Abstract
The non-thermal plasma (NTP) treatment of food products as an alternative for thermal processing has been investigated over the last few years. This quasi-neutral gas contains a wide variety of reactive oxygen and nitrogen species (RONS), which could be lethal for bacterial cells present in the product. However, apart from only targeting bacteria, the RONS will also interact with components present in the food matrix. Therefore, these food components will protect the microorganisms, and the NTP treatment efficiency will decrease. This effect was investigated by supplementing a plain agar medium with various representative food matrix components. After inoculation with Escherichia coli O157:H7 (STEC) MB3885, the plates were treated for 30 s by a multi-hollow surface dielectric barrier discharge (MSDBD) generated in either dry air or air at 75% humidity, at constant power (25.7 ± 1.7 W). Subsequently, the survival of the cells was quantified. It has been found that the addition of casein hydrolysate (7.1 ± 0.2 m%), starch (2.0 m%), or soybean oil (4.6 m%) decreased the inactivation effect significantly. Food products containing these biomolecules might therefore need a more severe NTP treatment. Additionally, with increasing humidity of the plasma input gas, ozone levels decreased, and the bactericidal effect was generally less pronounced.
1. Introduction
Thermal treatment of food products for preservation purposes has been used for around 6000 years, initially by drying and smoking of the foods. Later, with the industrial revolution, thermal pasteurization and sterilization technologies were developed, increasing the preservation power even more [1]. However, in recent times, the consumer has shown an increased interest in minimally processed foods that have the characteristics of freshness but without compromising on safety [2]. As conventional thermal treatment processes result in the loss of heat-sensitive nutritional components and changes in texture and organoleptic qualities, non-thermal techniques have been developed to respond to consumer demand [3,4]. Technologies such as high-pressure processing, pulsed electric field, and irradiation are non-thermal and established concepts in the food industry, but in the last years, a growing interest is also shown in non-thermal plasma (NTP) for bacterial inactivation [5]. PlAgri (2022) reported that the annual production of scientific documents in the field of plasma technology in the food industry increased from one to 255 in the period from 2003 to 2020 [6]. Surowsky et al., (2015) even mentioned around 800 publications dealing with non-thermal plasma-based microbial inactivation in foods in 2013 [7].
The concept of plasma was first described by Langmuir (1928) to define the gas region containing balanced charges of ions and electrons [8]. Plasma reactivity is the result of the presence of free electrons and radicals, ions, excited atoms and molecules, reactive oxygen and nitrogen species (RONS), and electromagnetic radiation (UV photons and visible light) [9,10,11,12]. Although the electrons in a non-thermal plasma are at a temperature of 104–105 °C, the heavy particles are close to ambient temperature. Apart from avoiding heat degradation, the advantages of NTP for food treatment are its energy efficiency [13], the possibility to operate at atmospheric pressure (so avoiding the need for vacuum equipment) [14], and the fact that it could be created from gasses that are conventionally used for modified atmosphere packaging (MAP) [15]. Additionally, for the treatment of solid foods, NTP acts only on the surface of the product, retaining the nutritional qualities on the inside [16].
The bactericidal effect of NTP treatment is the result of various modes of action. NTP irradiation is said to cause the denaturation of membrane proteins, being detrimental to the survival and duplication of the cell [17]. Reactive oxygen species will oxidize and/or damage essential biomolecules such as DNA, proteins, enzymes, lipids, and fatty acids (e.g., in the cell membrane). The cell membrane and cell wall will be disintegrated by chemical alterations and the breaking of important bonds, including C-O, C-N, and C-C. This will cause cell leakage and loss of cell functionality [18]. On the other hand, there is no scientific consensus on the contribution of UV photons in NTP to bacterial decontamination as they show bactericidal potential in various ways but are easily absorbed by the gas atoms and molecules at atmospheric pressure [19,20].
Earlier research by De Baerdemaeker et al., (2022) and Huang et al., (2020) showed that the bactericidal effect of NTP decreased when bacteria were inoculated and treated on real food products compared to agar plates. This could be explained by, among other things, the roughness of the food surface, protecting the bacterial cells [21,22]. Han et al., (2020) showed that this negative correlation between surface roughness and bacterial inactivation was linear [23]. Furthermore, Ziuzina et al., (2015) reported the entrance of bacterial cells into the pores of plant leaves, reducing the antimicrobial efficacy of NTP [24]. However, as food constituents such as lipids and proteins are prone to oxidation by ROS [25,26], it would be expected that those components also affect NTP’s efficacy. Nevertheless, there are only limited studies on the effect of the food matrix. In the current study, the aim is to explore this matrix effect by investigating the impact of various food components on bacterial inactivation using non-thermal plasmas generated from the air at both low and high relative humidity (RH) based on the humidity of gasses recommended for food packaging [27] and the composition of the plasma.
