Advanced QuEChERS Method Using Core-Shell Magnetic Molecularly Imprinted Polymers (Fe3O4@MIP) for the Determination of Pesticides in Chlorophyll-Rich Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Fe3O4 Nanoparticles (NPs)
2.3. Synthesis of Methacrylic Acid (MAA)-Modified
2.4. Preparation of the Fe3O4@MIPs and Fe3O4@NIPs
2.5. Adsorption Capacity
2.6. Sample Preparation
2.7. GC-MS/MS Analysis
2.8. UPLC-MS/MS Analysis
3. Results and Discussion
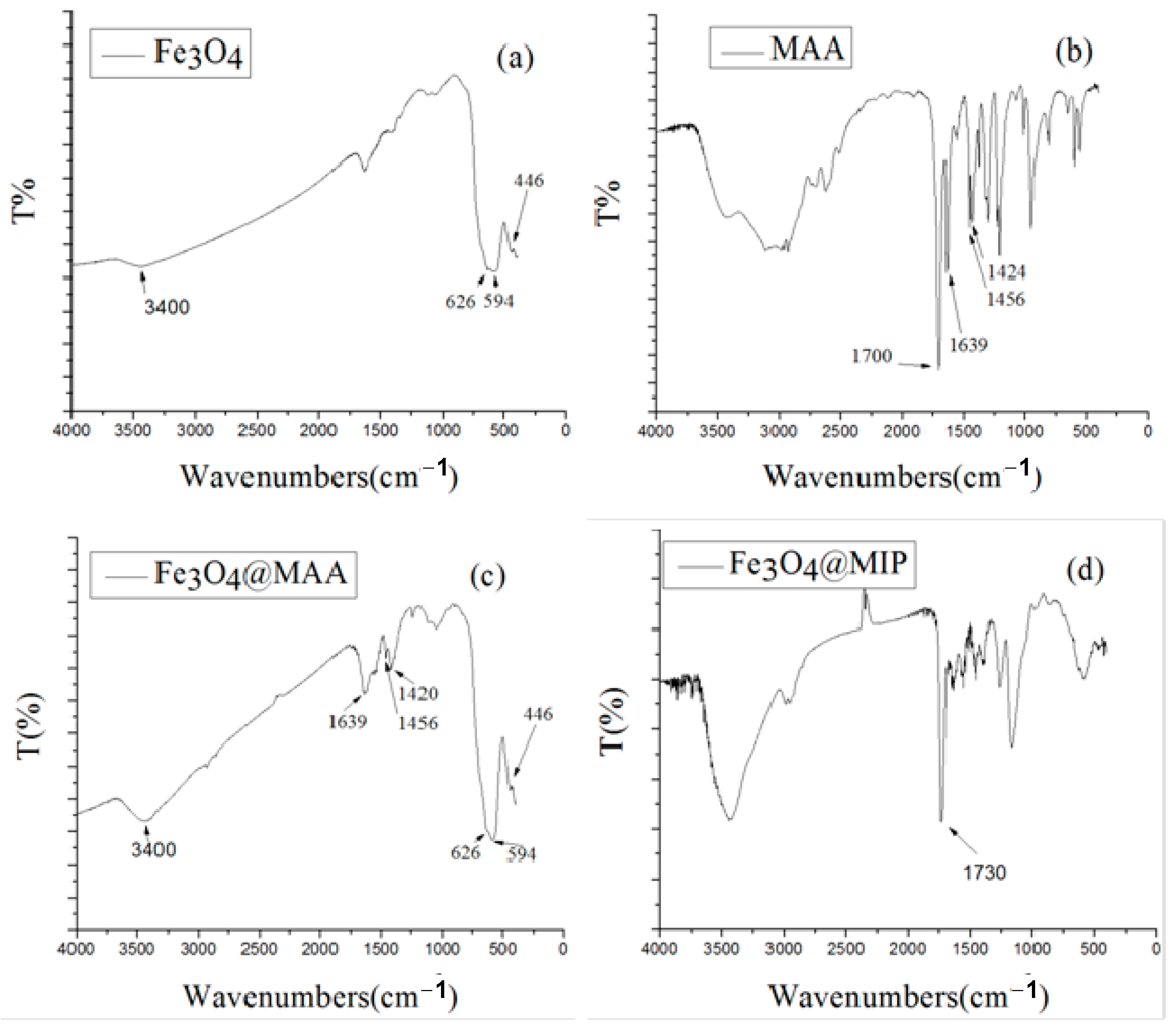
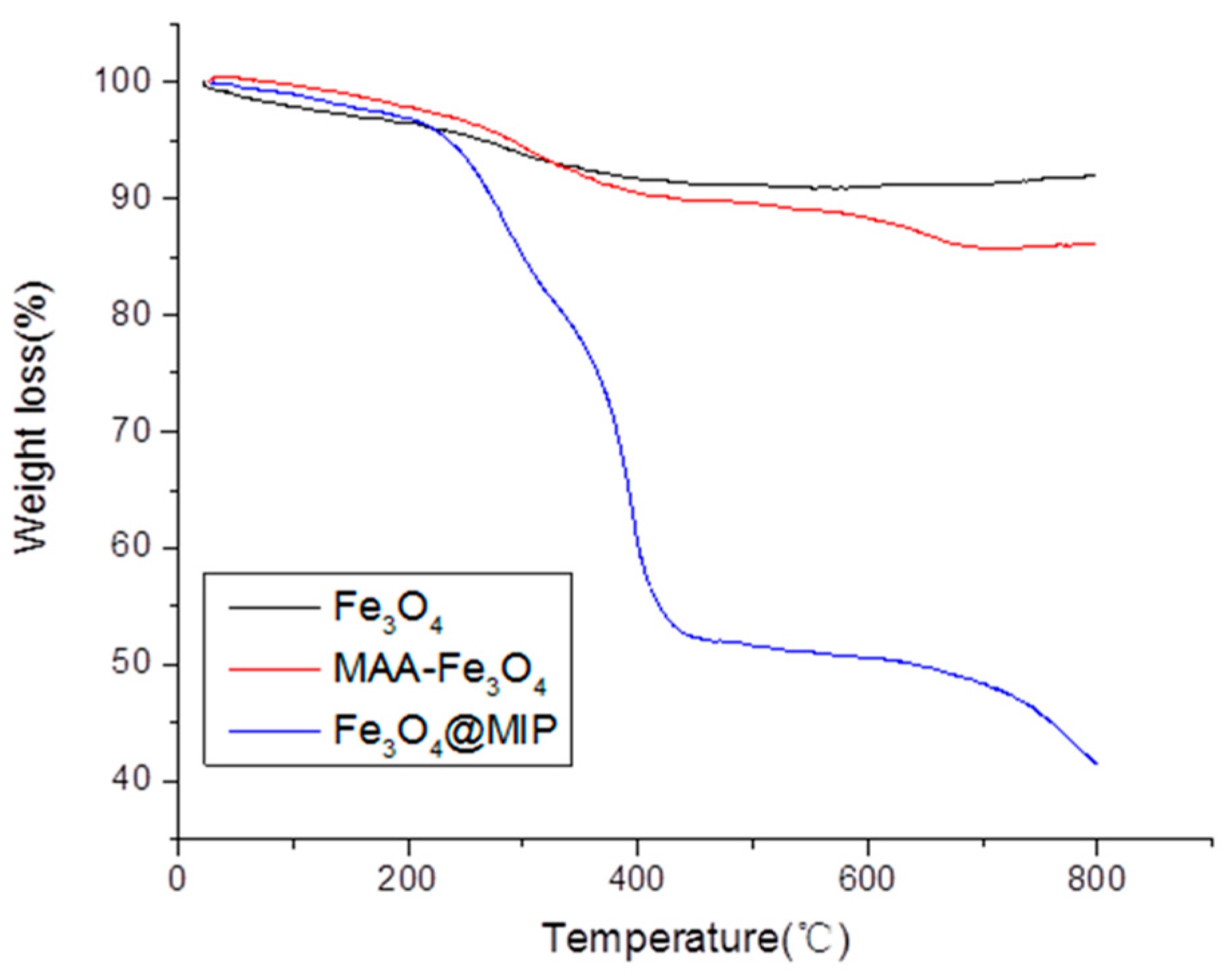
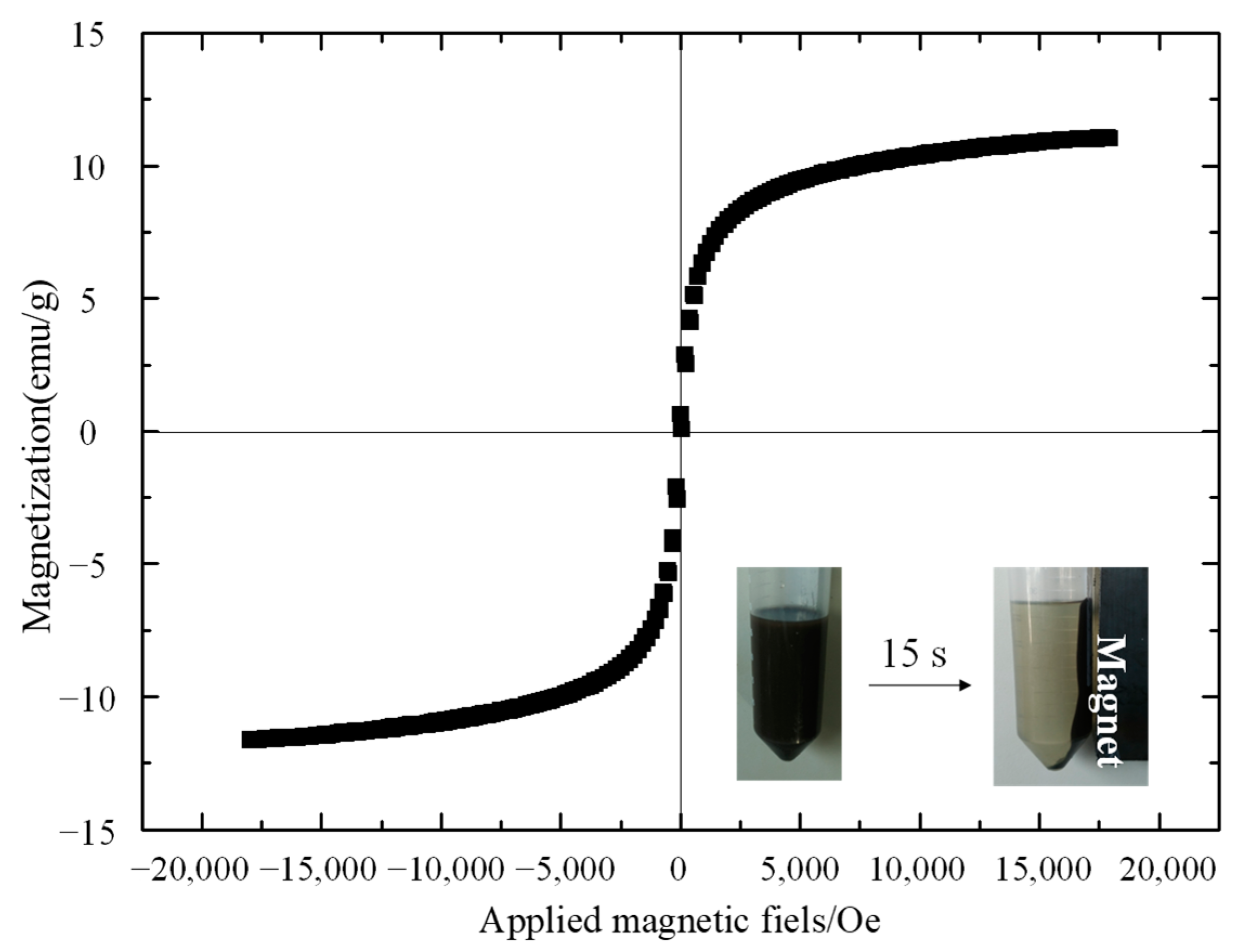
3.1. Preparation and Characterization of Fe3O4@MIP
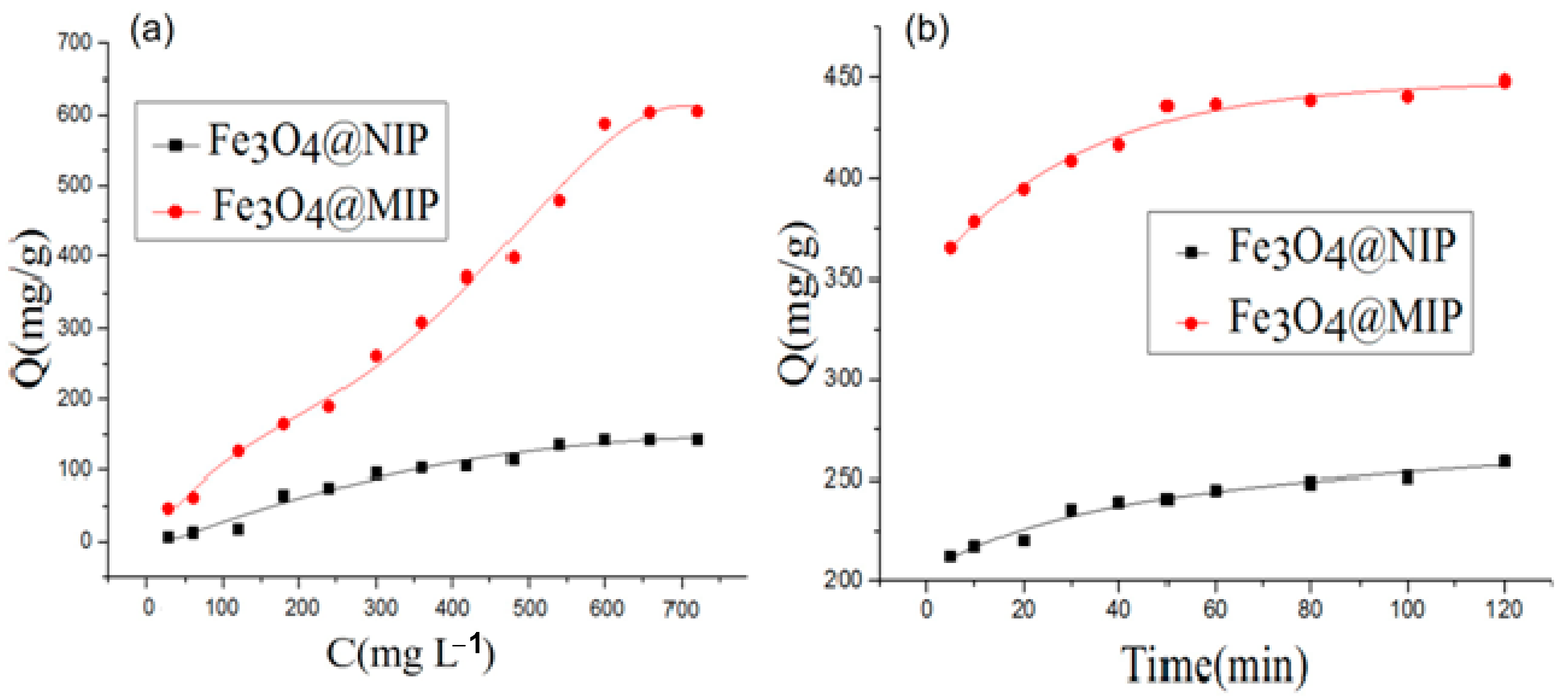
3.2. Adsorption Capacity of Chlorophyll
3.3. Purification Effect of the QuEChERS Method Modified with Fe3O4@MIP
3.4. Linearity, Limit of Detection (LOD), and Limit of Quantification (LOQ)
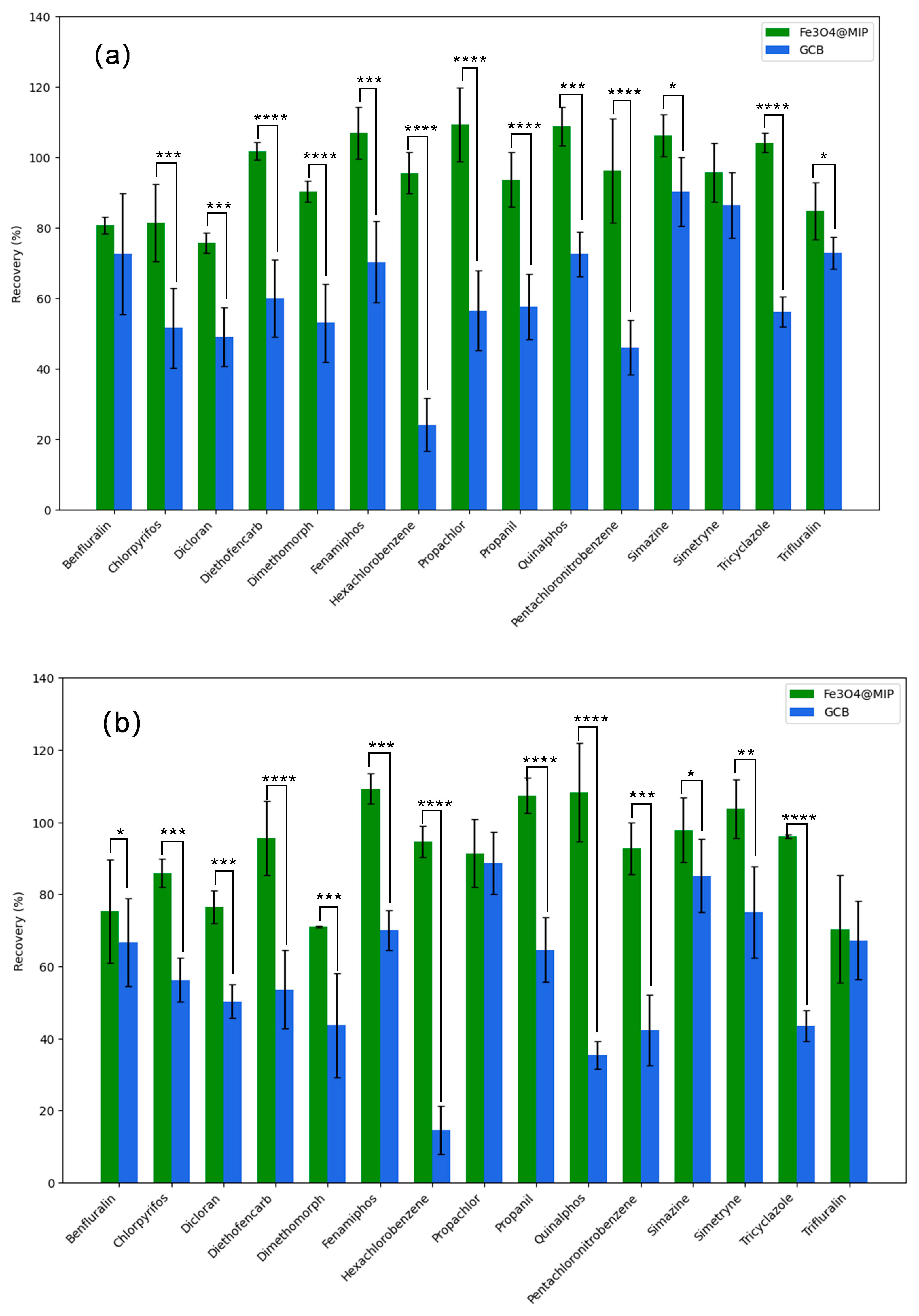
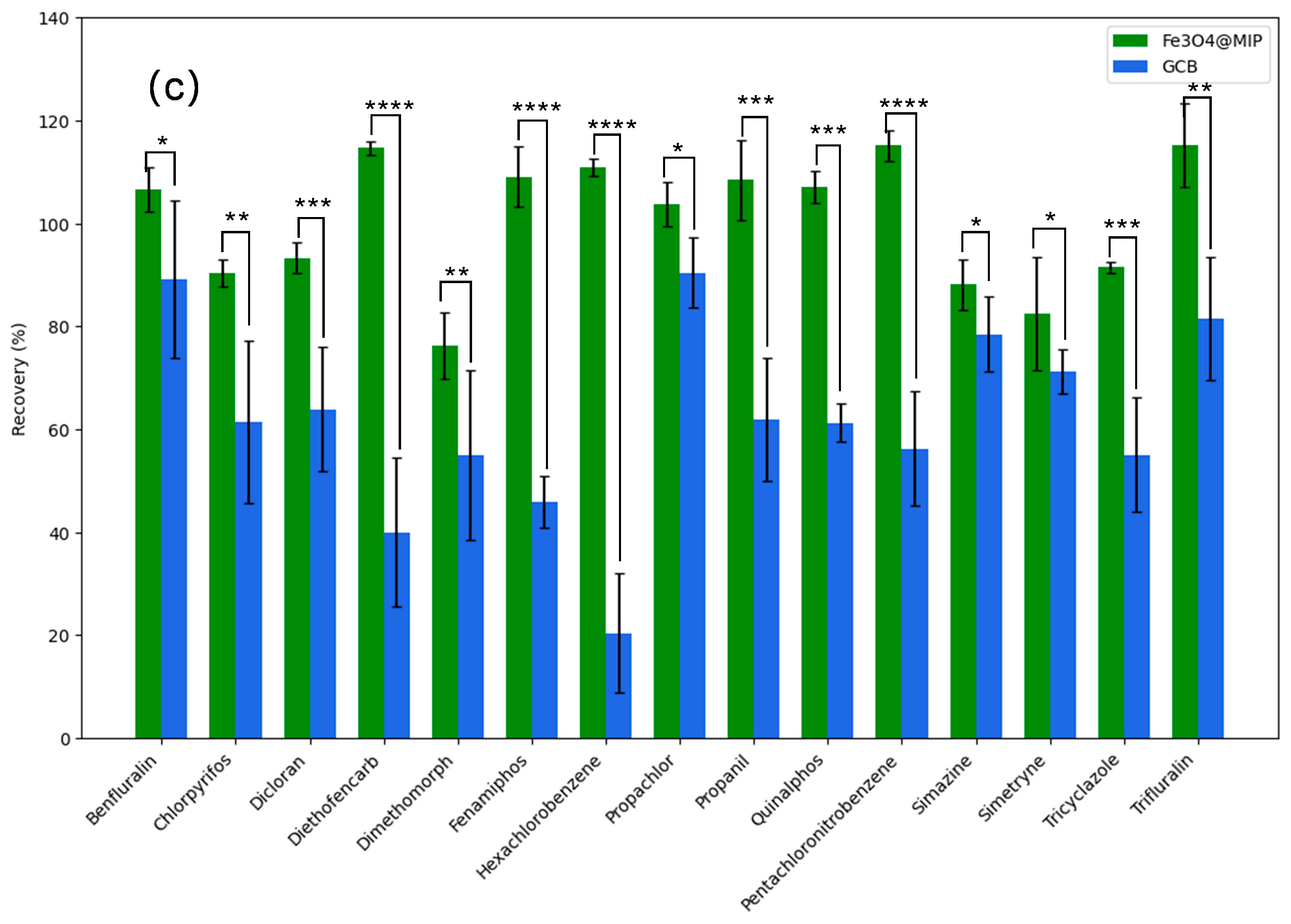
3.5. Effect of Fe3O4@MIP and GCB as Adsorbents on Planar and Aromatic Pesticide Recovery
