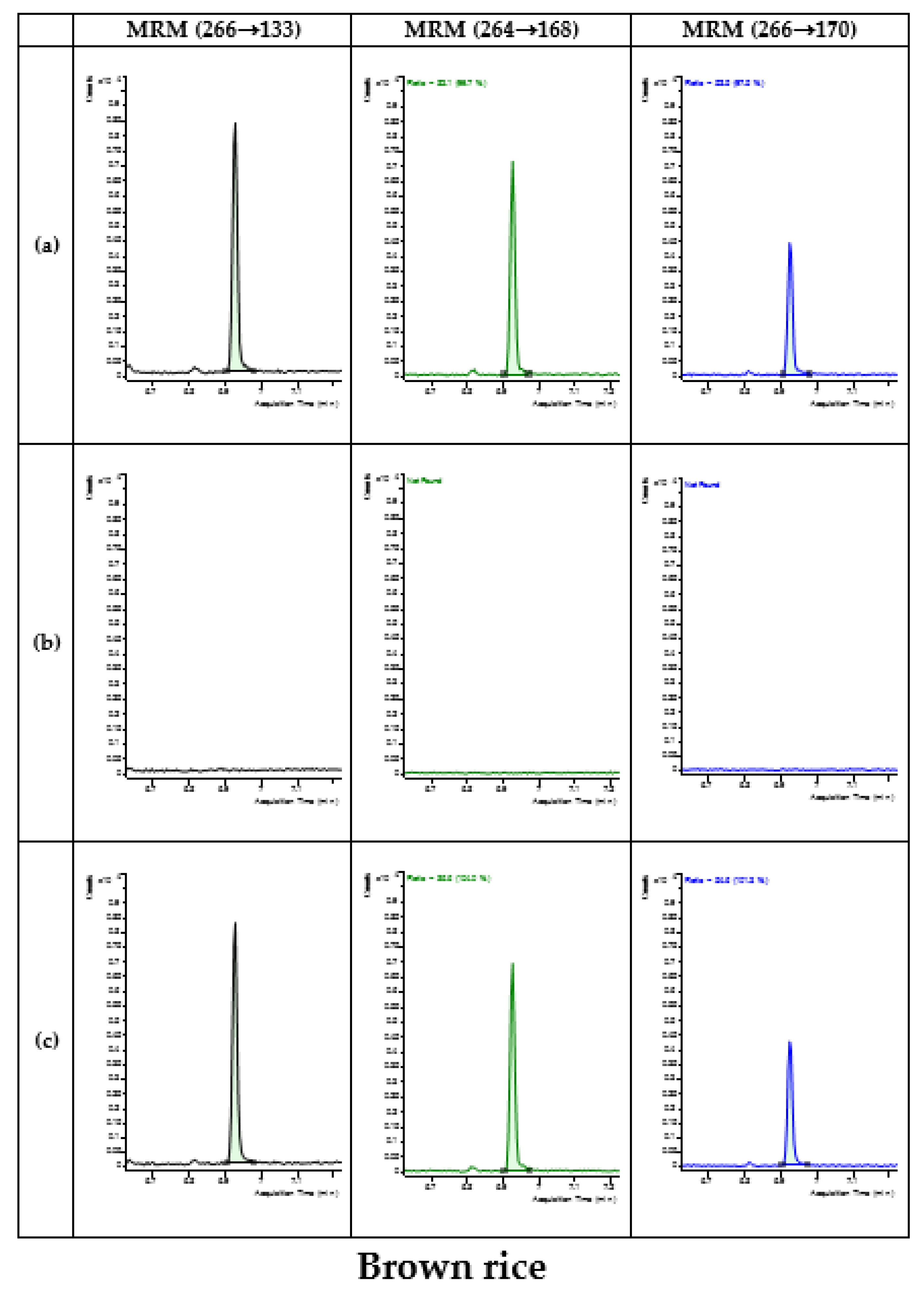
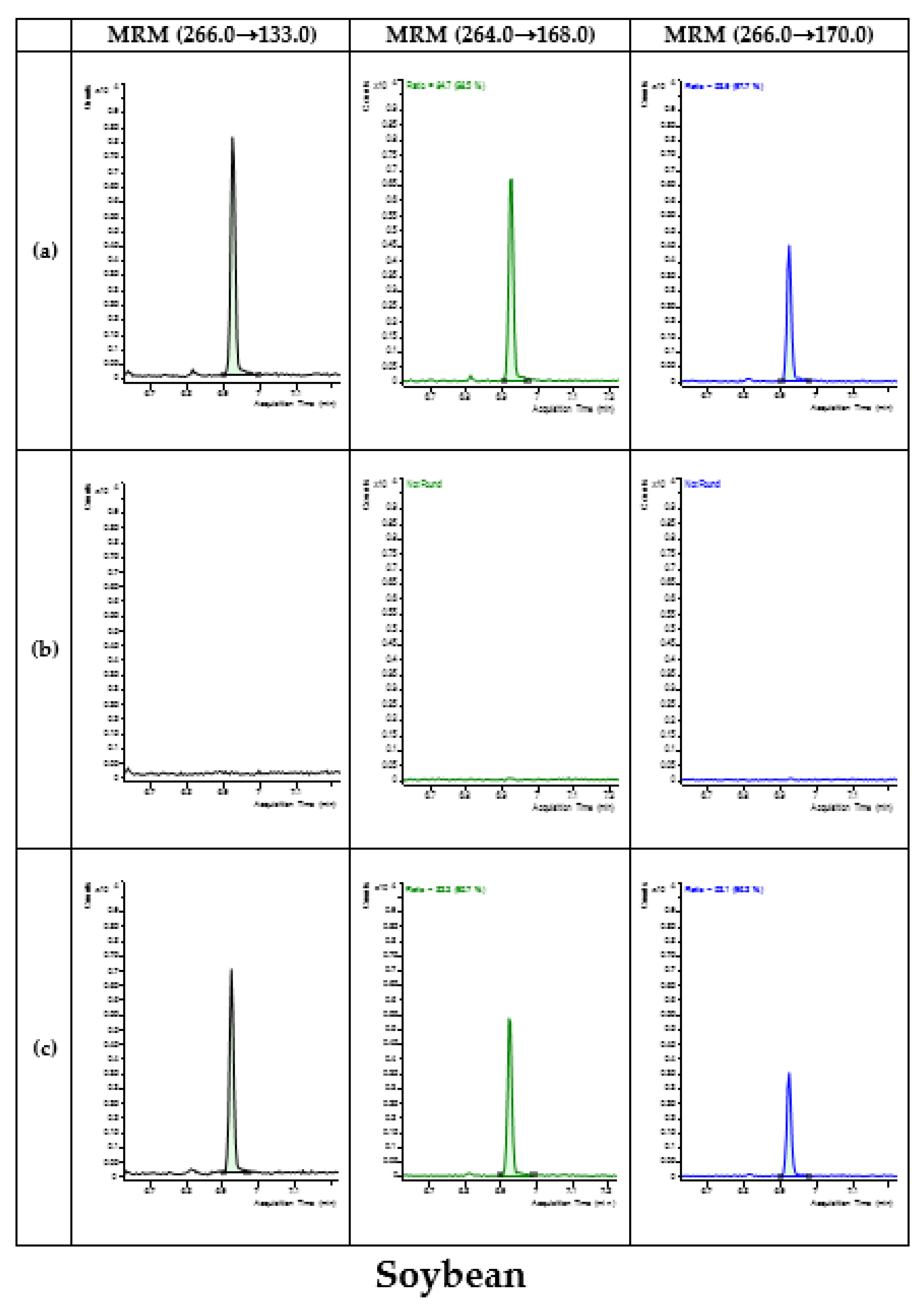
3.4. Application of Developed Method in Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dias, M.C. Phytotoxicity: An Overview of the Physiological Responses of Plants Exposed to Fungicides. J. Bot. 2012, 2012, 135479. [Google Scholar] [CrossRef]
- Lawruk, T.S.; Gueco, A.M.; Jourdan, S.W.; Scutellaro, A.M.; Fleeker, J.R.; Herzog, D.P.; Rubio, F.M. Determination of chlorothalonil in water and agricultural products by a magnetic particle-based enzyme immunoassay. J. Agric. Food Chem. 1995, 43, 1413–1419. [Google Scholar] [CrossRef]
- Downing, H.F.; Delorenzo, M.E.; Fulton, M.H.; Scott, G.I.; Madden, C.J.; Kucklick, J.R. Effects of the agricultural pesticides atrazine, chlorothalonil, and endosulfan on South Florida microbial assemblages. Ecotoxicology 2004, 13, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Inventory of Evaluations Performed by the Joint Meeting on Pesticide Residues (JMPR), JMPR_2009_Report. Available online: https://apps.who.int/pesticide-residues-jmpr-database/pesticide?name=CHLOROTHALONIL (accessed on 3 May 2023).