2. Materials and Methods
First, agar media supplemented with various food components at different levels representative of their potential presence in food products were prepared. The plates were inoculated with a standardized amount of Escherichia coli cells, and the NTP was treated at constant power (25.7 ± 1.7 W) and using air at 0% or 75% relative humidity (high levels and near absence of ozone, respectively, see Section 3.1), after which the bacterial cells were recovered, and surviving cells were quantified. A comparison of the recovery between the different components, their concentrations, and the humidity of the input gas allowed for understanding the effect of those components and relative humidity on bactericidal efficacy. Additionally, the plasma was characterized under the same conditions, and NTP inactivation on different supplemented media was evaluated with respect to the plasma composition.
2.1. Supplemented Agar Media Preparation
Agar plates were prepared, containing plain (pure) bacteriological agar (15 g/L, LP0011B, Oxoid), which was supplemented with one of the three concentrations of a certain major food matrix component (Table 1), each agar containing only one type of biomolecule. For all those components, changes in inactivation levels with increasing concentration were compared against a reference sample with no addition of the particular component but containing all chemicals facilitating its dissolution and undergoing the same preparation methods. For low-humidity plasma, all concentrations (reference, low, middle, and high) were analyzed, while the reference sample was only compared with the highest concentration when applying high-humidity plasma. Seven different components from five classes were added to the agars: proteins (casein hydrolysate (22090, Sigma-Aldrich, St. Louis, MO, USA)), carbohydrates (glucose (CL00.0710, Chem-Lab, Zedelgem, Belgium) and soluble starch (S9765, Sigma-Aldrich)), lipids (refined soybean oil and stripped soybean oil (AH slaolie, Albert Heijn, Zaandam, The Netherlands)), salt (NaCl (CL00.1429, Chem-Lab)), and anti-oxidants (β-carotene (C9750, Sigma-Aldrich)). Casein is a protein with very little secondary and tertiary structure [28], meaning all the amino acids are available, and none are structurally protected from interaction with the RONS. With the hydrolyzed protein, also referred to as peptone from casein, this is even more the case. Soybean oil consists of a good mixture of saturated fatty acids (SFA, 15%), mono-unsaturated fatty acids (MUFA, 23%), and poly-unsaturated fatty acids (PUFA, 62%) [29,30]. The refined oil was prepared by removing only oxidation products already present in commercial soybean oil by means of column chromatography on silica gel (1.15101, Millipore, Burlington, MA, USA). The stripped oil was, in addition, stripped of naturally occurring anti-oxidants (column chromatography with aluminum oxide (11503, Thermo Fisher Scientific, Waltham, MA, USA) following the silica gel column chromatography). Column chromatography was carried out using the method described by Wang et al., (2018) [31].

Table 1.
Concentrations of the food matrix components in the supplemented agar media applied in this study based on their presence in real-life food products. Seven different components from five classes (indicated in bold) were added to the agars.
For the glucose and NaCl, the procedure for medium preparation was identical. Plain agar medium and a solution of the food constituent, both at double concentration, were autoclaved and filter sterilized (0.45 µm pore size, 296–4545, Thermo Fisher Scientific, Waltham, MA, USA), respectively, and subsequently aseptically mixed at a 1:1 ratio. A casein medium was prepared by making a casein hydrolysate solution at a double concentration in distilled water and adapting the pH to 10.5 by the addition of a 0.1 M and 10 M NaOH (71690, Sigma-Aldrich) solution. This casein solution was sonicated for 30 min at 50 °C and filter sterilized (0.2 µm pore size, 596–4520, Thermo Fisher Scientific, Waltham, MA, USA), and the loss of precipitate was quantified by analyzing the weight of the filter after drying. The sterile solution was mixed with plain double-strength agar medium (1:1), and the pH was reduced to 8.5 in a sterile manner with a 1 M and 6 M HCl (30721, Sigma-Aldrich) solution. For the starch agar medium, plain agar medium and a concentrated starch solution were mixed and sterilized (autoclaved). Soybean oil, whether stripped or unstripped of anti-oxidants, was mixed with Tween 20 (233362500, Acros Organics, Morris Plains, NJ, USA) while heating and kept at 48 °C before the addition of a sterile agar medium of higher strength to form a homogeneous mixture. The anti-oxidant β-carotene was first suspended in a mixture of dimethyl sulfoxide (DMSO, CL00.0422, Sigma-Aldrich) and Tween 20 and subsequently added to a sterile agar medium. To ensure an equal concentration of DMSO (5.8 mL/100 g medium) and Tween 20 (1.2 mL/100 g medium) over all supplemented agar media, an extra amount of the mixture of those components (without β-carotene) was required for some agar media and the reference.