3.6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.; Hoque, M.S.; Bhowmik, S.; Ferdousi, S.; Kabiraz, M.P.; van Brakel, M.L. Monitoring of pesticide residues from fish feed, fish and vegetables in Bangladesh by GC-MS using the QuEChERS method. Heliyon 2021, 7, e06390. [Google Scholar] [CrossRef]
- Khezri, A.; Ansari, M.; Amirahmadi, M.; Shahidi, M.; Mohamadi, N.; Kazemipour, M. Pesticide residues in dates using a modified QuEChERS method and GC-MS/MS. Food Addit. Contam. B 2022, 15, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; An, Q.; Zhang, C.; Pan, C.; Zhang, Z. Comparison of Sin-QuEChERS nano and d-SPE methods for pesticide multi-residues in lettuce and Chinese chives. Molecules 2020, 25, 3391. [Google Scholar] [CrossRef] [PubMed]
- Mastovska, K.; Dorweiler, K.J.; Lehotay, S.J.; Wegscheid, J.S.; Szpylka, K.A. Pesticide multiresidue analysis in cereal grains using modified QuEChERS method combined with automated direct sample introduction GC-TOFMS and UPLC-MS/MS techniques. J. Agric. Food Chem. 2010, 58, 5959–5972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wong, J.W.; Hayward, D.G.; Sheladia, P.; Krynitsky, A.J.; Schenck, F.J.; Webster, M.G.; Ammann, J.A.; Ebeler, S.E. Multiresidue pesticide analysis of wines by dispersive solid-phase extraction and ultrahigh-performance liquid chromatography- tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 4019–4029. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Qiu, X.; Feng, C.; Lin, Y.; Xiong, L.; Wen, Y.; Wang, D.; Wang, G. Simultaneous determination of 45 pesticides in fruit and vegetable using an improved QuEChERS method and on-line gel permeation chromatography–gas chromatography/mass spectrometer. J. Chromatogr. B 2012, 895, 17–24. [Google Scholar] [CrossRef]
- Farooq, S.; Wu, H.; Nie, J.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Khan, R.; Asim, M. Application, advancement and green aspects of magnetic molecularly imprinted polymers in pesticide residue detection. Sci. Total Environ. 2022, 804, 150293. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Lu, H.; Xu, S. Preparation of magnetic hollow molecularly imprinted polymers for detection of triazines in food samples. J. Agric. Food Chem. 2016, 64, 5110–5116. [Google Scholar] [CrossRef] [PubMed]
- Boulanouar, S.; Mezzache, S.; Combès, A.; Pichon, V. Molecularly imprinted polymers for the determination of organophosphorus pesticides in complex samples. Talanta 2018, 176, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, H.; Cai, J.; Duan, Y.; Liu, Y. Development of fluorescence sensing material based on CdSe/ZnS quantum dots and molecularly imprinted polymer for the detection of carbaryl in rice and Chinese cabbage. J. Agric. Food Chem. 2015, 63, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.S.; Wu, M.S.; Zuo, H.G.; Jiang, C.; Jin, S.F.; Lu, Y.C.; Yang, H. Core–shell magnetic molecularly imprinted polymers as sorbent for sulfonylurea herbicide residues. J. Agric. Food Chem. 2015, 63, 3634–3645. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hussain, S.; Wong, A.; Foguel, M.V.; Goncalves, L.M.; Gurgo, M.I.P.; Sotomayor, M.d.P.T. Synthesis and characterization of magnetic-molecularly imprinted polymers for the HPLC-UV analysis of ametryn. React. Funct. Polym. 2018, 122, 175–182. [Google Scholar] [CrossRef]
- Shao, W.C.; Zang, Y.Y.; Ma, H.Y.; Ling, Y.; Kai, Z.P. Concentrations and related health risk assessment of pesticides, phthalates, and heavy metals in strawberries from Shanghai, China. J. Food Protect. 2021, 84, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. Pesticide Manual, 19th ed.; BCPC Publications: Berkshire, UK, 2021. [Google Scholar]