- Codex Allimentarius Commission. Pesticide Database-Maximum Residue Limits. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=81 (accessed on 3 May 2023).
- The Japan Food Chemical Research Foundation (JFCRF). Table of MRLs for Agricultural Chemicals. Available online: https://db.ffcr.or.jp/front/pesticide_detail?id=23300 (accessed on 3 May 2023).
- The Code of Federal Regulations. Protection of Environment. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-180/subpart-C/section-180.275 (accessed on 3 May 2023).
- Foodsafetykorea. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 3 May 2023).
- Seok, J.H.; Kim, G.S. Impact of the positive list system (PLS) on the banana market in Korea. J. Asia Pac. Econ. 2020, 25, 718–732. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety. Food Code (No.2022-7, 2022.1.27.). Available online: https://www.mfds.go.kr/brd/m_211/view.do?seq=14662&srchFr=&srchTo=&srchWord=%EC%8B%9D%ED%92%88%EC%9D%98+%EA%B8%B0%EC%A4%80+%EB%B0%8F+%EA%B7%9C%EA%B2%A9&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=2 (accessed on 25 September 2023).
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Zhao, L.; Liu, F. Determination of chlorothalonil residue in cabbage by a modified QuEChERS-based extraction and gas chromatography–mass spectrometry. Food Anal. Methods 2016, 9, 656–663. [Google Scholar] [CrossRef]
- Valles, N.B.; Retamal, M.; Martínez-Uroz, M.A.; Mezcua, M.; Fernandez-Alba, A.R.; De Kok, A. Determination of chlorothalonil in difficult-to-analyse vegetable matrices using various multiresidue methods. Analyst 2012, 137, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Lee, H.S.; Park, J.S.; Lee, S.J.; Shin, H.S.; Chung, Y.M.; Choi, H.N.; Jung, Y.H.; Oh, J.H.; Yun, S.S. Determination of residual triflumezopyrim insecticide in agricultural products through a modified QuEChERS method. Foods 2021, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission (CAC). Guidelines on Good Laboratory Practice in Pesticide Residue Analysis, CAC/GL 40-1993; Rev.1-2003; CAC: Rome, Italy, 2003. [Google Scholar]
- Ministry of Food and Drug Safety. Practical Manual on the Analysis of Residual Pesticides in Food; Guidelines on Standard Procedures for Preparation of Test Methods; National Institute of Food and Drug Safety Evaluation: Osong, Republic of Korea, 2016.