Since the distance between the sample and electrodes has been shown to affect NTP bactericidal potential (data not shown), the height and, therefore, the volume of (supplemented) agar medium in all plates was standardized to ensure equal treatment. For all different samples, small Petri dishes (Ø 5.5 cm) were filled with 10 mL of the respective supplemented agar medium. Plates with soybean oil or β-carotene were stored for a maximum of 1 week in an anaerobic environment (AnaeroGen, AN0025A, Thermo Fisher Scientific, Waltham, MA, USA) to prevent oxidation of the biomolecules. All plates were kept at 4 °C until further use.
2.2. Plasma Treatment
Plasma was generated using the multi-hollow surface dielectric barrier discharge (MSDBD) setup shown in Figure 1. The MSDBD consists of two gold mesh electrodes placed at a distance of 0.20 mm with the interelectrode space filled with a dielectric barrier (Al2O3). An airflow (synthetic air, 14746, Air Products), whether or not humidified to 75% RH (near absence of ozone, see Section 3.1) by bubbling through a water column of ca. 89 cm, of 5 standard L/min (slm) went perpendicular through 260 holes (Ø 1.00 mm), arranged symmetrically in a hexagonal configuration in the electrode system. This gas flow was controlled by mass flow controllers (Bronkhorst). The discharge was generated by a high voltage pulse generator (Redline Technologies) at alternating current (frequency of 64.01 kHz) and supplied energy to the system at constant power (25.7 ± 1.7 W). Samples were NTP treated for 30 s at a distance of 18 mm from the electrodes, after which the active atmosphere was removed by flushing the reactor for 2 more minutes before opening.
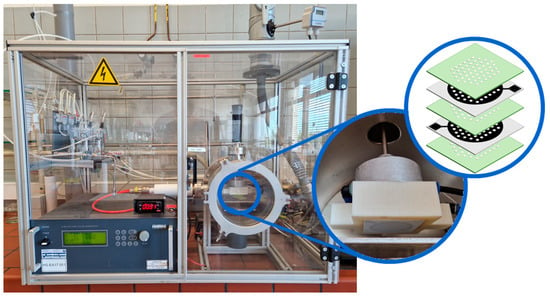
Figure 1.
Multi-hollow surface dielectric barrier discharge setup used in the current study. On the right, a detailed image and schematic overview of the electrode system are presented (green = dielectric; black = electrode).
Under those conditions, the temperature increase of the plasma exhaust, measured with an iButton data logger (Maxim Integrated), was limited to ca. 8.5 °C, which proved the non-thermal properties of the applied NTP. The plasma-treated gas was analyzed by Fourier-transformed infrared spectroscopy (FTIR) using a Matrix-MG2 Bruker FTIR spectrometer, enabling the quantitative analysis of the concentrations of NO, NO2, N2O5, N2O, and ozone. The detection was performed by a collection of the effluent gas and stable products at a 3 m distance from the plasma reactor. The optical multi-pass gas cell of 5.0 m length was used to measure the absorption, and the absolute calibrations were performed by Bruker. Spectra were obtained with an average of 50 scans with a resolution of 0.5 cm−1. The system was flushed thoroughly with air for at least 15 min in between measurements.
2.3. Strain Preparation
Escherichia coli O157:H7 (STEC) MB3885 was obtained from the FMFP (Ghent University) culture collection as cryobeads in a 15% glycerol (CL00.0736, Chem-Lab) in brain heart infusion (BHI, CM1135, Oxoid) broth at −75 °C. Two beads were transferred to fresh BHI and incubated for 2 days at 37 °C. A pure culture, obtained by the four-quadrant streak method (37 °C, 1 day) on tryptone soy agar (TSA, CM0131, Oxoid), was transferred to TSA slants (37 °C, 1 day) and kept as such for no more than 6 weeks (4 °C).