- He, Z.; Zhao, L.; Liu, X.; Xu, Y. The application of in-source fragmentation in ultra-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for pesticide residue analysis. J. Chromatogr. A 2020, 1633, 461637. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, S.; Wang, L.; Peng, Y.; Luo, M.; Wang, W.; Liu, X. Multiresidue analysis of 213 pesticides in leek and garlic using QuEChERS-based method and gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef] [PubMed]
- China Pesticide Information Network, Announcement No. 2386 of the Ministry of Agriculture, the People’s Republic of China. 2016. Available online: http://www.chinapesticide.org.cn/ssfxpg/9556.jhtml (accessed on 17 November 2022).




| Analytes | r2 | Linear Range (mg L−1) | LOD a (mg kg−1) | LOQ b (mg kg−1) |
|---|---|---|---|---|
| Benfluralin | 0.9906 | 0.001–0.4 | 0.001 | 0.005 |
| Chlorpyrifos | 0.9998 | 0.001–0.4 | 0.001 | 0.005 |
| Dicloran | 0.9962 | 0.001–0.4 | 0.001 | 0.005 |
| Diethofencarb | 0.9972 | 0.001–0.4 | 0.001 | 0.005 |
| Dimethomorph | 0.9964 | 0.002–0.4 | 0.002 | 0.005 |
| Fenamiphos | 0.9924 | 0.001–0.4 | 0.001 | 0.005 |