- EU Reference Laboratories for Residues of Pesticides. Modified QuEChERS-Method for the Analysis of Chlorothalonil in Fruits and Vegetables; EU Reference Laboratories for Residues of Pesticides: Valencia, Spain, 2022. [Google Scholar]
- Li, P.; Duan, Y.; Ge, H.; Zhang, Y.; Wu, X. Multiresidue analysis of 113 pesticides in different maturity levels of mangoes using an optimized QuEChERS method with GC-MS/MS and UHPLC-MS/MS. Food Anal. Methods 2018, 11, 2742–2757. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.; Lee, J.; Lee, J.; Kim, B.J.; Kim, J.H. Simultaneous analysis of 310 pesticide multiresidues using UHPLC-MS/MS in brown rice, orange, and spinach. Chemosphere 2018, 207, 519–526. [Google Scholar] [CrossRef]
- Lee, J.; Kim, L.; Shin, Y.; Lee, J.; Lee, J.; Kim, E.; Moon, J.-K.; Kim, J.-H. Rapid and simultaneous analysis of 360 pesticides in brown rice, spinach, orange, and potato using microbore GC-MS/MS. J. Agric. Food Chem. 2017, 65, 3387–3395. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 980–1010. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. The Pesticide Manual, 17th ed.; BCPC: Hampshire, UK, 2015; pp. 196–197. [Google Scholar]
- Heo, H.M.; Jo, H.W.; Chang, H.R.; Moon, J.K. Development of Simultaneous Analytical Method for Imidazolinone Herbicides from Livestock Products by UHPLC-MSMS. Foods 2022, 11, 1781. [Google Scholar] [CrossRef]
- Pogăcean, M.O.; Hlihor, R.M.; Gavrilescu, M. Monitoring pesticides degradation in apple fruits and potential effects of residues on human health. J. Environ. Eng. Landsc. Manag. 2014, 22, 171–182. [Google Scholar] [CrossRef]
- Tan, G.H.; Ali, A.; Siddiqui, Y. Current strategies, perspectives and challenges in management and control of postharvest diseases of papaya. Sci. Hortic. 2022, 301, 111139. [Google Scholar] [CrossRef]
- Hwang, J.I.; Seok, D.R.; Kim, J.E. Effects of cuticular waxes on permeation of fungicides azoxystrobin and chlorothalonil into apples. Appl. Biol. Chem. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- Jones, J.G.; Korir, R.C.; Walter, T.L.; Everts, K.L. Reducing chlorothalonil use in fungicide spray programs for powdery mildew, anthracnose, and gummy stem blight in melons. Plant Dis. 2020, 104, 3213–3220. [Google Scholar] [CrossRef] [PubMed]

| Analyte | CAS No. | Molecular Formula | Structure | Vapor Point (mPa) |
|---|---|---|---|---|
| Chlorothalonil | 1897-45-6 | C8Cl4N2 |  | 0.076 |
| Analyte | Molecular Weight (g/mol) | Exact Mass (g/mol) | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (eV) |
|---|---|---|---|---|---|
| Chlorothalonil | 265.9 | 263.9 | 266 | 133 (a) | 45 |
| 170 | 30 | ||||
| 264 | 168 | 30 |
| Sorbent Type | Recovery (%) | CV a (%) |
|---|---|---|
| MgSO4 150 mg, PSA 25 mg | 93.3 | 10.4 |
| MgSO4 150 mg, C18 25 mg | 83.2 | 10.8 |
| MgSO4 150 mg, GCB 2.5 mg | 58.2 | 9.0 |
| MgSO4 150 mg, PSA 25 mg, C18 25 mg | 102.7 | 5.1 |
| Matrix | Equation | R2 | LOD | LOQ | Matrix Effect (%) |
|---|---|---|---|---|---|
| Solvent | y = 8622.5x + 6773 | 0.9968 | 0.003 | 0.01 | - |