The day before every experiment, a loopful of the subculture was transferred to fresh BHI broth and incubated at 37 °C for ca. 1 day. One mL of the homogenized cell suspension was brought in an Eppendorf tube and centrifuged (8000 rpm, 5 min), after which the supernatant was replaced by fresh peptone physiological solution (PPS). This centrifugation and washing step was repeated twice more, resulting in an inoculum at a high concentration. Finally, the cell suspension was diluted to obtain an inoculum at a concentration of ca. 7 log CFU/mL. Actual concentrations and purity were determined by the standard pour-plating technique with both TSA and RAPID’E.coli 2 Medium (3564024, Bio-Rad Laboratories Inc., Hercules, CA, USA).
2.4. Sample Handling
The (supplemented) media plates were inoculated with 0.1 mL (ca. 6 log CFU/sample) of the diluted inoculum and dried for 15 min by air. Consequently, the plates were NTP treated (see Section 2.2). Cells were recovered from the (supplemented) agar medium by making a tenfold dilution with PPS and extraction in a Stomacher device. Survival of STEC was quantified by the standard pour-plating technique with TSA.
2.5. Statistical Analysis
All tests regarding inactivation levels on agar media supplemented with different concentrations of the food components were performed in triplicates. Inactivation was determined as the difference in survival of treated and their respective untreated plates. Homoscedasticity was determined by Levene’s test (based on the median) with α = 0.01. For plasma generated from dry air (comparison of four concentration levels from the same component), ANOVA (post-hoc Tukey or Games-Howell in case of homo- or heteroscedasticity, respectively) was used for analyzing the significance (α = 0.05) of the differences in inactivation levels. For humid air plasma (comparison of two concentration levels from the same component), the same comparison was made by means of the independent sample t-test. For both levels of RH, the inactivation on agars supplemented with monomeric glucose was compared with the inactivation on starchy (polymeric glucose) agars at the same concentration by using the independent sample t-test (α = 0.05). The same statistical tests were performed to compare both types of oily agars. Finally, for every component at a certain concentration, the effect of relative humidity of the plasma input gas was analyzed by the independent sample t-test (α = 0.05).
3. Results
Plain agar plates were supplemented with various food matrix components in different concentrations, inoculated with STEC, and treated with non-thermal plasma in a multi-hollow surface dielectric barrier discharge. Plasma was generated from either dry air (0% RH) or humid air (75% RH).
3.1. Plasma Characterization
The concentrations of various long-living RONS (N2O, N2O5, NO, NO2 and O3) generated during the first 50 s of treatment were measured for input air with increasing RH using Fourier-transform infrared spectroscopy. These characterization results are shown in Figure 2. With increasing %RH, the ozone concentration in the plasma drops remarkably, from 796 ± 1 ppm at 0% RH to 9 ± 1 ppm at 100% RH. On the other hand, the concentrations of NO and even more so NO2 increase with increasing %RH. N2O levels remain quasi-constant, while N2O5 decreases with increasing RH and is already absent at 75% RH. These decreasing ozone and N2O5 concentrations and increasing levels of NO2 and NO at increasing RH will have an impact on the antibacterial efficiency of the MSDBD treatment. This motivates the choice to compare the bactericidal potential of plasma at 0% and 75% RH with an ozone-rich atmosphere and the near absence of ozone in dry and humid air plasma, respectively. Next to this, an RH of 75% is representative of the relative humidity of the headspace of packaged microbiologically unstable food products.
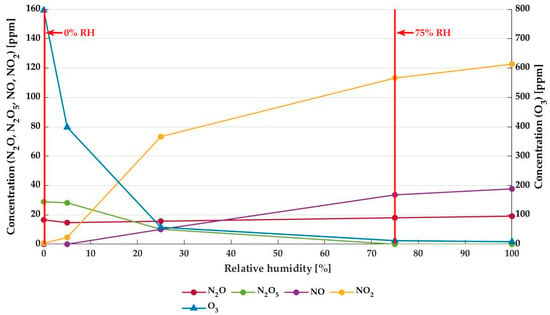
Figure 2.
