Lipid Profile in Multiple Sclerosis: Functional Capacity and Therapeutic Potential of Its Regulation after Intervention with Epigallocatechin Gallate and Coconut Oil
Abstract
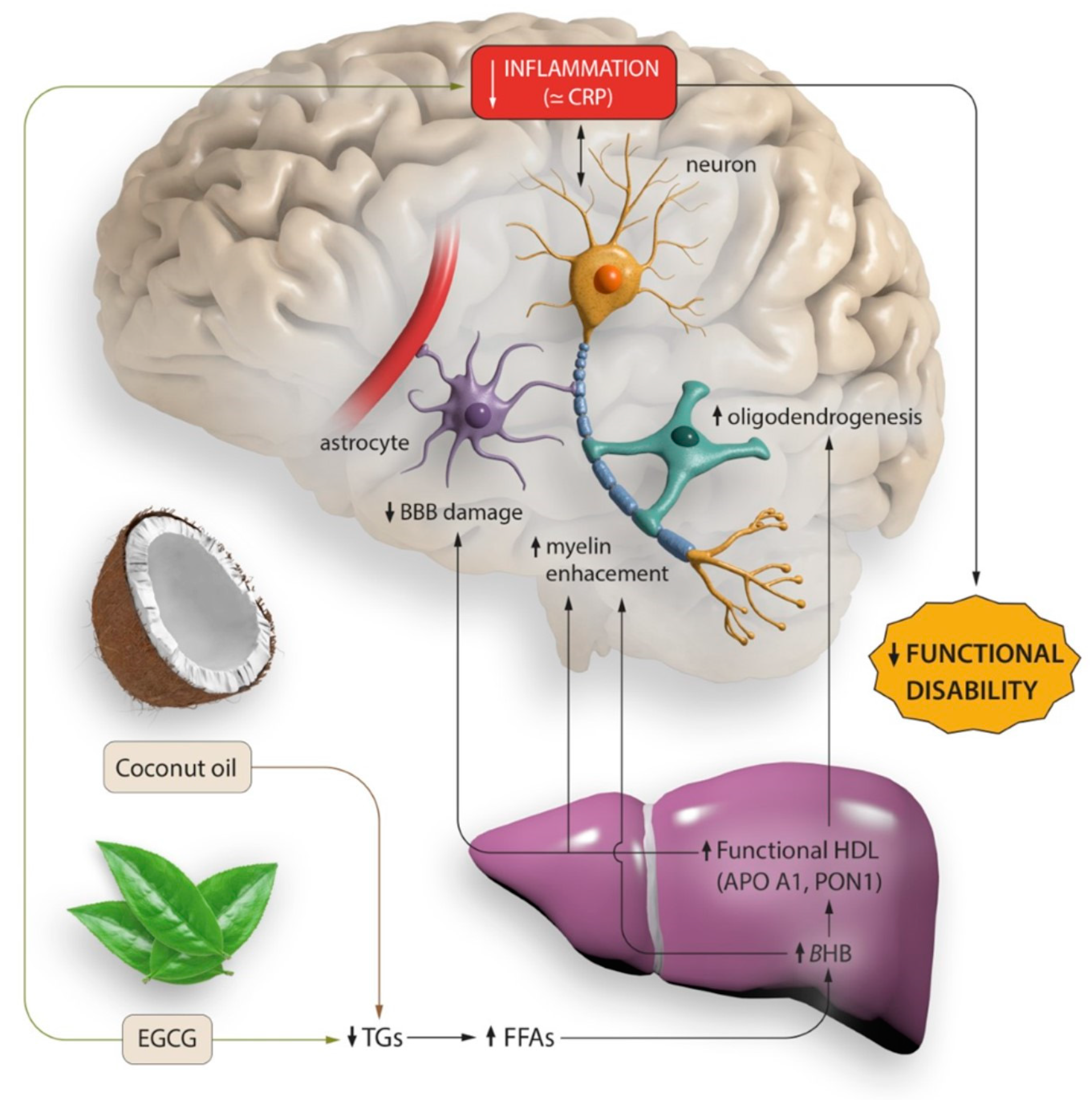
:1. Introduction
2. Materials and Methods
3. Results
3.1. Lipid Profile in Relation to Anthropometric Parameters
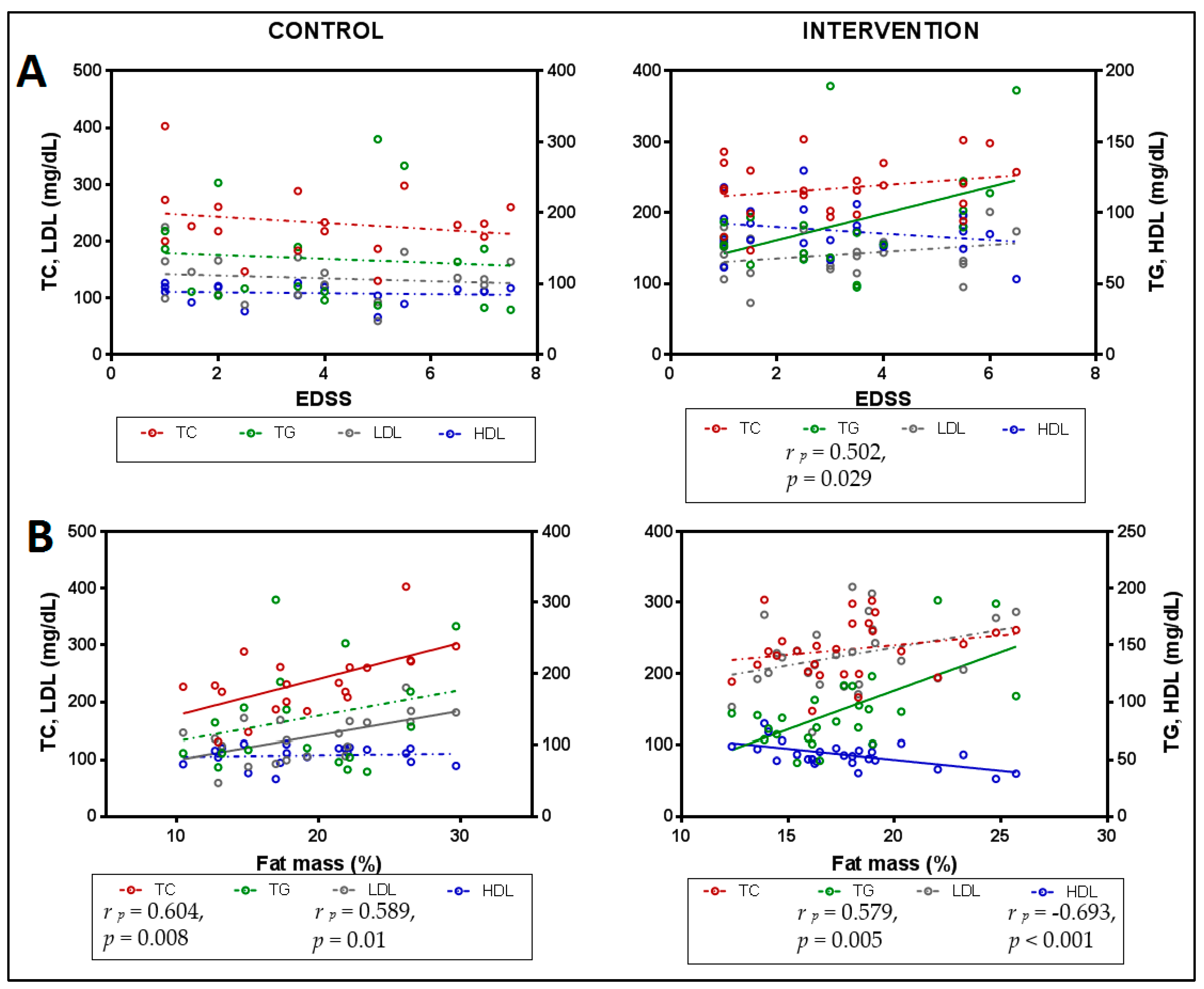
3.2. Lipid Profile in Relation to Functionality
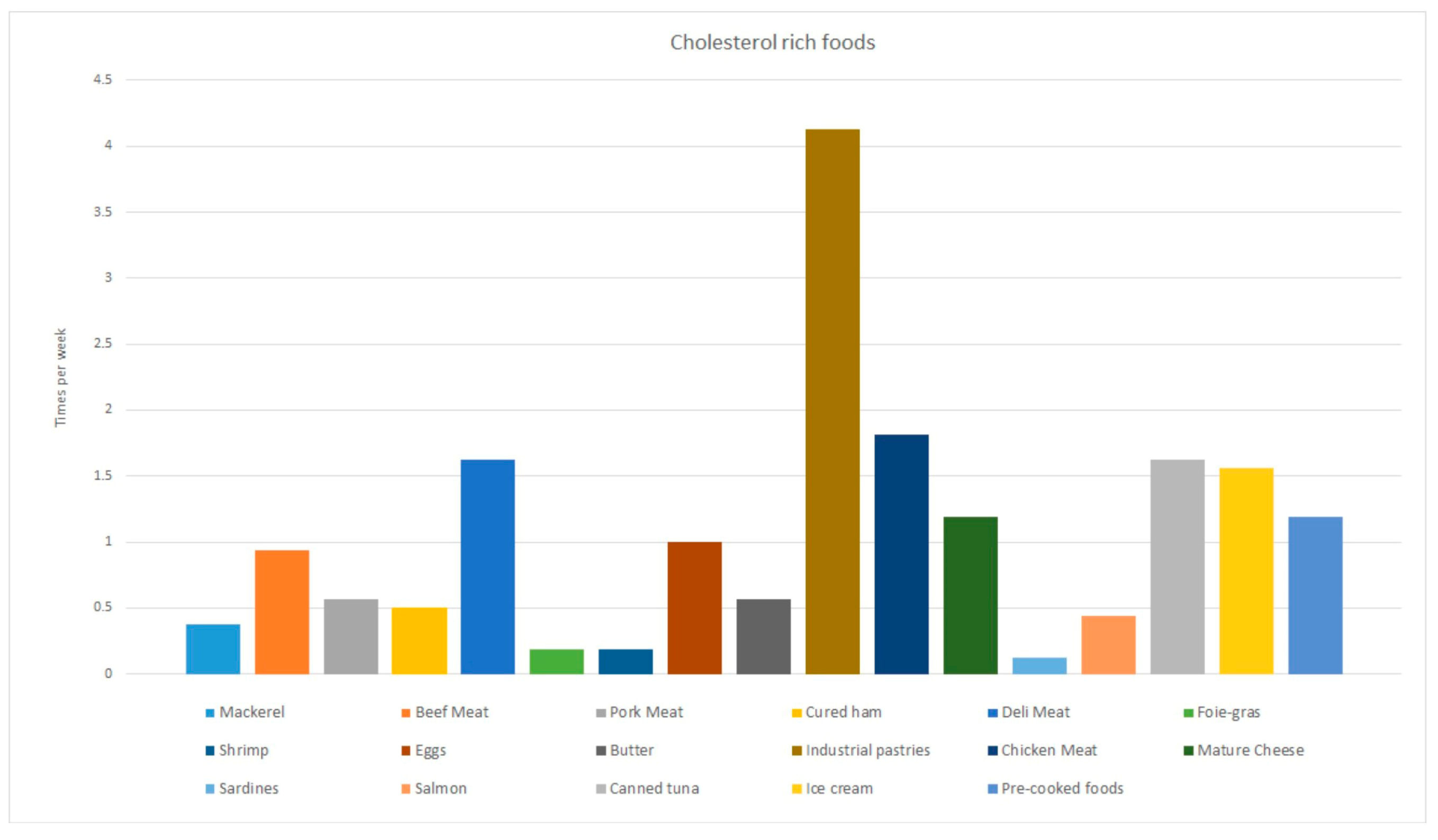
3.3. Nutritional Habits of the Study Population
3.4. Relationship between Nutritional Habits and Lipid Profile
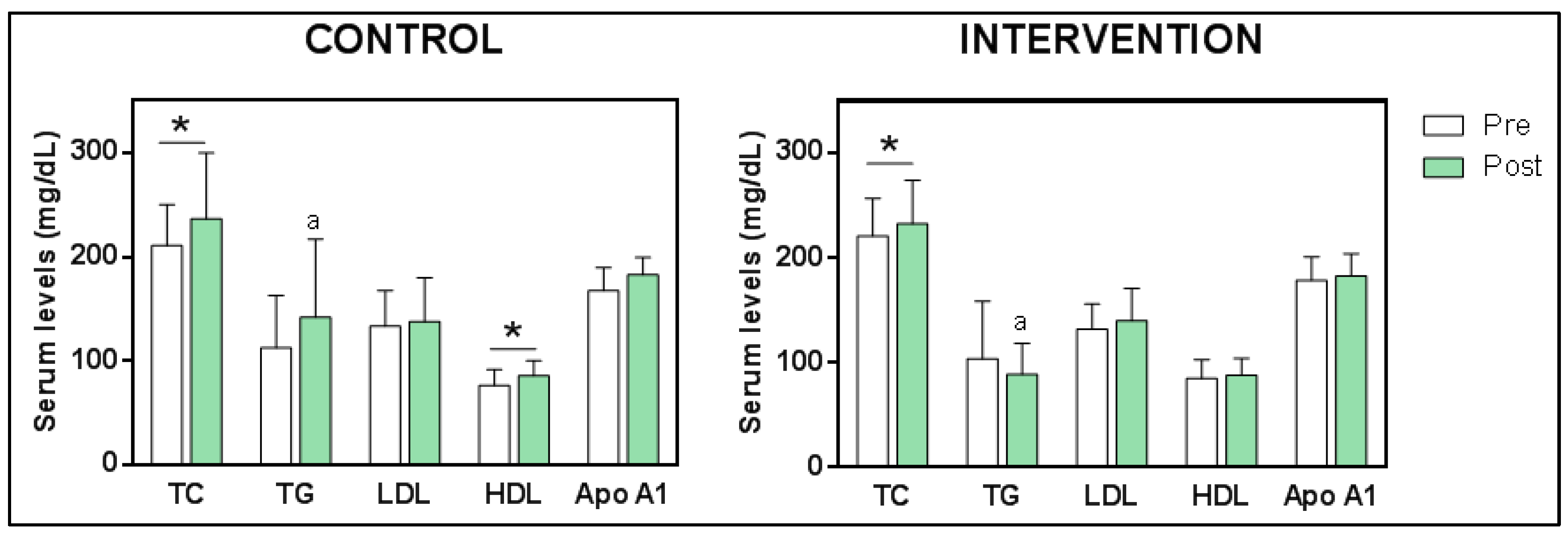
3.5. Effect of the Intervention
3.5.1. Changes in Blood Lipoprotein Levels
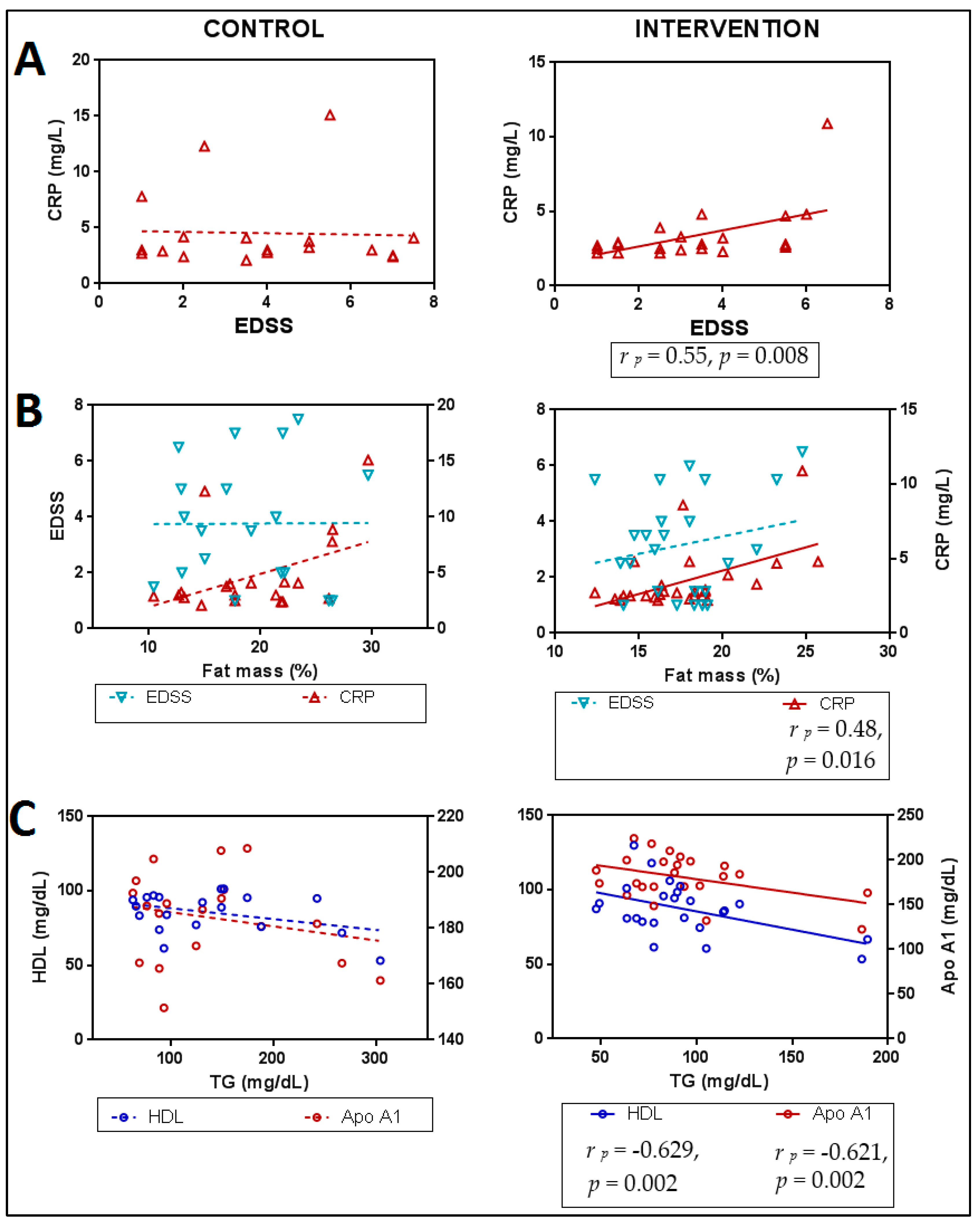
3.5.2. Relationships between Lipid Profile, Functionality, Anthropometry and Inflammation Markers after Intervention
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiorini, A.; Koudriavtseva, T.; Bucaj, E.; Coccia, R.; Foppoli, C.; Giorgi, A.; Schininà, M.E.; Di Domenico, F.; De Marco, F.; Perluigi, M. Involvement of Oxidative Stress in Occurrence of Relapses in Multiple Sclerosis: The Spectrum of Oxidatively Modified Serum Proteins Detected by Proteomics and Redox Proteomics Analysis. PLoS ONE 2013, 8, e65184. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.J.P.; Widrick, J.J. Cross-Bridge Mechanisms of Muscle Weakness in Multiple Sclerosis. Muscle Nerve 2003, 27, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mörk, S.; Bö, L. Axonal Transection in the Lesions of Multiple Sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Adamczyk-Sowa, M. New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis. Oxid. Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Fossati, V.; Bonora, M.; Giorgi, C.; Marchi, S.; Missiroli, S.; Rusielewicz, T.; Wieckowski, M.R.; Pinton, P. Mitochondria in Multiple Sclerosis: Molecular Mechanisms of Pathogenesis. Int. Rev. Cell Mol. Biol. 2017, 328, 49–103. [Google Scholar]
- Rezaeimanesh, N.; Moghadasi, A.N.; Sahraian, M.A.; Eskandarieh, S. Dietary Risk Factors of Primary Progressive Multiple Sclerosis: A Population-Based Case-Control Study. Mult. Scler. Relat. Disord. 2021, 56, 103233. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Bogdan Petcu, E.; Surugiu, R.; Fang, W.; Dumbrava, D.-A. Dietary Habits, Lifestyle Factors and Neurodegenerative Diseases. Neural Regen. Res. 2019, 15, 394–400. [Google Scholar] [CrossRef]
- Lorincz, B.; Jury, E.C.; Vrablik, M.; Ramanathan, M.; Uher, T. The Role of Cholesterol Metabolism in Multiple Sclerosis: From Molecular Pathophysiology to Radiological and Clinical Disease Activity. Autoimmun. Rev. 2022, 21, 103088. [Google Scholar] [CrossRef]
- Liu, J.-P.; Tang, Y.; Zhou, S.; Toh, B.H.; McLean, C.; Li, H. Cholesterol Involvement in the Pathogenesis of Neurodegenerative Diseases. Mol. Cell. Neurosci. 2010, 43, 33–42. [Google Scholar] [CrossRef]
- Sicras-Mainar, A.; Ruíz-Beato, E.; Navarro-Artieda, R.; Maurino, J. Comorbidity and Metabolic Syndrome in Patients with Multiple Sclerosis from Asturias and Catalonia, Spain. BMC Neurol. 2017, 17, 134. [Google Scholar] [CrossRef]
