Abstract
In this study, the effects of Lentilactobacillus buchneri (L. buchneri: CCTCC M 2023228) and Kazachstania bulderi (K. bulderi: CCTCC M 2023227) on the quality characteristics and volatile flavor substances in fermented red sour soup were explored based on natural fermentation. Compared to natural fermentation (nitrite: 5.5 mg/kg; amino acid nitrogen: 0.17 g/100 g; lycopene: 63.73 µg/mL), three fortified fermentation methods using L. buchneri, K. bulderi, and both strains together significantly reduced the concentrations of nitrite (2.62, 2.49, and 2.37 mg/kg), amino acid nitrogen (0.03 g/100 g, 0.02 g/100 g, and 0.05 g/100 g), and lycopene (26.64, 32.45, and 51.89 µg/mL). Total acid content (11.53 g/kg) and lactic acid bacteria count (285.9 ± 1.65 × 106 CFU/mL) were the elements most significantly increased by fortified fermentation with L. buchneri relative to other fermentation methods. A total of 99 volatile compounds were determined in red sour soup and could be roughly classified into alcohols, aldehydes, ketones, and esters. Fortified fermentation with two strains and fortified fermentation with K. bulderi increased the content of methyl butanoate and 3-hydroxybutan-2-one-acetoin (D). This study confirmed the effects of L. buchneri and K. bulderi on the quality and flavor of fermented red sour soup and provided a theoretical basis for the fortified fermentation of red sour soup.
1. Introduction
Red sour soup is a condiment made from tomatoes and red peppers and supplemented with some ginger, salt, and white wine [1]. It originates from the southwestern region of Guizhou, China, and has a history of more than one thousand years. Fermented red sour soup contains many organic acids, minerals, amino acids, capsaicin, and lycopene [2] and plays an important role in preventing nonalcoholic fatty liver disease, regulating intestinal flora, scavenging free radicals, enhancing immunity, and maintaining the acid–base balance [3,4,5]. Currently, red sour soup has become an essential food for daily cooking in southwest China [1] and is widely used in the production of hot pot seasonings and sour fish products.
Flavor, color, and aroma are three key quality indicators of foods [6]. Volatile aroma compounds significantly affect the flavor and overall evaluation of foods [7]. Studies have revealed that the flavor of fermented foods is closely related to microbial communities [8,9,10,11]. Fermenters affect the diversity and structure of microbial communities during fermentation [12]. The fermentation agents of fermented foods have been explored. Liu et al. [13] used a mixture of L. paracasei and K. marxianus to increase the acidity and aroma of rice sour soup and shorten its fermentation time. Song et al. [14] reported that inoculation with L. plantarum and P. pentosaceus increased the content of amino acids, alcohols, and aldehydes in sauerkraut. In Zheng et al. [15] and our previous studies [16], we found that the total acid content of the fortified fermented red sour soup reached the maximum level and was higher than the T/TSSP standard for fermented fruit and vegetable juices (pulps) and their products on the fifth and sixth days [17]. Fermentation agents play an important role in improving food quality. However, fortified fermentation with the dominant strains from red sour soup has seldom been explored.
Lactic acid bacteria (LAB) and yeasts are the most commonly used microorganisms in food fermentation. Niamahr et al. [18] used LAB and yeasts to ferment yogurt and improved the physical and chemical properties of the yogurt. Zhong et al. [19] found that the co-fermentation of LAB and non-saccharomyces cerevisiae affected the antioxidant properties, aroma compounds, and total acids of mango juice. Various LAB and yeasts can increase the lactic acidity, ester aroma, and mellow aroma of fermented products [20]. Lactobacillus and yeasts are the main strains in the fermentation of red sour soup [1] and largely affect its flavor and quality. To date, L. plantarum NR1-7, Bifidobacteriµm animalis subsp. lactis BZ11, and Candida utilis RY have been used as fermenters in red sour soup to shorten the fermentation time of red sour soup and increase the content of lycopene, 6-gingerin, capsaicin, and lactic acid [21].
Gas chromatography–ion-mobility spectrometry (GC-IMS) is a powerful technique for the separation and sensitive detection of volatile organic compounds and has the characteristics of fast response speed, high sensitivity, convenient operation, and low cost [7]. It has a wide range of applications in foods and can be used to determine adulterated foods, stale foods, and volatile metabolites in the process of food processing [22,23,24].
The traditional fermentation process of red sour soup is affected by raw materials, microbial community, fermentation time, and fermentation temperature and the fortified fermentation of red sour soup has seldom been reported. Fermentation with the dominant bacteria screened from red sour soup may help to improve its quality and flavor. Therefore, we investigated the effects of dominant LAB and/or yeasts on the physicochemical properties, organic acids, lycopene, and volatile flavor components of red sour soup. This study provided a theoretical basis for the fortified fermentation of red sour soup.
2. Materials and Methods
2.1. Materials
Ingredients for preparing sour soup, including tomatoes, chillies, garlic, ginger, salt, and glutinous rice, were purchased from Shiban Logistics Park, Huaxi District, Guiyang City, Guizhou Province. Lentilactobacillus buchneri (L. buchneri: CCTCC M 2023228) and Kazachstania bulderi (K. bulderi: CCTCC M 2023227) are the strains in red sour soup screened by our group. De Man–Rogosa–Sharpe (MRS), modified Chalmers agar (MC) and yeast extract peptone dextrose medium (YPD agar medium (YPD) and YPED broth medium (YPED)) were purchased from Shanghai Bo Microbiology Technology Co. (Shanghai, China). Lycopene standard, tartaric acid, citric acid, and lactic acid were purchased from Beijing Solaibao Technology Co. (Beijing, China). Formaldehyde and sodium hydroxide were purchased from Xilong Science Co. (Shenzhen, China). Phenolphthalein, zinc acetate, p-aminobenzenesulfonic acid, naphthalene ethylenediamine hydrochloride, and sodium borate were purchased from Shanghai Yien Chemical Technology Co. (Shanghai, China). Sodium chloride, anhydrous ethanol, potassium ferricyanide, etc., were purchased from Tianjin Fuyu Fine Chemical Co. (Tianjin, China).