Characterization of NTP by FTIR in the first 50 s of plasma generation as a function of relative humidity of the input air. The left axis shows the concentration of N2O, N2O5, NO, and NO2 (circles); on the right axis, the concentration of ozone (O3) in the plasma is projected (triangles).
3.2. NTP Inactivation with Dry and Humid Air Plasma
Inactivation levels of STEC when using dry air for plasma generation are shown in Figure 3. Without the addition of food components, inactivation reached levels of around 3.8 log CFU/sample, except for β-carotene (2.7 log CFU/sample, possibly due to the reaction between DMSO used for making a stable agar media and OH radicals in the plasma [32]). However, all those reference media reached the limit of detection (LOD) for at least one of three repeats (with the same exception), so inactivation could, in reality, be even higher. Agar medium supplemented with casein did show a reduced bactericidal effect with increasing protein concentration, although this was only significant (p ≤ 0.05) for the highest level of casein addition (7.1 ± 0.2 m%). For starch agar, the decrease in inactivation potential was even more explicit, and even at the lowest concentration (2.0 m%) evaluated, the effect of starch on the inactivation of STEC was statistically significant (p ≤ 0.05). Middle and high-starch concentrations did not differ significantly from each other. In contrast to the starch polysaccharide, its monomeric building blocks (glucose) did not significantly alter the NTP inactivation of STEC (p > 0.05), even at concentrations as high as 18.0 m%. This resulted in a significant difference (p ≤ 0.05) between inactivation on high-starch and high-glucose agar media. Additionally, STEC inactivation in the presence of lipids differed significantly from the reference sample, and this was for all investigated concentrations (low, middle, and high). However, for all tested concentrations (except the middle one), there was no significant difference between stripped and refined oil. Finally, in analogy to media supplemented with glucose, the addition of β-carotene or NaCl did not result in any significant changes in bactericidal effect (p > 0.05), although for the latter component, a slight but not statistically relevant decrease in inactivation was noticed.
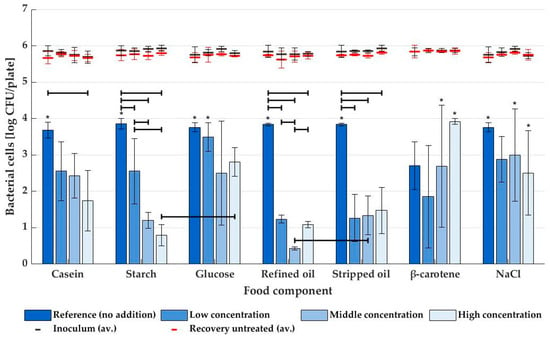
Figure 3.
Average NTP inactivation levels (and standard deviations) of STEC on agar media supplemented with various food matrix components (n = 3), with plasma generated from dry air. An asterisk (*) indicates that inactivation reached LOD for at least one repeat. All components were tested at low, middle, and high concentrations (see Table 1) and compared against a reference sample without addition. The average inoculum and number of cells recovered from untreated samples are given as black and red lines, respectively. Significant differences between inactivation levels are indicated with black horizontal lines.
Levels of inactivation for plasma generated from humid air at 75% RH are shown as red bars in Figure 4. A significant effect (p ≤ 0.05) of casein addition was observed, even resulting in a complete drop of bactericidal potential to approximately 0 log CFU/sample. Just as for dry air plasma, it was statistically shown that the polysaccharide starch chain negatively affected the decontamination efficiency (p ≤ 0.05), while this was not the case for the monomeric glucose units (p > 0.05). Furthermore, the effect of refined oil and stripped oil was quasi-identical: a decrease from >3.0 log CFU to ca. 1.0 log CFU inactivation after the addition of the lipid component. Finally, neither β-carotene nor NaCl impacted the humid air plasma inactivation.
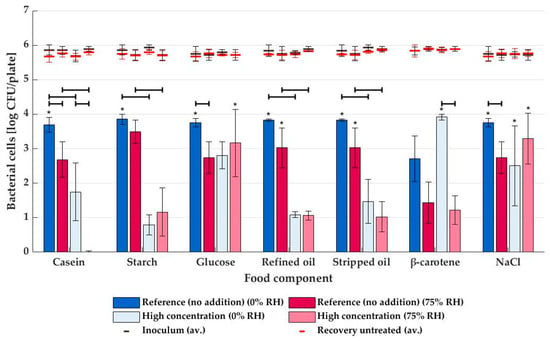
Figure 4.