- Wens, I.; Dalgas, U.; Stenager, E.; Eijnde, B.O. Risk Factors Related to Cardiovascular Diseases and the Metabolic Syndrome in Multiple Sclerosis—A Systematic Review. Mult. Scler. 2013, 19, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Tettey, P.; Simpson, S.; Taylor, B.; Blizzard, L.; Ponsonby, A.-L.; Dwyer, T.; Kostner, K.; van der Mei, I. An Adverse Lipid Profile Is Associated with Disability and Progression in Disability, in People with MS. Mult. Scler. J. 2014, 20, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Zivadinov, R.; Mahfooz, N.; Carl, E.; Drake, A.; Schneider, J.; Teter, B.; Hussein, S.; Mehta, B.; Weiskopf, M.; et al. Serum Lipid Profiles Are Associated with Disability and MRI Outcomes in Multiple Sclerosis. J. Neuroinflamm. 2011, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Gafson, A.R.; Thorne, T.; McKechnie, C.I.J.; Jimenez, B.; Nicholas, R.; Matthews, P.M. Lipoprotein Markers Associated with Disability from Multiple Sclerosis. Sci. Rep. 2018, 8, 17026. [Google Scholar] [CrossRef]
- Rádiková, Ž.; Penesová, A.; Vlček, M.; Havranová, A.; Siváková, M.; Šiarnik, P.; Žitňanová, I.; Imrich, R.; Turčáni, P.; Kollár, B. Lipoprotein Profiling in Early Multiple Sclerosis Patients: Effect of Chronic Inflammation? Lipids Health Dis. 2020, 19, 49. [Google Scholar] [CrossRef]
- Banks, W.A.; Farr, S.A.; Salameh, T.S.; Niehoff, M.L.; Rhea, E.M.; Morley, J.E.; Hanson, A.J.; Hansen, K.M.; Craft, S. Triglycerides Cross the Blood-Brain Barrier and Induce Central Leptin and Insulin Receptor Resistance. Int. J. Obes. 2018, 42, 391–397. [Google Scholar] [CrossRef]
- Meyers, L.; Groover, C.J.; Douglas, J.; Lee, S.; Brand, D.; Levin, M.C.; Gardner, L.A. A Role for Apolipoprotein A-I in the Pathogenesis of Multiple Sclerosis. J. Neuroimmunol. 2014, 277, 176–185. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Interactions of Lipids, Lipoproteins, and Apolipoproteins with the Blood-Brain Barrier. Pharm. Res. 2021, 38, 1469–1475. [Google Scholar] [CrossRef]
- Cascella, M.; Bimonte, S.; Muzio, M.R.; Schiavone, V.; Cuomo, A. The Efficacy of Epigallocatechin-3-Gallate (Green Tea) in the Treatment of Alzheimer’s Disease: An Overview of Pre-Clinical Studies and Translational Perspectives in Clinical Practice. Infect. Agents Cancer 2017, 12, 36. [Google Scholar] [CrossRef]
- Renaud, J.; Nabavi, S.F.; Daglia, M.; Nabavi, S.M.; Martinoli, M.G. Epigallocatechin-3-Gallate, a Promising Molecule for Parkin-Son’s Disease? Rejuvenation Res 2022, 18, 257–269. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Shahpiri, Z.; Bahramsoltani, R.; Nia, M.M.; Najafi, F.; Rahimi, R. Efficacy and Tolerability of Phytomedicines in Mul-Tiple Sclerosis Patients: A Review. CNS Drugs 2017, 31, 867–889. [Google Scholar] [CrossRef] [PubMed]
- Bezard, J.; Bugaut, M.; Clement, G. Triglyceride Composition of Coconut Oil. J. Am. Oil Chem. Soc. 1971, 48, 134–139. [Google Scholar] [CrossRef]
- Kovács, Z.; Brunner, B.; Ari, C. Beneficial Effects of Exogenous Ketogenic Supplements on Aging Processes and Age-Related Neurodegenerative Diseases. Nutrients 2021, 13, 2197. [Google Scholar] [CrossRef]
- Sun, W.; Wen, M.; Liu, M.; Wang, Q.; Liu, Q.; Li, L.; Siebert, H.-C.; Loers, G.; Zhang, R.; Zhang, N. Effect of β-Hydroxybutyrate on Behavioral Alterations, Molecular and Morphological Changes in CNS of Multiple Sclerosis Mouse Model. Front. Aging Neurosci. 2022, 14, 1075161. [Google Scholar] [CrossRef]