2.2. Strains, Culture Media, and Growth Conditions
L. buchneri and K. bulderi were screened by our group in Guizhou red sour soup. LAB and yeasts were cultured according to the method of Liu et al. [25] with minor modifications. L. buchneri was inoculated in MRS agar at 37 °C for 1 d and purified for 3 generations. Then, the purified L. buchneri was inoculated in MRS broth at 37 °C and cultured at 150 RPM for 30 h to obtain the primary seed liquid, which was then inoculated into MRS broth at 3% inoculation rate so that the secondary seed liquid could be obtained under the same conditions. K. bulderi was inoculated in YPD at 30 °C for 24 h and purified for three generations. After that, the purified K. bulderi was inoculated in YEPD at 30 °C and cultured at 150 RPM for 24 h to obtain primary seed liquid, which was then inoculated into YEPD at 3% inoculation rate so that the secondary seed medium could be obtained under the same conditions.
2.3. Preparation of Red Sour Soup Samples
Red sour soup samples were prepared according to a previous report, with minor modifications [26]. Firstly, tomatoes were mixed with other ingredients, including chillies (36%), salt (13%), glutinous rice (6%), ginger (3%), and garlic (2%), to obtain a sour soup mixture. Afterwards, fermentation was carried out with four fermentation agents: fermentation with L. buchneri (3%, mass ratio) alone (MLQ), fermentation with K. bulderi (5%, mass ratio) alone (MYQ), fermentation with a mixture of L. buchneri (3% mass ratio) and K. bulderi (5% mass ratio) (MSQ), and natural fermentation (MZ). Fermentation was carried out in triplicate and samples were acquired on days 1 and 5 of fermentation.
2.4. Physical, Microbial and Chemical Determination
The pH values of samples were measured with a pH meter (Testo 205, Titisee-Neustadt, Germany) [27]. Lactobacillus counting was performed based on the method of Liu et al. [16]. Firstly, the samples were diluted with sterile saline 10 times in series, and two to three suitable dilutions were selected and spread on MC agar plates and MRS agar. The plates were incubated aerobically at 36 ± 1 °C for 72 ± 2 h and anaerobically at 36 ± 1 °C for 72 ± 2 h, respectively. Yeast counting was performed according to GB 4789.15-2016 Counting of Moulds and Yeasts. The samples were coated on Bengal red agar plates after treatment and incubated at 28 ± 1 °C for 5 d. The counting results were recorded to calculate the total number of yeasts. Total acid content and amino nitrogen content were determined according to the acidity meter method in GB 5009.235–2016 Determination of Amino Nitrogen in Foods [22]. Nitrite content was determined according to GB5009.33-2016. Organic acid content was determined based on a previous method [26], with minor modifications, under the following conditions: column temperature of 35 °C, detection wavelength of 210 nm, injection volume of 10 μL, and flow rate of 0.8 mL/min. Lycopene content was determined according to a previous method [28].
2.5. GC-IMS Analysis
Flavors collected from different red sour soup samples after different fermentation days were detected with an Agilent 490 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with an automatic sampling device and an IMS instrument (Flavor Spec®, Analytical Sensor Systems, Dortmund, Germany). Firstly, 1 g of each red sour soup sample was weighed and added to a 20 mL headspace bottle. The samples were incubated for 15 min (80 °C, 500 RPM). After incubation, 200 μL of headspace sample was automatically withdrawn with a syringe and injected into the GC injector with N2 as the carrier gas (column temperature of 60 °C and drift tube temperature of 45 °C). The flow rate was set at 2 mL/min for 2 min, then increased to 10 mL/min within 3 min, increased to 100 mL/min within 20 min, and maintained for 5 min.
2.6. Calculation of Relative Odor Activity Value (ROAV)
On the basis of relative quantification, the ROAV values of aroma compounds in water were calculated according to a previous method [11]. The equation for each relative odor activity value is provided as follows:
where Ci denotes the relative content of aroma compounds in the sour soup (%); Ti is the aroma threshold of the compound in water (μg/kg); Cmax and Tmax, respectively, denote the relative content and aroma threshold of the compound that contributes most to the overall flavor of the sample.
ROAV = Ci/Cmax × Tmax/Ti × 100,
2.7. Statistical Analysis
The analytical software used in the experiment was composed of VOCal and three plug-ins. VOCal was used for viewing analytical spectra and the qualitative and quantitative analysis of data. Reporter plug-in was used for the direct comparison of spectral differences between samples. A gallery plot was used for the fingerprint comparison of volatile organic compounds between different samples. The dynamic PCA plug-in was used for dynamic principal component analysis. One-way analysis of variance (ANOVA) was performed using SPSS software (Version 24, SPSS Inc., Chicago, IL, USA) to determine statistical significance and Duncan’s multiple range test was performed to detect significant inter-sample differences (p < 0.05). Origin 8.5 (Origin Lab Corp, Hampton (HPT), USA) was used for data plotting. TB tools version 1.082 (Heatmap Illustrator, toolbox for biologists, China) was used to create heat maps.
3. Results
3.1. Physical and Chemical Indicators
Among all physical indicators, pH is an important parameter indicating the growth status of microorganisms and the accumulation of metabolites, which have an important impact on the quality of fermented foods. The pH values of MZ and MLQ samples decreased from 4.45 ± 0.01 and 4.25 ± 0.01 to 3.91 ± 0.01 and 3.5 ± 0.01, respectively (Figure 1a). After five days of fermentation, the pH values of MYQ and MSQ increased from 4.29 ± 0.01 and 4.14 ± 0.02 to 4.41 ± 0.01 and 4.61 ± 0.02, respectively. LAB can convert sugars into lactic acid and other organic acids [29], thus decreasing the pH value of MLQ samples. Additionally, a lower pH could inhibit the growth of complex microorganisms, thus reducing nitrite levels [30].
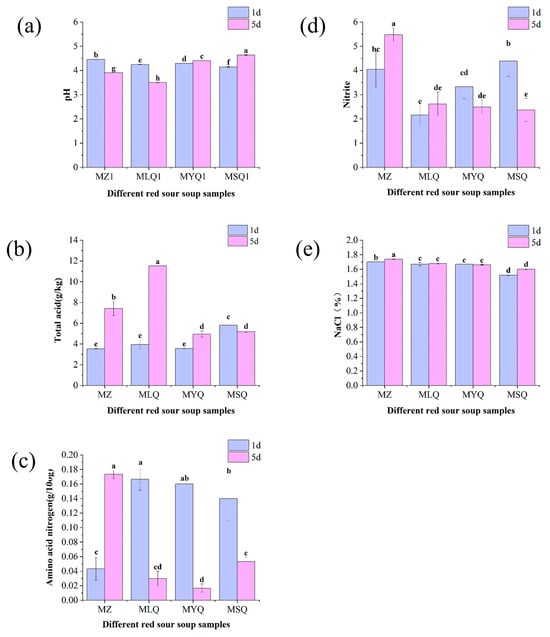
Figure 1.