| Hexachlorobenzene | 0.9907 | 0.001–0.4 | 0.001 | 0.005 |
| Pentachloronitrobenzene | 0.9946 | 0.001–0.4 | 0.001 | 0.005 |
| Propachlor | 0.9928 | 0.001–0.4 | 0.001 | 0.005 |
| Propanil | 0.9946 | 0.002–0.4 | 0.002 | 0.005 |
| Quinalphos | 0.9910 | 0.002–0.4 | 0.002 | 0.005 |
| Simazine | 0.9971 | 0.001–0.4 | 0.001 | 0.005 |
| Simetryne | 0.9927 | 0.001–0.4 | 0.001 | 0.005 |
| Tricyclazole | 0.9998 | 0.001–0.4 | 0.001 | 0.005 |
| Trifluralin | 0.9921 | 0.002–0.4 | 0.002 | 0.005 |
| Analytes | 0.005 mg kg−1 | 0.02 mg kg−1 | 0.1 mg kg−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4@MIP | GCB | Fe3O4@MIP | GCB | Fe3O4@MIP | GCB | |||||||
| Recovery(%) | RSD(%) | Recovery (%) | RSD (%) | Recovery (%) | RSD(%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |
| Benfluralin | 80.73 | 2.4 | 72.69 | 17.2 | 75.21 | 14.3 | 66.6 | 9.2 | 106.53 | 4.3 | 89.03 | 15.3 |
| Chlorpyrifos | 81.46 | 10.9 | 51.64 | 11.3 | 85.9 | 4 | 56.26 | 6.1 | 90.28 | 2.7 | 61.44 | 15.7 |
| Dicloran | 75.79 | 2.9 | 49.01 | 8.3 | 76.43 | 4.7 | 50.27 | 4.7 | 93.26 | 3 | 63.88 | 12 |
| Diethofencarb | 101.81 | 2.6 | 60.07 | 11 | 95.66 | 10.3 | 53.63 | 10.8 | 114.6 | 1.3 | 40.01 | 14.5 |