- Goto, T.; Saito, Y.; Morikawa, K.; Kanamaru, Y.; Nagaoka, S. Epigallocatechin Gallate Changes MRNA Expression Level of Genes Involved in Cholesterol Metabolism in Hepatocytes. Br. J. Nutr. 2012, 107, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.S.; Butt, M.S.; Sultan, M.T.; Mushtaq, Z.; Ahmad, S.; Dewanjee, S.; Feo, V.; Zia-Ul-Haq, M. Preventive Role of Green Tea Catechins from Obesity and Related Disorders Especially Hypercholesterolemia and Hyperglycemia. J. Transl. Med. 2015, 13, 79. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Sua, M.; Arola-Arnal, A. Resveratrol and EGCG Bind Directly and Distinctively to MiR-33a and MiR-122 and Modulate Divergently Their Levels in Hepatic Cells. Nucleic Acids Res. 2014, 42, 882–892. [Google Scholar] [CrossRef]
- Sharman, M.J.; Kraemer, W.J.; Love, D.M.; Avery, N.G.; Gómez, A.L.; Scheett, T.P.; Volek, J.S. A Ketogenic Diet Favorably Affects Serum Biomarkers for Cardiovascular Disease in Normal-Weight Men. J. Nutr. 2002, 132, 1879–1885. [Google Scholar] [CrossRef]
- Dashti, H.M.; Mathew, T.C.; Khadada, M.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I.; Al-Zaid, N.S. Beneficial Effects of Ke-Togenic Diet in Obese Diabetic Subjects. Mol. Cell. Biochem. 2007, 302, 249–256. [Google Scholar] [CrossRef]
- Casanova, E.; Salvadó, J.; Crescenti, A.; Gibert-Ramos, A. Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 532. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Koutnik, A.P.; D’Agostino, D.P.; Egan, B. Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends Endocrinol. Metab. 2019, 30, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Dowis, K.; Banga, S. The Potential Health Benefits of the Ketogenic Diet: A Narrative Review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, M.; Cuerda Ballester, M.; Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; López-Rodríguez, M.M.; Ceron, J.J.; Tvarijonaviciute, A.; Navarro, M.Á.; Moreno, M.L.; et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients 2020, 12, 3792. [Google Scholar] [CrossRef]
- Rodríguez, I.T.; Ballart, J.F.; Pastor, G.C.; Jordà, E.B.; Val, V.A. Validation of a Short Questionnaire on Frequency of Dietary Intake: Reproducibility and Validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar] [PubMed]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary Assessment Methods: Dietary Records. Nutr. Hosp. 2015, 31, 38–45. [Google Scholar] [CrossRef]
- Dapcich, V.; Salvador, G.; Ribas, L.; Pérez, C.; Aranceta, J.; Serra, L. Guía de La Alimentación Saludable [Healthy Diet Guide]. Available online: https://www.nutricioncomunitaria.org/es/noticia/guia-de-alimentacion-saludablesenc (accessed on 7 October 2022).
- Aend, S.L.U. Easy Diet; Academia Española de Nutrición y Dietética: Barcelona, Spain, 2023. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Oliveira, B.C.d.L.; Bellozi, P.M.Q.; Reis, H.J.; de Oliveira, A.C.P. Inflammation as a Possible Link Between Dyslipidemia and Alzheimer’s Disease. Neuroscience 2018, 376, 127–141. [Google Scholar] [CrossRef]