Effect of different fermentation methods on pH (a), total acid (b), amino acid nitrogen (c), nitrite (d), and NaCI (e) in red sour soup samples. Different small letters indicate significant differences between samples of different red sour soups (p < 0.05).
Total acid is an important indicator of the sourness of fermented vegetables [31] and mainly related to organic acid metabolism and the degradation of raw proteins and starches by microorganisms. Figure 1b indicates the changes in total acid content in different red sour soup samples from day 1 to day 5 of fermentation. Except for the total acid content of MSQ samples, which decreased, the total acid content of other samples increased. Especially, the total acid content of MLQ samples increased most significantly from 3.56 ± 0.05 g/kg on day 1 to 11.53 ± 0.00 g/kg on day 5. These data met the industrial standard of sour soup (T/TSSP 014–2022), in which the total acid should be more than 5 g/kg (0.5 g/100 mL) [17]. The total acid content was mainly ascribed to the accumulation of organic acids (such as lactic acid) [13].
Amino acid nitrogen affects the umami flavor of fermented vegetables. During the fermentation of red sour soup, proteins were hydrolyzed into various amino acids, which endowed the red sour soup with a good flavor. Amino acid nitrogen content showed significant differences between the various samples (p < 0.05, Figure 1c). The amino acid nitrogen content of the MZ sample was 0.04 ± 0.02 c on the first day of fermentation and increased to 0.17 ± 0.01 g/100 g on the fifth day. Natural fermentation had more miscellaneous bacteria and the fermentation was slow, so amino acid nitrogen mostly accumulated on the fifth day. The amino acid nitrogen content of the MLQ, MYQ, and MSQ samples decreased from 0.17 ± 0.02 g/100 g, 0.16 ± 0 g/100 g, and 0.14 ± 0.03 g/100 g to 0.03 ± 0.01 g/100 g, 0.02 ± 0.01 g/100 g, and 0.05 ± 0.01 g/100 g, respectively, because the microbial proliferation in the early stages of fortified fermentation in these samples was intensive and produced higher levels of amino acid nitrogen than natural fermentation.
Nitrite formation and accumulation in fermented or salted vegetables often causes food safety problems [32]. The nitrite content of the MZ and MLQ samples increased from 4.05 ± 0.74 mg/kg and 2.17 ± 0.47 mg/kg to 5.48 ± 0.28 mg/kg and 2.62 ± 0.50 mg/kg, respectively (Figure 1d), because the nitrate reductase secreted by some miscellaneous bacteria on the surface of raw materials reduced the nitrate in raw materials into nitrite. The nitrite content of the MYQ and MSQ samples decreased from 3.33 ± 0.49 mg/kg and 4.39 ± 0.63 mg/kg to 2.50 ± 0.29 mg/kg and 2.37 ± 0.47 mg/kg, respectively, probably due to the effect of yeasts on nitrite reduction. The accumulation of organic acids produced by the inoculated fermentation of LAB significantly reduces the nitrite content of fermented foods [33]. In this study, the inoculation of LAB in fortified fermentation samples had no effect on reducing nitrite due to insufficient fermentation time (Figure 1e). Sodium chloride content showed no significant change from day 1 to day 5 (p > 0.05) in all the samples except the MZ sample.
3.2. Counting of Microbial Cells
The colony counts of bacteria, LAB, and yeasts in red sour soup samples on day 1 and day 5 of fermentation are shown in Table 1. The total number of bacteria, LAB, and yeast colonies detected in each sample increased significantly. After 5 days of fermentation, the cell densities of bacteria (149.33 ± 4.04 × 106 CFU/mL) and LAB (285.9 ± 1.65 × 106 CFU/mL) in the MSQ sample were higher than those in the other three samples, and the highest cell density of yeasts was found in the MYQ sample on day 5 of fermentation. It was noteworthy that the colony number of bacteria in the MLQ sample on day 5 of fermentation was much larger than that of LAB because the environment of the MLQ1 sample was more suitable for the growth and survival of miscellaneous bacteria. The growth of yeasts was influenced by the LAB fermentation agent. In the MSQ red sour soup sample, the total number of LAB was 5.5 times of that of yeast because of the antagonism between LAB and yeasts under the condition of nutrient competition [34].

Table 1.
The number of microbial colonies in red sour soup samples produced by different fermentation methods.
3.3. Changes in Organic Acids in Different Red Sour Soup Samples
Organic acids are mainly responsible for the flavor of fermented products and their composition and content largely determine the acidity of the final products [35]. Therefore, in this study, the content of seven organic acids (oxalic acid, tartaric acid, malic acid, lactic acid, acetic acid, citric acid, and succinic acid) was examined in different red sour soup samples on day 1 and day 5 of fermentation. Oxalic or tartaric acid was not detected in any sample or only a small content of oxalic or tartaric acid was detected (Table 2). The content of oxalic and tartaric acids showed no significant differences between samples (p > 0.05). The content of malic acid did not differ significantly between samples after five days of fermentation. The content of lactic acid in MLQ samples increased significantly from 2.91 ± 0.33 g/kg on day 1 to 13.55 ± 11.77 g/kg on day 5, whereas the content of lactic acid in MSQ samples decreased from 3.37 ± 1.06 g/kg on day 1 to 0.22 ± 0.19 g/kg on day 5, probably due to the interaction between LAB and yeasts. The content of acetic acid in all samples increased during fermentation. Especially, the content of acetic acid in the MSQ sample increased from 1.22 ± 0.77 g/kg to 3.41 ± 0.29 g/kg. The citric acid content of all samples decreased significantly (p < 0.05). Citric acid content in the MSQ sample decreased from 4.33 ± 0.06 g/kg to 0 g/kg because the metabolism of citric acid by LAB produced lactic acid, diacetyl, or other substances [36]. Succinic acid was not detected in MZ samples and the content of succinic acid detected in the remaining samples increased on day 5.