| Brown rice | y = 10,469x – 12,291 | 0.9959 | 21.4 | ||
| Soybean | y = 4192.4x + 8532.3 | 0.9939 | −51.4 | ||
| Pepper | y = 6765.7x − 3063.6 | 0.9961 | −36.0 | ||
| Potato | y = 5795.2x + 3682.6 | 0.9971 | −21.5 | ||
| Mandarin | y = 5317.3x + 12,987 | 0.9964 | −32.8 |
| Matrix | Fortification (mg/kg) | Intra-Day | Inter-Day | Ave. a | |||
|---|---|---|---|---|---|---|---|
| Recovery (%) | CV b (%) | Recovery (%) | CV b (%) | Recovery (%) | CV b (%) | ||
| Brown rice | 0.01 | 89.6 | 7.0 | 101.1 | 4.1 | 95.4 | 8.5 |
| 0.1 | 100.8 | 6.0 | 91.5 | 1.7 | 96.2 | 6.8 | |
| 0.5 | 109.2 | 5.8 | 89.3 | 2.6 | 99.3 | 14.2 | |
| Soybean | 0.01 | 96.8 | 11.6 | 79.6 | 17.7 | 88.2 | 13.8 |
| 0.1 | 72.0 | 3.1 | 86.5 | 13.0 | 79.3 | 12.9 | |
| 0.5 | 81.7 | 5.5 | 92.7 | 7.3 | 87.2 | 8.9 | |
| Mandarin | 0.01 | 112.4 | 5.7 | 87.2 | 12.5 | 99.8 | 17.9 |
| 0.1 | 96.5 | 11.6 | 93.9 | 2.8 | 95.2 | 1.9 | |
| 0.5 | 87.9 | 7.7 | 96.8 | 11.6 | 92.4 | 6.8 | |
| Potato | 0.01 | 93.7 | 12.5 | 87.9 | 8.6 | 90.8 | 4.5 |
| 0.1 | 89.3 | 8.3 | 99.3 | 12.2 | 94.3 | 7.5 | |
| 0.5 | 77.5 | 12.1 | 89.6 | 7.5 | 83.5 | 10.2 | |
| Pepper | 0.01 | 89.0 | 1.3 | 111.9 | 6.2 | 100.5 | 16.1 |
| 0.1 | 93.7 | 7.4 | 114.4 | 3.9 | 104.1 | 14.1 | |
| 0.5 | 84.7 | 7.5 | 94.2 | 3.6 | 89.5 | 7.5 | |
| Type | Commodity | No. of Samples | No. of Detected Samples | Concentration (mg/kg) |
|---|---|---|---|---|
| Fruits | Apple | 31 | 8 | 0.01–0.10 |
| Banana | 16 | - | - | |
| Mandarin | 26 | - | - | |
| Persimmon | 18 | - | - | |
| Vegetables | Cucumber | 22 | 1 | 0.04 |
| Green onion | 17 | - | - | |
| Onion | 30 | - | - | |
| Radish | 29 | - | - | |
| Tomato | 24 | - | - | |
| Watermelon | 16 | 1 | 0.08 | |
| Korean melon | 16 | - | - | |
| Cereals | Rice | 34 | - | - |
| Potatoes | Potato | 28 | - | - |
| Sweet potato | 19 | - | - | |
| Legumes | Soybean | 14 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, D.-Y.; Bae, J.-Y.; Park, C.-W.; Jang, G.-H.; Choe, W.-J. Determination of Modified QuEChERS Method for Chlorothalonil Analysis in Agricultural Products Using Gas Chromatography–Mass Spectrometry (GC-MS/MS). Foods 2023, 12, 3793. https://doi.org/10.3390/foods12203793
Yun D-Y, Bae J-Y, Park C-W, Jang G-H, Choe W-J. Determination of Modified QuEChERS Method for Chlorothalonil Analysis in Agricultural Products Using Gas Chromatography–Mass Spectrometry (GC-MS/MS). Foods. 2023; 12(20):3793. https://doi.org/10.3390/foods12203793
Chicago/Turabian StyleYun, Da-Young, Ji-Yeon Bae, Chan-Woong Park, Gui-Hyun Jang, and Won-Jo Choe. 2023. "Determination of Modified QuEChERS Method for Chlorothalonil Analysis in Agricultural Products Using Gas Chromatography–Mass Spectrometry (GC-MS/MS)" Foods 12, no. 20: 3793. https://doi.org/10.3390/foods12203793
APA StyleYun, D.-Y., Bae, J.-Y., Park, C.-W., Jang, G.-H., & Choe, W.-J. (2023). Determination of Modified QuEChERS Method for Chlorothalonil Analysis in Agricultural Products Using Gas Chromatography–Mass Spectrometry (GC-MS/MS). Foods, 12(20), 3793. https://doi.org/10.3390/foods12203793