- Martins, I. Overnutrition Determines LPS Regulation of Mycotoxin Induced Neurotoxicity in Neurodegenerative Diseases. Int. J. Mol. Sci. 2015, 16, 29554–29573. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (Version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Re. Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef] [PubMed]
- Fellows Maxwell, K.; Wahls, T.; Browne, R.W.; Rubenstein, L.; Bisht, B.; Chenard, C.A.; Snetselaar, L.; Weinstock-Guttman, B.; Ramanathan, M. Lipid Profile Is Associated with Decreased Fatigue in Individuals with Progressive Multiple Sclerosis Following a Diet-Based Intervention: Results from a Pilot Study. PLoS ONE 2019, 14, e0218075. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, A.L.; Rodríguez-Méndez, A.J.; Gallardo-Vidal, L.S.; García-Gasca, T.; Alatorre-Cruz, J.M.; García-Solís, P.; López Reyes, J.; Solís-Saínz, J.C. Lipid Profile: Causal Relationship on Cognitive Performance in Multiple Sclerosis? Mol. Biol. Rep. 2020, 47, 9667–9676. [Google Scholar] [CrossRef] [PubMed]
- Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; Moreno, M.L.; Tvarijonaviciute, A.; Navarro, M.Á.; López-Rodríguez, M.M.; Ortí, J.E.d.l.R. The Relation between Eating Habits and Abdominal Fat, Anthropometry, PON1 and IL-6 Levels in Patients with Multiple Sclerosis. Nutrients 2020, 12, 744. [Google Scholar] [CrossRef]
- Canhada, S.L.; Vigo, Á.; Luft, V.C.; Levy, R.B.; Alvim Matos, S.M.; del Carmen Molina, M.; Giatti, L.; Barreto, S.; Duncan, B.B.; Schmidt, M.I. Ultra-Processed Food Consumption and Increased Risk of Metabolic Syndrome in Adults: The ELSA-Brasil. Diabetes Care 2023, 46, 369–376. [Google Scholar] [CrossRef]
- Boshra, H.; Awad, M.; Hussein, M.; Elyamani, E. Vascular Dysfunction and Dyslipidemia in Multiple Sclerosis: Are They Correlated with Disease Duration and Disability Status? Egypt. Heart J. 2022, 74, 9. [Google Scholar] [CrossRef]
- Martin, C.L.; Phillips, B.A.; Kilpatrick, T.J.; Butzkueven, H.; Tubridy, N.; McDonald, E.; Galea, M.P. Gait and Balance Impairment in Early Multiple Sclerosis in the Absence of Clinical Disability. Mult. Scler. J. 2006, 12, 620–628. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Ibáñez, V.; Sancho, D.; Lopez-Rodríguez, M.M.; Drehmer, E.; de la Rubia Ortí, J.E. The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of IL-6, Anxiety and Disability in Multiple Sclerosis Patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef]
- Gutiérrez-Salmeán, G.; Meaney, E.; Lanaspa, M.A.; Cicerchi, C.; Johnson, R.J.; Dugar, S.; Taub, P.; Ramírez-Sánchez, I.; Villarreal, F.; Schreiner, G.; et al. A Randomized, Placebo-Controlled, Double-Blind Study on the Effects of (−)-Epicatechin on the Triglyceride/HDLc Ratio and Cardiometabolic Profile of Subjects with Hypertriglyceridemia: Unique in Vitro Effects. Int. J. Cardiol. 2016, 223, 500–506. [Google Scholar] [CrossRef]
- Donat-Vargas, C.; Sandoval-Insausti, H.; Rey-García, J.; Moreno-Franco, B.; Åkesson, A.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. High Consumption of Ultra-Processed Food Is Associated with Incident Dyslipidemia: A Prospective Study of Older Adults. J. Nutr. 2021, 151, 2390–2398. [Google Scholar] [CrossRef]