Table 2.
Changes in organic acids in different red sour soup samples.
3.4. Changes in Lycopene Content in Different Red Sour Soup Samples
Natural lycopene is the main pigment in ripe tomatoes and plays an important role in photosynthesis [37]. Natural lycopene is mostly located in the trans-structure [38], but its cis-structure is more bioactive and has a higher absorption rate [39]. The total lycopene content in the MZ sample increased to 63.73 ± 0.72μg/mL after five days of fermentation and was significantly higher than that in other samples (p < 0.05, Table 3). The total lycopene content in the MLQ sample decreased, but the content of cis-lycopene increased from 2.44 ± 0.03 μg/mL to 4.48 ± 0.01 μg/mL and the proportion of cis-lycopene in the MLQ sample (16.82 ± 0.36%) was the largest among all the samples. The total lycopene content or the proportion of cis-lycopene in MSQ samples did not change significantly (p ≥ 0.05) on day 1 or day 5 of fermentation. The total lycopene content and the proportion of cis-lycopene in MYQ samples also increased from 16.74 ± 0.26 μg/mL on day 1 to 30.15 ± 2.99μg/mL on day 5 and the proportion of cis-lycopene in MYQ samples also increased from 3.47 ± 0.78% to 7.11 ± 0.34%.

Table 3.
Changes in lycopene in different red sour soup samples.
3.5. HS-GC-IMS Topographic Plots of Different Red Sour Soup Samples during Fermentation
In this study, HS-GC-IMS was used to determine the flavor substances of different sour soup in various fermentation stages. Figure 2 shows the three-dimensional spectra and two-dimensional top views obtained by HS-GC-IMS. The composition of volatile substances in different samples could be compared visually. A white color indicated a lower intensity of volatile compounds and a red color indicated their higher intensity. On the first day of fermentation, MSQ had the highest signal intensity and natural fermentation had the lowest signal intensity (Figure 2b), while signal intensity showed no significant difference between MLQ and MYQ. On the fifth day of fermentation, the signal intensity of MZQ was the highest and the signal intensity of MSQ became the lowest.
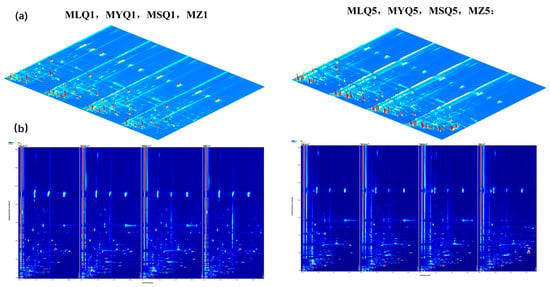
Figure 2.
Topography of volatile flavor compounds in red sour soup prepared by different fermentation methods. (a) Three-dimensional spectra of the composition of volatile substances; (b) spectrum of comparative differences in the composition of volatile substances.
3.6. Comparison of Fingerprint Profiles of Volatile Compounds in Different Red Sour Soup Samples
To further identify the differences of volatile compounds among different sour soups, all the volatile compounds identified in the GC-IMS spectra were selected to generate volatile fingerprints using the Reporter plug-in (Figure 3). Each row of the fingerprint profile shows entire signal peaks of sour soup samples and each column of the fingerprint profile shows the signal intensities of the same compounds present in sour soup samples (Figure 3). In total, 119 compounds were qualitatively detected in fingerprint profiles (Figure 3). Among these volatiles, ninety-nine compounds were identified based on the GC-IMS database and NIST database (Table 4), including twenty-six alcohols, twenty-two aldehydes, eighteen ketones, seventeen esters, four ethers, three pyrazines, eight terpenoids, and one furan.
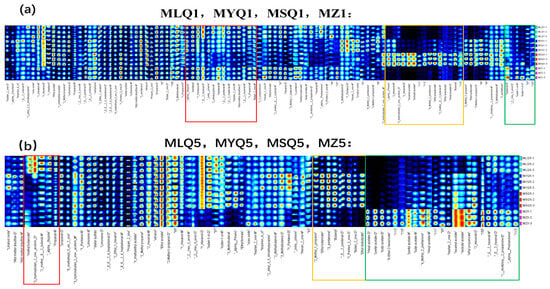
Figure 3.
The fingerprints of different red sour soup samples; (a) indicates the first day of fermentation; (b) indicates the fifth day of fermentation.

Table 4.
GC-IMS global regional aggregation parameters obtained from different sour soup samples.
Alcohols are the main volatile components in fresh fruits and contribute to a green and fresh odor [40]. In this study, alcohols were the most abundant compounds detected in red sour soup samples based on GC-IMS, including 26 compounds. The content of alcohols decreased in red sour soup samples on the fifth day compared to the first day (p < 0.05, Figure 4), probably due to the chemical reactions between alcohols and free fatty acids which formed esters during fermentation [41]. The content of esters increased in each sample (Figure 4). The content of some alcohols differed between samples. The content of (Z)-Hex-3-enol (D), butan-1-ol (M), and butan-1-ol (D) in MZ samples was higher than that in other samples on the first day of fermentation. The content of 1-Pentanol (M), (E)-2-pentenal (M), and 1-Propanol (M) in MLQ, MYQ, and MZ samples was significantly higher than that in MSQ samples on the first day of fermentation.
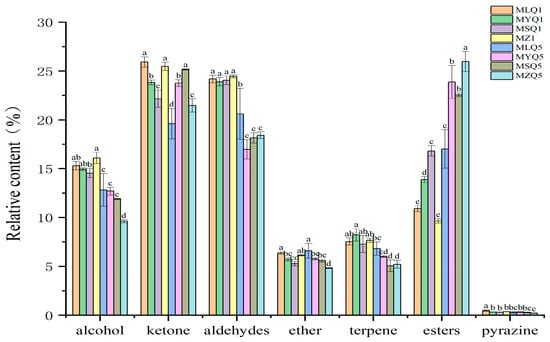
Figure 4.
Relative content of volatile flavor components in different red sour soup samples. Different small letters indicate significant differences between samples of different red sour soups (p < 0.05).