Average NTP inactivation levels (and standard deviations) of STEC on agar media supplemented with various food matrix components (n = 3), with plasma generated from dry air (0% RH, blue bars) and humid air (75% RH, red bars). An asterisk (*) indicates that inactivation reached LOD for at least one repeat. All components were tested at high concentrations (see Table 1) and compared against a reference sample without addition. The average inoculum and number of cells recovered from untreated samples are given as black and red lines, respectively. Significant differences between inactivation levels are indicated with black horizontal lines.
For non-thermal plasma treatment on unsupplemented (reference) media, STEC inactivation was always lower when humidified air was used for plasma generation (see Figure 4). However, this was only significant (p ≤ 0.05) for the casein-, glucose- and NaCl-reference media. Although these food matrix components themselves were not present in the reference media, the additional chemicals used during media preparation (see Table 1) and the sometimes rather high standard deviation might have played a role. When the food constituents were added in high concentrations, only for the casein- and β-carotene-supplemented media did the NTP inactivation differ significantly at 75% RH compared to 0% RH.
4. Discussion
The main long-living RONS were characterized using FTIR spectroscopy. As shown in Figure 2, the most abundant long-living RONS in the dry air MSDBD exhaust was ozone. Its production in non-thermal air plasma happens by various pathways, although the mechanism is rather complex due to the presence of nitrogen [13,33]. The main reaction for ozone formation involves O2, atomic oxygen (O), and molecular oxygen or nitrogen (M). O was formed due to electron impact dissociation of molecular O2. This simple mechanism, given by Equations (1) and (2), does not include reactions with excited species or other molecules (e.g., NO and NO2), nor does it take into account ozone self-destruction reactions [33].
e + O2 → O + O + e,
O + O2 + M → O3 + M,
The ozone concentration drops drastically with the increasing humidity of the carrier gas. According to Patil et al., (2014), this could be attributed to the quenching/attachment of electrons to H2O, leading to water dissociation and a direct ozone reaction with the water molecules. It has been shown by optical absorption spectroscopy (OAS) that increasing %RH results in a lower ozone concentration due to the formation of N2O5, peroxides (mainly H2O2), HNO4, OH and to a lesser extent also N2O4 and HONO [34]. Nevertheless, in the current study, the concentration of N2O5 decreased (Figure 2), while the other RONS mentioned were not measured due to the limitation of the IR detection of the short-living RONS. It is said that the electronic dissociation of water in a non-thermal plasma leads to the formation of an OH radical, which rapidly reacts with ozone to form the peroxy-radical HO2. The latter component itself also reacts with ozone molecules, again forming OH and O2 [13]. Correspondingly, it is expected that the chemistry initiated by NTP in conditions of dry air and humid air should be substantially different. In the case of dry air, the main mechanism will be ozone driven, whereas active nitrogen species and peroxide radicals are defining the chemistry at high RH.
The bactericidal potential of non-thermal plasma technology for the treatment of (contaminated) solid (food) surfaces has been proven before [21,35,36,37]. Nevertheless, research has also shown that this microbial inactivation is lower when treating real food products compared to agar plates [38,39]. The reason for this observation can most probably not be attributed to one factor but is rather a combination of several elements. Han et al., (2020) have shown that surface roughness is negatively correlated with the NTP bacterial inactivation rate [23], and Ziuzina et al., (2015) found how bacteria could enter stomata on the produce surface, which protects them from inactivation by NTP [24]. However, it has been shown by De Baerdemaeker et al., (2022), although to a limited extent, that the composition of the food matrix also has a major influence on the bactericidal effect of non-thermal plasma [21].
The effect of proteins and their amino acids was investigated in the current study by supplementing casein hydrolysate (peptone from casein) to plain agar medium. As seen in Figure 3 and Figure 4, concentrations as high as 7.1 ± 0.2 m% had a significantly negative impact on the MSDBD plasma efficacy. Cataldo (2003) observed the interaction of ozone with some individual amino acids, mainly tryptophan, but also methionine, cystine, tyrosine, and phenylalanine [40]. According to Liu et al., (2019), these amino acids make up ca. 16.1 m% of the casein molecule [41]. These interactions between amino acids and the RONS might render the reactive plasma components unavailable for interaction with microorganisms, which explains the decrease in NTP inactivation efficacy on protein-rich media. In NTP generated from the air with high humidity, the ozone concentration drops close to zero, and NO2 seems to be most abundantly present (98.23 ppm). This nitrogen species, proven to be bactericidal [42], interacts strongly with tryptophan and, to a lesser extent, tyrosine [43], which might cause the bactericidal effect to disappear completely at 75% RH.