- Balazs, Z.; Panzenboeck, U.; Hammer, A.; Sovic, A.; Quehenberger, O.; Malle, E.; Sattler, W. Uptake and Transport of High-density Lipoprotein (HDL) and HDL-associated A-tocopherol by an in Vitro Blood–Brain Barrier Model. J. Neurochem. 2004, 89, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Wang, C.; Nyegaard, S.; Heit, B.; Fairn, G.D.; Lee, W.L. SR-BI Mediated Transcytosis of HDL in Brain Microvascular Endothelial Cells Is Independent of Caveolin, Clathrin, and PDZK1. Front. Physiol. 2017, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Swaminathan, S.K.; Curran, G.L.; Poduslo, J.F.; Lowe, V.J.; Li, L.; Kandimalla, K.K. Apolipoprotein A-I Crosses the Blood-Brain Barrier through Clathrin-Independent and Cholesterol-Mediated Endocytosis. J. Pharmacol. Exp. Ther. 2019, 369, 481–488. [Google Scholar] [CrossRef]
- Fellows, K.; Uher, T.; Browne, R.W.; Weinstock-Guttman, B.; Horakova, D.; Posova, H.; Vaneckova, M.; Seidl, Z.; Krasensky, J.; Tyblova, M.; et al. Protective Associations of HDL with Blood-Brain Barrier Injury in Multiple Sclerosis Patients. J. Lipid Res. 2015, 56, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.A.; Levin, M.C. Importance of Apolipoprotein A-I in Multiple Sclerosis. Front. Pharmacol. 2015, 6, 278. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Li, Y.; Cui, X.; Zheng, X.; Roberts, C.; Lu, M.; Elias, S.B.; Chopp, M. Niaspan Treatment Improves Neurological Functional Recovery in Experimental Autoimmune Encephalomyelitis Mice. Neurobiol. Dis. 2008, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.K.; Zhou, A.L.; Ahlschwede, K.M.; Curran, G.L.; Lowe, V.J.; Li, L.; Kandimalla, K.K. High-Density Lipoprotein Mimetic Peptide 4F Efficiently Crosses the Blood-Brain Barrier and Modulates Amyloid- β Distribution between Brain and Plasma. J. Pharmacol. Exp. Ther. 2020, 375, 308–316. [Google Scholar] [CrossRef]
- Cuerda-Ballester, M.; Proaño, B.; Alarcón-Jimenez, J.; de Bernardo, N.; Villaron-Casales, C.; Lajara Romance, J.M.; de la Rubia Ortí, J.E. Improvements in Gait and Balance in Patients with Multiple Sclerosis after Treatment with Coconut Oil and Epigallocatechin Gallate. A Pilot Study. Food Funct. 2023, 14, 1062–1071. [Google Scholar] [CrossRef]
- Kim, S.-N.; Kwon, H.-J.; Akindehin, S.; Jeong, H.; Lee, Y.-H. Effects of Epigallocatechin-3-Gallate on Autophagic Lipolysis in Adipocytes. Nutrients 2017, 9, 680. [Google Scholar] [CrossRef]
- Doğan, K.; Şeneş, M.; Karaca, A.; Kayalp, D.; Kan, S.; Gülçelik, N.E.; Aral, Y.; Yücel, D. HDL Subgroups and Their Paraoxonase-1 Activity in the Obese, Overweight and Normal Weight Subjects. Int. J. Clin. Pract. 2021, 75, 14969. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin Gallate Decreases Plasma Triglyceride, Blood Pressure, and Serum Kisspeptin in Obese Human Subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Abdullah; Tian, W.; Song, M.; Cao, Y.; Xiao, J. Co-Delivery of EGCG and Lycopene via a Pickering Double Emulsion Induced Synergistic Hypolipidemic Effect. Food Funct. 2022, 13, 3419–3430. [Google Scholar] [CrossRef]
- Mu, J.; Wang, T.; Li, M.; Guan, T.; Guo, Y.; Zhang, X.; Zhang, G.; Kong, J. Ketogenic Diet Protects Myelin and Axons in Diffuse Axonal Injury. Nutr. Neurosci. 2022, 25, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood–Brain Barrier Permeability of Green Tea Catechin Metabolites and Their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zheng, Y.; Zhang, X.; Hu, X.; Wang, Y.; Zhang, S.; Zhang, D.; Nie, H. Novel Immunoregulatory Properties of EGCG on Reducing Inflammation in EAE. Front. Biosci. 2013, 18, 332–342. [Google Scholar] [CrossRef]