Aldehydes are mainly produced by unsaturated fatty acids through enzymatic and microbial oxidation and Strecker degradation [42] and often have pleasant, grassy, malty, and fruity flavors and aromas. Aldehydes have a low flavor threshold and a strong impact on the overall flavor of foods [43]. In this study, a total of seventeen aldehydes were detected by GC-IMS, including eight dimers. After five days of fermentation, the aldehyde content in each sample decreased compared to the first day (p < 0.05, Figure 4) because aldehydes were reduced into alcohols or oxidized into acids by microbial action [44] or as a result of lactic acid fermentation [45]. After one day of fermentation, MSQ samples contained much higher levels of 2-Hexenal (M), 2-methyl-(E)-2-butenal (D), and butanal, which endow red sour soup with clear, fruity, and nutty aromas, respectively, than the other three samples [45,46]. On the fifth day of fermentation, MZ samples contained higher levels of 2-Hexenal (M) and alpha-Phellandrene than the other three fermentation samples.
Ketones were the third major group of volatile flavor compounds determined in GC-IMS assays and eighteen ketones were found, including six dimers. Ketones are mainly produced by lipid oxidation, the Maillard reaction, and amino acid degradation [45] and endow fermented products with a unique fruitiness and aroma [47]. The reduction in the content of ketones in MLQ and MZ samples was ascribed to LAB. Silapeux et al. [48] also found that the inoculation of LAB in fermented fenugreek leaves could reduce the content of aldehydes and ketones and increase the content of esters. The content of 4-methyl-2-pentanone and 3-hydroxybutan-2-one-acetoin-D in the MSQ sample was higher than that in the other three samples in the first day of fermentation. On the fifth day of fermentation, the content of 3-pentanone, 1-Penten-3-one (D), and Butan-2-one (D), which endow the red sour soup with fruity, pungent, and garlicky flavors, in MLQ and MSQ samples was higher than that in the other two samples [49,50]. In contrast, the content of 4-methyl-2-pentanone, heptan-2-one (D), and 1-(Acetyloxy)-2-propanone (D) in the MZ sample was higher than that in the fortified fermentation samples. Heptan-2-one is produced by linoleic acid decomposition and has the odor of blue cheese [13].
In this study, a total of seventeen esters, including three dimers, were detected in the red sour soup samples based on GC-IMS. Esters are considered to be important flavor contributors in red sour soup [51]. Esters were not the first major class of compounds in this study and even small changes in ester content directly affected the sensory quality of the red sour soup due to the low flavor threshold of esters [52]. Different red sour soup samples showed an increased ester content on the fifth day of fermentation. Esters have pleasant, sweet, and fruity flavors [53] and are the main products in chili fermentation [54]. The ester content in MSQ samples on day 1 of fermentation was higher than that in other samples and the main esters included isoamyl acetate, isobutyl acetate, ethyl propanoate, and ethyl isobutyrate, which endow red sour soup with the flavors of various fruits such as banana and apple [55]. On the fifth day of fermentation, the content of some esters, mainly including Hexyl acetate (D), pentyl acetate (D), butyl acetate (D), and (E)-Ethyl-2-hexenoate, in MZ samples was significantly higher than that in the fortified fermentation samples. The MZ sample had a lot of miscellaneous bacteria and slow fermentation, so that the esters were only accumulated significantly on the fifth day.
Other compounds are explored below. Terpenes were mainly derived from raw materials and supporting materials, such as tomato, red pepper, and ginger, with floral and woody aromas [21]. The content of alpha-Fenchene in MSQ samples was significantly higher on the first day of fermentation. Ethers, with a low threshold and high contribution to the flavor of cooking wine, especially those containing benzene rings, mostly have strong and pleasant aromas and endow foods with special flavors [56]. Four ethers were detected in this study. Furan compounds are mainly generated by a Maillard reaction between amino acids and reducing sugars, and by a pyrolysis reaction between amino acids and thiamin, and have a strong meat flavor [57]. Only 2-pentylfuran was detected in this study.
3.7. Principal Component Analysis of Volatile Flavor Compounds in Different Red Sour Soup Samples
PCA is a multivariate statistical analysis method in which several valid variables are selected through multivariate linear transformation. It is usually used to analyze the relationship between the observed variables. The PCA model can be chosen as a separation model when the cumulative contribution reaches 60% [58]. In this study, PCA was used to analyze the variation of identified VOC in red sour soup samples (Figure 5). The cumulative variance contribution of the first component PC1 (first principal component) (58%) and the second component PC2 (second principal component) (23%) was 81% on day 1 of fermentation. PC1 and PC2 showed high similarity between two samples, MLQ1 and MZ1. However, PC1 and PC2 showed significant differences between the remaining samples. On day 5 of fermentation, the cumulative variance contribution of the first component PC1 (first principal component) (58%) and the second component PC2 (second principal component) (23%) was 81%. PC1 and PC2 showed high similarity between two samples, MSQ5 and MYQ5. However, PC1 and PC2 showed significant differences between the remaining samples.
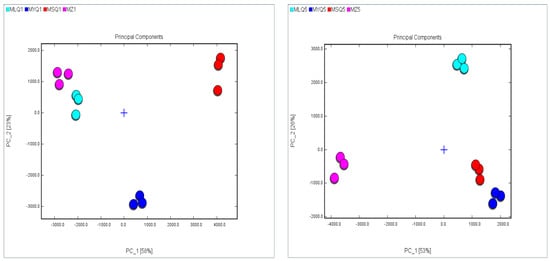
Figure 5.
PCA based on the signal intensity obtained from different red sour soup samples.