| Dimethomorph | 90.29 | 3 | 52.98 | 21 | 71.01 | 0.2 | 43.65 | 33.3 | 76.27 | 26.4 | 54.89 | 30.1 |
| Fenamiphos | 107.03 | 7.4 | 70.31 | 11.5 | 109.29 | 4.2 | 69.99 | 5.5 | 109.04 | 5.8 | 45.96 | 5 |
| Hexachlorobenzene | 95.59 | 5.8 | 24.08 | 7.5 | 94.61 | 4.3 | 14.51 | 6.7 | 110.83 | 1.7 | 20.36 | 11.6 |
| Pentachloronitrobenzene | 96.18 | 14.8 | 46.01 | 7.8 | 92.63 | 7.2 | 42.18 | 9.8 | 115.07 | 3.1 | 56.27 | 11 |
| Propachlor | 109.27 | 10.5 | 56.53 | 21.2 | 91.38 | 9.5 | 88.65 | 8.5 | 103.63 | 4.3 | 90.35 | 6.8 |
| Propanil | 93.68 | 7.8 | 57.65 | 9.2 | 107.35 | 4.9 | 64.6 | 9 | 108.39 | 7.7 | 61.91 | 11.9 |
| Quinalphos | 108.92 | 5.5 | 72.61 | 6.3 | 108.21 | 13.6 | 35.4 | 3.8 | 107.07 | 3.2 | 61.28 | 3.6 |
| Simazine | 106.19 | 6 | 90.22 | 9.8 | 97.8 | 9 | 85.13 | 10.1 | 88.06 | 5 | 78.42 | 7.3 |
| Simetryne | 95.75 | 8.4 | 86.47 | 9.3 | 103.67 | 8.1 | 75.01 | 12.6 | 82.35 | 11 | 71.2 | 4.3 |
| Tricyclazole | 104.19 | 2.8 | 56.19 | 4.3 | 96.05 | 0.4 | 43.5 | 4.3 | 91.44 | 1.1 | 55.01 | 11.1 |