- Cai, J.; Jing, D.; Shi, M.; Liu, Y.; Lin, T.; Xie, Z.; Zhu, Y.; Zhao, H.; Shi, X.; Du, F.; et al. Epigallocatechin Gallate (EGCG) Attenuates Infrasound-Induced Neuronal Impairment by Inhibiting Microglia-Mediated Inflammation. J. Nutr. Biochem. 2014, 25, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Caminhotto, R.d.O.; Komino, A.C.M.; de Fatima Silva, F.; Andreotti, S.; Sertié, R.A.L.; Boltes Reis, G.; Lima, F.B. Oral β-Hydroxybutyrate Increases Ketonemia, Decreases Visceral Adipocyte Volume and Improves Serum Lipid Profile in Wistar Rats. Nutr. Metab. 2017, 14, 31. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Cabezas Perez, R.J.; Ávila Rodríguez, M.F.; Rosero Salazar, D.H. Exogenous Antioxidants in Remyelination and Skeletal Muscle Recovery. Biomedicines 2022, 10, 2557. [Google Scholar] [CrossRef]
- Alipour, A.; van Oostrom, A.J.H.H.; Izraeljan, A.; Verseyden, C.; Collins, J.M.; Frayn, K.N.; Plokker, T.W.M.; Elte, J.W.F.; Castro Cabezas, M. Leukocyte Activation by Triglyceride-Rich Lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Pirillo, A.; Ammirati, E.; Catapano, A.L. Emerging Role of High Density Lipoproteins as a Player in the Immune System. Atherosclerosis 2012, 220, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.B.; Cho, H.-J.; Choi, M.; Seok, J.M.; Shin, H.Y.; Kim, B.J.; Min, J.-H. Low High-Density Lipoprotein Cholesterol and High Triglycerides Lipid Profile in Neuromyelitis Optica Spectrum Disorder: Associations with Disease Activity and Disability. Mult. Scler. Relat. Disord. 2020, 40, 101981. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Gustavsen, S.; Gisselø Lauridsen, K.; Chenoufi Hasselbalch, I.; Sellebjerg, F.; Bach Søndergaard, H.; Bang Oturai, A. Neutrophil-to-lymphocyte Ratio and CRP as Biomarkers in Multiple Sclerosis: A Systematic Review. Acta Neurol. Scand. 2021, 143, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Soilu-Hanninen, M.; Koskinen, J.O.; Laaksonen, M.; Hanninen, A.; Lilius, E.M.; Waris, M. High Sensitivity Measurement of CRP and Disease Progression in Multiple Sclerosis. Neurology 2005, 65, 153–155. [Google Scholar] [CrossRef]
- Shu, Y.; Li, R.; Qiu, W.; Chang, Y.; Sun, X.; Fang, L.; Chen, C.; Yang, Y.; Lu, Z.; Hu, X.; et al. Association of Serum Gamma-Glutamyltransferase and C-Reactive Proteins with Neuromyelitis Optica and Multiple Sclerosis. Mult. Scler. Relat. Disord. 2017, 18, 65–70. [Google Scholar] [CrossRef]
- Sajja, A.; Park, J.; Sathiyakumar, V.; Varghese, B.; Pallazola, V.A.; Marvel, F.A.; Kulkarni, K.; Muthukumar, A.; Joshi, P.H.; Gianos, E.; et al. Comparison of Methods to Estimate Low-Density Lipoprotein Cholesterol in Patients with High Triglyceride Levels. JAMA Netw. Open 2021, 4, e2128817. [Google Scholar] [CrossRef]
| Overall (n = 45) | Male (n = 15) | Female (n = 30) | ||
|---|---|---|---|---|
| M ± SD | M ± SD | M ± SD | p-Value | |
| Age (y) | 47 ± 12.6 | 46.9 ± 13.4 | 47 ± 12.5 | 0.979 t |
| Time since diagnosis (y) | 13.1 ± 9.6 | 15.5 ± 9.1 | 11.9 ± 9.7 | 0.122 u |