3.8. ROAV Analysis of Key Volatile Compounds in Different Red Sour Soup Samples
ROAV values indicate the contribution of key aromas to the overall flavor. ROAV is usually used to assess the impact of individual volatile compounds on the overall aroma. Compounds with ROAV ≥ 1 are considered key volatile aroma compounds with a high contribution to flavor [59]. On the first day of fermentation, 31, 32, 32, and 31 key volatile aroma compounds were found in MLQ, MYQ, MSQ, and MZ samples, respectively, and 28 aroma compounds were the same in three samples. On the fifth day of fermentation, 22, 22, 22, and 21 key volatile aroma components were found in MLQ, MYQ, MSQ, and MZ samples, respectively, and 21 aroma compounds were the same in three samples. On the fifth day of fermentation, hexyl acetate (D) was the key volatile compound (ROAV = 1.81) in the MZ sample and the ROAV of hexyl acetate (D) was less than 0.1 in the other samples (Table 5 and Figure 6). Hexyl acetate (D) endows red sour soup with apple and fruit flavors [60]. Propanal-M (ROAV = 9.74) and 2-Methyl propanal (ROAV = 139.26) contributed more to the aroma of MLQ samples than to that of other samples (Figure 6). 2-Methyl propanal has a fatty and mushroom-like odor. Isoamyl acetate, isobutyl acetate, ethyl isobutyrate, and butanal contributed significantly more to the aroma of MYQ and MSQ samples than to that of MLQ and MZ samples (Figure 6); isoamyl acetate and isobutyl acetate endow red sour soup with banana and fruity flavors and sweet and apple flavors, respectively [61]. Isoamyl acetate also shows a wide range of antibacterial activity against filamentous fungi [62]. Ethyl isobutyrate endows red sour soup with a strawberry flavor.

Table 5.
The ROAV values of volatile flavor compounds in different red sour soup samples.
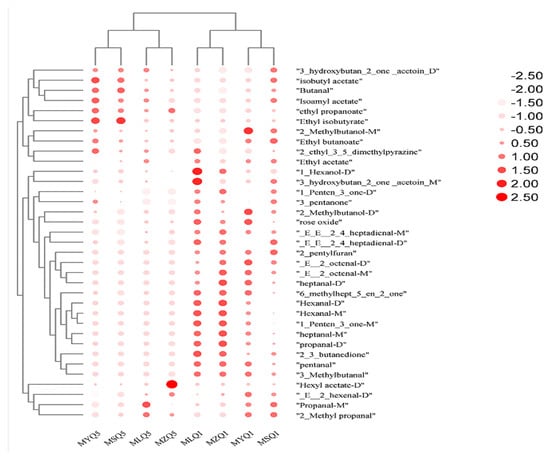
Figure 6.
Heat accumulation map based on ROAV values of volatile flavor components in different red sour soup samples.
4. Conclusions
In this study, the effects of fortified fermentation with L. buchneri and/or K. bulderi on the basic physicochemical properties and volatile flavor substances of red sour soup were investigated. Compared to natural fermentation, fortified fermentation decreased the amino acid nitrogen content. Compared to the inoculation of L. buchneri and natural fermentation alone, the mixed fortified fermentation and the inoculation of K. bulderi could reduce the content of nitrite and increase the content of 3-pentanone and butyraldehyde. The inoculation of L. buchneri in fortified fermentation significantly increased the content of lactic acid. In addition, compared to fortified fermentation, natural fermentation had a higher content of lycopene and esters. Therefore, we will use omics technology (e.g., transcriptomics and metabolomics) to explore the results of fortified fermentation with the two strains. These results contribute to the understanding of the effect of fermentation agents on the fermentation of red sour soup and provide technical support for the industrial production of red sour soup.
Author Contributions
N.L.: Methodology, Writing—original draft, Investigation, Funding acquisition. X.L.: Methodology, Software, Formal analysis, Data curation, Writing—original draft. Y.H.: Methodology, Investigation, Validation, Data curation. L.Q.: Conceptualization, Investigation, Writing—review and editing, Supervision, Project administration. A.B. and W.Q.: Resources. S.M.: Conceptualization, Supervision, Writing—review and editing, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Project of Guizhou Province, Qian Ke He Zhicheng [2022] Zhongdian 001, Qian Ke He Zhicheng [2022] Zhongdian 003, Guizhou University, Gui Da Te Gang He Zi (2022) 39, and China Scholarship Council (201906670006).
Data availability Statement
Data will be made available on request.
Conflicts of Interest
The authors have declared no conflict of interest for this article.
References
- Li, D.F.; Duan, F.X.; Tian, Q.M.; Zhong, D.J.; Wang, X.Y.; Jia, L.R. Physiochemical, microbiological and flavor characteristics of traditional Chinese fermented food Kaili Red Sour Soup. LWT 2021, 142, 110933. [Google Scholar] [CrossRef]
- Yang, H.; Xie, J.; Wang, N.; Zhou, Q.; Lu, Y.; Qu, Z.; Wang, H. Effects of Miao sour soup on hyperlipidemia in high-fat diet-induced obese rats via the AMPK signaling pathway. Food Sci. Nutr. 2021, 9, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Byun, B.Y.; Bai, X.; Mah, J.-H. Occurrence of biogenic amines in Doubanjiang and Tofu. Food Sci. Biotechnol. 2013, 22, 55–62. [Google Scholar] [CrossRef]
- Fang, Z.; Hongfei, Z.; Junyu, Z.; Dziugan, P.; Shanshan, L.; Bolin, Z. Evaluation of probiotic properties of Lactobacillus strains isolated from traditional Chinese cheese. Ann. Microbiol. 2015, 65, 1419–1426. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Z.; Zhang, L.; Jiang, Y.; Zhu, J. Dynamic changes of quality and flavor characterization of Zhejiang rosy vinegar during fermentation and aging based on untargeted metabolomics. Food Chem. 2023, 404, 134702. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Liang, H.; He, Z.; Wang, X.; Song, G.; Chen, H.; Lin, X.; Ji, C.; Zhang, S. Bacterial profiles and volatile flavor compounds in commercial Suancai with varying salt concentration from Northeastern China. Food Res. Int. 2020, 137, 109384. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lu, Z.-M.; Shi, J.S.; Xu, Z.H. Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Sci. Rep. 2016, 6, 26818. [Google Scholar] [CrossRef]
- Xiao, M.; Xiong, T.; Peng, Z.; Liu, C.; Huang, T.; Yu, H.; Xie, M. Correlation between microbiota and flavours in fermentation of Chinese Sichuan Paocai. Food Res. Int. 2018, 114, 123–132. [Google Scholar] [CrossRef]
- Liu, N.; Pan, J.; Miao, S.; Qin, L. Microbial community in Chinese traditional fermented acid rice soup (rice-acid) and its correlations with key organic acids and volatile compounds. Food Res. Int. 2020, 137, 109672. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Qin, L.; Lu, X.; Zhao, Y.; Miao, S. Fortified Fermented Rice-Acid Can Regulate the Gut Microbiota in Mice and Improve the Antioxidant Capacity. Nutrients 2021, 13, 4219. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; He, Z.; Wang, X.; Zhao, M.; Cao, X.; Lin, X.; Ji, C.; Zhang, S.; Liang, H. Improving the quality of Suancai by inoculating with Lactobacillus plantarum and Pediococcus pentosaceus. Food Res. Int. 2021, 148, 110581. [Google Scholar] [CrossRef]
- Sha-Sha, Z.; Ping, H.U.J.C.C. Study on Quality Change of Red Sour Soup Fermented by Lactic Acid Bacteria. China Condiment 2019, 44, 65–70. (In Chinese) [Google Scholar]
- Liu, N.; Hu, Y.; Qin, L.; Bao, A.; Qin, W.; Miao, S. Flavor and quality characteristics of Guizhou red sour soup prepared by different artificially fortified fermentation methods. Lwt 2023, 186, 115247. [Google Scholar] [CrossRef]
- Fermented Fruits and Vegetables Liquid and Its Products (T/TSSP 014—2022). Available online: https://www.ttbz.org.cn/upload/file/20220718/6379375792229400983301077.pdf (accessed on 29 August 2023).