| Trifluralin | 84.9 | 8 | 72.84 | 4.5 | 70.37 | 15 | 67.22 | 10.8 | 115.16 | 8.2 | 91.43 | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kai, Z.-P.; Hou, M.-X.; Zhu, J.-J.; Jiang, Z.-P.; Chen, S.-S. Advanced QuEChERS Method Using Core-Shell Magnetic Molecularly Imprinted Polymers (Fe3O4@MIP) for the Determination of Pesticides in Chlorophyll-Rich Samples. Foods 2023, 12, 3742. https://doi.org/10.3390/foods12203742
Kai Z-P, Hou M-X, Zhu J-J, Jiang Z-P, Chen S-S. Advanced QuEChERS Method Using Core-Shell Magnetic Molecularly Imprinted Polymers (Fe3O4@MIP) for the Determination of Pesticides in Chlorophyll-Rich Samples. Foods. 2023; 12(20):3742. https://doi.org/10.3390/foods12203742
Chicago/Turabian StyleKai, Zhen-Peng, Meng-Xia Hou, Jing-Jing Zhu, Zhong-Ping Jiang, and Shan-Shan Chen. 2023. "Advanced QuEChERS Method Using Core-Shell Magnetic Molecularly Imprinted Polymers (Fe3O4@MIP) for the Determination of Pesticides in Chlorophyll-Rich Samples" Foods 12, no. 20: 3742. https://doi.org/10.3390/foods12203742
APA StyleKai, Z.-P., Hou, M.-X., Zhu, J.-J., Jiang, Z.-P., & Chen, S.-S. (2023). Advanced QuEChERS Method Using Core-Shell Magnetic Molecularly Imprinted Polymers (Fe3O4@MIP) for the Determination of Pesticides in Chlorophyll-Rich Samples. Foods, 12(20), 3742. https://doi.org/10.3390/foods12203742