| BMI (kg/m2) | 25.6 ± 5.8 | 25.2 ± 7.1 | 25.7 ± 5.3 | 0.613 u |
| TC (mg/dL) | 217.4 ± 37.6 | 216.5 ± 44.2 | 217.9 ± 34.3 | 0.900 t |
| TG (mg/dL) | 107.1 ± 50.2 | 104.6 ± 38.6 | 108.5 ± 55.8 | 0.911 u |
| LDL (mg/dL) | 132 ± 27.7 | 134 ± 34.6 | 130.9 ± 23.6 | 0.725 t |
| HDL (mg/dL) | 83.2 ± 19.2 | 79 ± 22.1 | 85.6 ± 17.4 | 0.059 u |
| Apo A1 (mg/dL) | 175.6 ± 23.8 | 167.8 ± 25 | 179.9 ± 22.4 | 0.037 u |
| MS type | N (%) | n (%) | n (%) | p-value |
| Relapsing–Remitting | 34 (75.6) | 9 (60) | 25 (83.3) | 0.09 f |
| Secondary-Progressive | 11 (24.4) | 6 (40) | 5 (16.7) |
| Regression Coefficients: Fat Mass (%) | Regression Coefficients: Muscle Mass (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | t | p-Value | B | SE B | β | t | p-Value | |
| TC | 0.017 | 0.010 | 0.164 | 1.69 | 0.098 | −0.027 | 0.012 | −0.303 | −2.32 | 0.025 |
| R2 = 0.674; Fmodel = 15.32, p < 0.001; d | R2 = 0.407; Fmodel = 5.07, p = 0.001; b | |||||||||
| TG | −0.001 | 0.008 | −0.010 | −0.097 | 0.924 | 0.002 | 0.010 | 0.036 | 0.248 | 0.806 |
| LDL | 0.047 | 0.014 | 0.319 | 3.25 | 0.003 | −0.061 | 0.017 | −0.482 | −3.48 | 0.001 |
| HDL | −0.034 | 0.018 | −0.162 | −1.89 | 0.067 | 0.016 | 0.022 | 0.089 | 0.732 | 0.469 |
| R2 = 0.764; Fmodel = 15.66, p < 0.001; b, d | R2 = 0.528; Fmodel = 5.43, p < 0.001; b, c | |||||||||
| Regression coefficients: 10MWT | Regression coefficients: 2MWT | |||||||||
| B | SE B | β | t | p-value | B | SE B | β | t | p-value | |
| TC | −0.004 | 0.002 | −0.197 | −1.55 | 0.132 | −0.259 | 0.1 | −0.267 | −2.58 | 0.014 |
| R2 = 0.543; F model = 7.374, p < 0.001; a, b | R2 = 0.649; Fmodel = 13.279, p < 0.001; a, b | |||||||||
| TG | 0.001 | 0.002 | 0.049 | 0.311 | 0.758 | 0.106 | 0.087 | 0.144 | 1.21 | 0.233 |
| LDL | −0.001 | 0.004 | −0.047 | −0.315 | 0.755 | −0.489 | 0.16 | −0.359 | −3.05 | 0.004 |
| HDL | −0.01 | 0.004 | −0.288 | −2.22 | 0.035 | −0.337 | 0.185 | −0.176 | −1.82 | 0.077 |
| R2 = 0.588; F model = 5.731, p < 0.001; a, b | R2 = 0.71; Fmodel = 11.535, p < 0.001; a, b | |||||||||
| Regression coefficients: Berg | ||||||||||
| B | SE B | β | t | p-value | ||||||
| TC | −0.086 | 0.036 | −0.27 | −2.366 | 0.023 | |||||
| R2 = 0.54; F model = 8.811, p < 0.001 | ||||||||||
| TG | 0.047 | 0.032 | 0.2 | 1.463 | 0.153 | |||||
| LDL | −0.164 | 0.056 | −0.38 | −2.929 | 0.006 | |||||
| HDL | −0.019 | 0.071 | −0.03 | −0.263 | 0.794 | |||||
| R2 = 0.586; Fmodel = 6.87, p < 0.001; a, b | ||||||||||
| Regression Coefficients: Systemic LDL | Regression Coefficients: Systemic TC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | t | p-Value | B | SE B | β | t | p-Value | |
| Total lipids | 0.555 | 0.358 | 0.334 | 1.549 | 0.138 | 0.718 | 0.498 | 0.323 | 1.441 | 0.166 |
| R2 = 0.25; Fmodel = 1.269; p = 0.318 | R2 = 0.189; Fmodel = 0.886; p = 0.510 | |||||||||
| Saturated fats | 0.568 | 0.749 | 0.161 | 0.758 | 0.458 | 0.931 | 1.028 | 0.197 | 0.905 | 0.377 |
| R2 = 0.18; Fmodel = 0.836; p = 0.540 | R2 = 0.138; Fmodel = 0.606; p = 0.696 | |||||||||
| Cholesterol | 0.067 | 0.072 | 0.218 | 0.938 | 0.360 | 0.127 | 0.097 | 0.307 | 1.303 | 0.208 |
| R2 = 0.193; Fmodel = 0.090; p = 0.496 | R2 = 0.174; Fmodel = 0.802; p = 0.562 | |||||||||
| Regression coefficients: systemic HDL | Regression coefficients: systemic TG | |||||||||