- Niamah, A.K.; Al-fekaiki, D.F.; Thyab Gddoa Al-Sahlany, S.; Verma, D.K.; Patel, A.R.; Singh, S. Investigating the effect of addition of probiotic microorganisms (bacteria or yeast) to yoghurt on the viability and volatile aromatic profiles. J. Food Meas. Charact. 2023, 17, 5463–5473. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, R.; Zhang, M.; Chen, W.; Chen, H.; Chen, W. Effect of the Mixed Inoculation of Lactic Acid Bacteria and Non-Saccharomyces on the Quality and Flavor Enhancement of Fermented Mango Juice. Fermentation 2023, 9, 563. [Google Scholar] [CrossRef]
- Fujimoto, A.; Ito, K.; Itou, M.; Narushima, N.; Ito, T.; Yamamoto, A.; Hirayama, S.; Furukawa, S.; Morinaga, Y.; Miyamoto, T. Microbial behavior and changes in food constituents during fermentation of Japanese sourdoughs with different rye and wheat starting materials. J. Biosci. Bioeng. 2018, 125, 97–104. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Li, C.; He, L.; Wang, X.; Zeng, X. Mixed fermentation with Lactobacillus plantarum, Bifidobacterim animalis subsp. lactis and Candida utilis improves the fermentation quality of Hong Suan Tang. Food Chem. 2023, 402, 134488. [Google Scholar] [CrossRef]
- Wang, Z.L.; Mi, S.; Wang, X.H.; Mao, K.M.; Liu, Y.W.; Gao, J.; Sang, Y.X. Characterization and discrimination of fermented sweet melon juice by different microbial strains via GC-IMS-based volatile profiling and chemometrics. Food Sci. Hum. Wellness 2023, 12, 1241–1247. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B 2018, 1099, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, D.; Zanardi, S.; Dall’Asta, C.; Suman, M. Ion mobility spectrometry coupled to gas chromatography: A rapid tool to assess eggs freshness. Food Chem. 2019, 271, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Miao, S.; Qin, L. Screening and application of lactic acid bacteria and yeasts with L-lactic acid-producing and antioxidant capacity in traditional fermented rice acid. Food Sci. Nutr. 2020, 8, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Y.; Xu, L.; Lu, Q.-S.; Mou, Q. Optimization of Kaili red sour soup "tomato juice" fermentation process and fortified microflora. Food Sci. Technol. 2018, 43, 313–318. (In Chinese) [Google Scholar] [CrossRef]
- He, Y.; Li, G.; Li, Y.; Luo, X.; Luo, Q.; Shi, B.; Duan, Z.; Liu, N. Analysis of Microflora and Volatile Substances Change in Red Sour Soup during Fermentation. Sci. Technol. Food Ind. 2022, 43, 177–190. (In Chinese) [Google Scholar] [CrossRef]
- Sun, Q.; Yang, C.; Li, J.; Raza, H.; Zhang, L. Lycopene: Heterogeneous Catalytic E/Z Isomerization and In Vitro Bioaccessibility Assessment Using a Diffusion Model. J. Food Sci. 2016, 81, C2381–C2389. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A Holistic Review on Euro-Asian Lactic Acid Bacteria Fermented Cereals and Vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Yang, H.; Zou, H.; Qu, C.; Zhang, L.; Liu, T.; Wu, H.; Li, Y. Dominant Microorganisms during the Spontaneous Fermentation of Suan Cai, a Chinese Fermented Vegetable. Food Sci. Technol. Res. 2014, 20, 915–926. [Google Scholar] [CrossRef]
- Liang, H.; Chen, H.; Ji, C.; Lin, X.; Zhang, W.; Li, L. Dynamic and Functional Characteristics of Predominant Species in Industrial Paocai as Revealed by Combined DGGE and Metagenomic Sequencing. Front. Microbiol. 2018, 9, 2416. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, Y. Effects of microbial diversity on nitrite concentration in pao cai, a naturally fermented cabbage product from China. Food Microbiol. 2018, 72, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.-M.; Xue, W.-T.; Tan, S.-S.; Zhang, H.; Chang, X.-H. Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control 2008, 19, 50–55. [Google Scholar] [CrossRef]
- Gerardi, C.; Tristezza, M.; Giordano, L.; Rampino, P.; Perrotta, C.; Baruzzi, F.; Capozzi, V.; Mita, G.; Grieco, F. Exploitation of Prunus mahaleb fruit by fermentation with selected strains of Lactobacillus plantarum and Saccharomyces cerevisiae. Food Microbiol. 2019, 84, 103262. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, Z.; Hao, Y.; Zhang, L.; Ren, Y.; Zhang, Y.; Chen, Z.; Mandlaa. Correlation between bacterial communities and organic acids in the fermentation stage of traditional Chinese sour porridge. Int. J. Food Prop. 2020, 23, 1430–1440. [Google Scholar] [CrossRef]
- Garcia-Quintans, N.; Repizo, G.; Martin, M.; Magni, C.; Lopez, P. Activation of the diacetyl/acetoin pathway in Lactococcus lactis subsp. lactis bv. diacetylactis CRL264 by acidic growth. Appl. Environ. Microbiol. 2008, 74, 1988–1996. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. Lwt 2020, 127, 109323. [Google Scholar] [CrossRef]
- Varma, S.; Karwe, M.V.; Lee, T.-C. Effect of High Hydrostatic Pressure Processing on Lycopene Isomers. Int. J. Food Eng. 2010, 6, 5. [Google Scholar] [CrossRef]
- Honest, K.N.; Zhang, H.W.; Zhang, L. Lycopene: Isomerization Effects on Bioavailability and Bioactivity Properties. Food Rev. Int. 2011, 27, 248–258. [Google Scholar] [CrossRef]
- Perez, A.G.; Sanz, C.; Olias, R.; Olias, J.M. Lipoxygenase and hydroperoxide lyase activities in ripening strawberry fruits. J. Agric. Food Chem. 1999, 47, 249–253. [Google Scholar] [CrossRef]
- Guclu, G.; Keser, D.; Kelebek, H.; Keskin, M.; Sekerli, Y.E.; Soysal, Y.; Selli, S. Impact of production and drying methods on the volatile and phenolic characteristics of fresh and powdered sweet red peppers. Food Chem. 2021, 338, 128129. [Google Scholar] [CrossRef]
- Coelho, E.; Azevedo, M.; Teixeira, J.A.; Tavares, T.; Oliveira, J.M.; Domingues, L. Evaluation of multi-starter S. cerevisiae/D. bruxellensis cultures for mimicking and accelerating transformations occurring during barrel ageing of beer. Food Chem. 2020, 323, 126826. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, Y.; Wang, Y.; Li, L.; Li, C.; Zhao, Y.; Yang, S. Contribution of autochthonous microbiota succession to flavor formation during Chinese fermented mandarin fish (Siniperca chuatsi). Food Chem. 2021, 348, 129107. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Li, Y.; Zhu, H.; Liu, Y.; Quan, K. Effect of paracasei fermentation on the volatile flavors of mung beans. LWT 2021, 146, 111434. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhou, L.; Zhang, R. Study on the influences of ultrasound on the flavor profile of unsmoked bacon and its underlying metabolic mechanism by using HS-GC-IMS. Ultrason. Sonochemistry 2021, 80, 105807. [Google Scholar] [CrossRef]
- Qin, L.; Kang, W.; Zhang, Z.; Guo, A. Changes in C6 Volatile Aldehyde and Alcohol Components of Nectarine Fruits Analyzed by Headspace Solid-Phase Microextraction-gas Chromatography/Mass Spectrometry. Xiandai Shipin Keji 2015, 31, 301–307. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.H.; Cao, J.; Pei, Z.S.; Wei, P.Y.; Xiang, D.; Cao, X.Y.; Shen, X.R.; Li, C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef]
- Kamda, A.G.S.; Ramos, C.L.; Fokou, E.; Duarte, W.F.; Mercy, A.; Germain, K.; Dias, D.R.; Schwan, R.F. In vitro determination of volatile compound development during starter culture-controlled fermentation of Cucurbitaceae cotyledons. Int. J. Food Microbiol. 2015, 192, 58–65. [Google Scholar] [CrossRef]
- Hosoglu, M.I.; Karagul-Yuceer, Y.; Guneser, O. Aroma characterization of heterotrophic microalgae Crypthecodinium cohnii using solid-phase microextraction and gas chromatography- mass spectrometry/olfactometry during different growth phases. Algal Res. 2020, 49, 101928. [Google Scholar] [CrossRef]
- Liao, Y.; Ding, Y.; Wu, Y.; Du, Q.; Xia, J.; Jia, J.; Lin, H.; Benjakul, S.; Zhang, B.; Hu, Y. Analysis of volatile compounds and flavor fingerprint in hairtail (Trichiurus lepturus) during air-drying using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Front. Nutr. 2023, 9, 128129. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; He, L.; Li, C. Determination of the microbial communities of Guizhou Suantang, a traditional Chinese fermented sour soup, and correlation between the identified microorganisms and volatile compounds. Food Res. Int. 2020, 138, 109820. [Google Scholar] [CrossRef]
- Settanni, L.; Corsetti, A. The use of multiplex PCR to detect and differentiate food and beverage-associated microorganisms: A review. J. Microbiol. Methods 2007, 69, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Liang, W.; Liu, Y.; Gao, H. Identification and analysis of the flavor characteristics of unfermented stinky tofu brine during fermentation using SPME-GC-MS, e-nose, and sensory evaluation. J. Food Meas. Charact. 2020, 14, 597–612. [Google Scholar] [CrossRef]
- Tang, X.; Xia, Y.; Wu, C. Analysis of Volatile Components in Hot Pepper Juice during Fermentation. Food Sci. 2014, 35, 197–201. (In Chinese) [Google Scholar]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Lin, L.; Jiang, S.-T.; Lu, J.-F. Effects of two different sterilization methods on the nutritional composition and volatile components of shenxian (fairy) beans. Xiandai Shipin Keji 2015, 31, 245–253. [Google Scholar] [CrossRef]
- Xie, W.; Xu, X.-L.; Zhou, G.-H. Effects of Different Processing Procedures on Volatile Flavor Composition of Water Boiled Salted Duck. Food Sci. 2010, 31, 110–115. (In Chinese) [Google Scholar]
- Sebzalli, Y.M.; Wang, X.Z. Knowledge discovery from process operational data using PCA and fuzzy clustering. Eng. Appl. Artif. Intell. 2001, 14, 607–616. [Google Scholar] [CrossRef]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef]
- Kim, K.; Chun, I.J.; Suh, J.H.; Sung, J. Relationships between sensory properties and metabolomic profiles of different apple cultivars. Food Chem. X 2023, 18, 100641. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Yin, J.; Ma, N.; Tao, Y. Adjustment of impact odorants in Hutai-8 rose wine by co-fermentation of Pichia fermentans and Saccharomyces cerevisiae. Food Res. Int. 2022, 153, 1088128. [Google Scholar] [CrossRef]
- Ando, H.; Hatanaka, K.; Ohata, I.; Yamashita-Kitaguchi, Y.; Kurata, A.; Kishimoto, N. Antifungal activities of volatile substances generated by yeast isolated from Iranian commercial cheese. Food Control 2012, 26, 472–478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).