| B | SE B | β | t | p-value | B | SE B | β | t | p-value | |
| Total lipids | −0.106 | 0.212 | −0.119 | −0.501 | 0.622 | 0.871 | 0.651 | 0.297 | 1.339 | 0.197 |
| R2 = 0.095; Fmodel = 0.4; p = 0.843 | R2 = 0.246; Fmodel = 1.171; p = 0.361 | |||||||||
| Saturated fats | −0.290 | 0.421 | −0.153 | −0.689 | 0.499 | 1.497 | 1.337 | 0.238 | 1.119 | 0.278 |
| R2 = 0.106; Fmodel = 0.449; p = 0.809 | R2 = 0.225; Fmodel = 1.041; p = 0.424 | |||||||||
| Cholesterol | 0.008 | 0.041 | 0.048 | 0.194 | 0.848 | 0.163 | 0.125 | 0.292 | 1.297 | 0.211 |
| R2 = 0.085; Fmodel = 0.354; p = 0.873 | R2 = 0.241; Fmodel = 1.145; p = 0.373 | |||||||||
| Control Group | Intervention Group | ||
|---|---|---|---|
| n (%) | n (%) | p | |
| MS type | |||
| Relapsing-Remitting | 16 (80) | 18 (72) | 0.729 f |
| Secondary-Progressive | 4 (20) | 7 (28) | |
| Sex | |||
| Men n (%) | 10 (50) | 5 (20) | 0.034 c |
| Women n (%) | 10 (50) | 20 (80) | |
| M ± SD | M ± SD | p | |
| Age (y) | 48.9 ± 12.5 | 44 ± 11.5 | 0.176 t |
| Time since diagnosis (y) | 14.9 ± 9 | 11.7 ± 10 | 0.114 u |
| BMI (kg/m2) | 25.8 ± 6 | 25.9 ± 5.2 | 0.887 u |
| TC (mg/dL) | 210.8 ± 39.39 | 220.9 ± 35.96 | 0.873 u |
| TG (mg/dL) | 112.8 ± 50 | 103.6 ± 54.87 | 0.540 u |
| LDL (mg/dL) | 133.4 ± 34.1 | 131.9 ± 24.07 | 0.976 t |
| HDL (mg/dL) | 76.56 ± 15.22 | 84.81 ± 18.05 | 0.249 u |
| Apo A1 (mg/dL) | 167.6 ± 21.77 | 178.7 ± 22.54 | 0.126 u |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rubia Ortí, J.E.; Platero Armero, J.L.; Cuerda-Ballester, M.; Sanchis-Sanchis, C.E.; Navarro-Illana, E.; Lajara-Romance, J.M.; Benlloch, M.; Ceron, J.J.; Tvarijonaviciute, A.; Proaño, B. Lipid Profile in Multiple Sclerosis: Functional Capacity and Therapeutic Potential of Its Regulation after Intervention with Epigallocatechin Gallate and Coconut Oil. Foods 2023, 12, 3730. https://doi.org/10.3390/foods12203730
de la Rubia Ortí JE, Platero Armero JL, Cuerda-Ballester M, Sanchis-Sanchis CE, Navarro-Illana E, Lajara-Romance JM, Benlloch M, Ceron JJ, Tvarijonaviciute A, Proaño B. Lipid Profile in Multiple Sclerosis: Functional Capacity and Therapeutic Potential of Its Regulation after Intervention with Epigallocatechin Gallate and Coconut Oil. Foods. 2023; 12(20):3730. https://doi.org/10.3390/foods12203730
Chicago/Turabian Stylede la Rubia Ortí, Jose Enrique, Jose Luis Platero Armero, María Cuerda-Ballester, Claudia Emmanuela Sanchis-Sanchis, Esther Navarro-Illana, Jose María Lajara-Romance, María Benlloch, Jose Joaquín Ceron, Asta Tvarijonaviciute, and Belén Proaño. 2023. "Lipid Profile in Multiple Sclerosis: Functional Capacity and Therapeutic Potential of Its Regulation after Intervention with Epigallocatechin Gallate and Coconut Oil" Foods 12, no. 20: 3730. https://doi.org/10.3390/foods12203730
APA Stylede la Rubia Ortí, J. E., Platero Armero, J. L., Cuerda-Ballester, M., Sanchis-Sanchis, C. E., Navarro-Illana, E., Lajara-Romance, J. M., Benlloch, M., Ceron, J. J., Tvarijonaviciute, A., & Proaño, B. (2023). Lipid Profile in Multiple Sclerosis: Functional Capacity and Therapeutic Potential of Its Regulation after Intervention with Epigallocatechin Gallate and Coconut Oil. Foods, 12(20), 3730. https://doi.org/10.3390/foods12203730