The presence of glucose did not seem to have an immediate impact on the bactericidal effect of NTP treatment, neither at low nor high concentrations. Interestingly, in contrast to its monomeric units, the polymeric starch chain does impact the bactericidal efficacy of NTP treatment negatively, even at concentrations of only 2.0 m%. This shows the interaction between the polymer and the reactive plasma species, which could be the result of various pathways. The depolymerization process results in the formation of several fragments [44,45,46], and although it has been reported that NTP treatment can also induce cross-linking of the chain and/or increase its molecular weight (MW) [46,47,48], the degree of polymerization and MW of the starch molecules generally decrease during plasma treatment, depending on the type of starch and treatment dose [46,47,48,49]. Additionally, oxidation of the starch by the RONS will also induce changes to the starch molecules, as well as the introduction of functional groups by the RONS [45,46,50]. Again, the more interaction between the RONS and starch, the lower the bactericidal effect of NTP treatment will be, illustrated by a decrease in bacterial inactivation at higher starch concentrations in Figure 3 and Figure 4. Both ozone and NO2, the major components in dry and humid air plasma, respectively (see Figure 2), are known to interact with the polysaccharide [51,52], expressed by a similar reduction of NTP inactivation potential on starchy surfaces for dry and humid air plasmas. Additionally, peroxynitrite (ONOO−) and hydrogen peroxide (H2O2), two bactericidal agents [53,54,55] that are known to be present in humid air plasma or the gas-liquid interface [34,56], might increase the decontamination efficiency of the humid air NTP treatment. As H2O2 reaction with starch is very slow or requires high temperatures [57], this oxygen species can still react with bacteria, even on starchy surfaces. However, analysis of peroxynitrite and hydrogen peroxide was not possible by means of FTIR, so no results on their concentrations in the plasma are available. A remark needs to be made on the surface properties of high-starch media plates, as these media had a more gel-like and rough surface that could protect the bacterial cells from reactive plasma species.
Lipids are known to be prone to oxidation, even when exposed only to air [58]. Characterization of NTP used in the current study indicated the presence of ozone, NO2, and/or NO, all of which have been shown to impact the lipid oxidation process [26,58,59,60,61]. Due to this interaction between lipids and the RONS, the efficacy of the MSDBD treatment decreased both for low and high RH plasmas. It has been proven by Rød et al., (2012) that NTP treatment as short as five seconds could result in the formation of oxidation products [62]. Furthermore, there were generally no differences between inactivation levels when using soybean oil with or without its naturally occurring anti-oxidants, although it would be expected that those anti-oxidants would protect bacterial cells even more by capturing the RONS in the plasma. However, it would be too soon to conclude that these components intrinsically do not impact plasma inactivation, as their naturally occurring concentrations might be too low to have a considerable effect. It has been found that higher concentrations of α-tocopherol, an anti-oxidant present in soybean oil [63], do show a protective effect on bacteria during NTP treatment by scavenging ROS [64].
Analogously, no significant effect of β-carotene, another anti-oxidant, supplementation to the agar medium was observed. Since this vitamin A precursor is known to scavenge, e.g., ozone [65], a decrease in bacterial decontamination was expected. Possibly, the presence of DMSO in the agar impacts the effect of the β-carotene as the former has been shown to react with alkenes, abundantly present with β-carotene, with the formation of methyl sulfones. This reaction needs hydroxyl radicals, which could be supplied by the plasma discharge [66].
The addition of NaCl to the agar could impact NTP inactivation in different ways. Earlier research has shown that ozone and NaCl could form the bactericidal component hypochlorous acid [67,68]. On the other hand, the salt ions (presumably Cl-) may protect the microorganisms by increasing the solution density, resulting in a decreased movement of reactive plasma species and lower accessibility to bacterial cells [69]. Nevertheless, no significant effect on NTP efficacy was found by supplementing NaCl to the agars when using dry or humid air.
5. Conclusions
This study shows that various food matrix components do have an important effect on NTP inactivation efficacy against STEC. Treatment of (food) products with a significant lipid or starch content (>4.5 and 2.0 m%, respectively) will result in an important reduction of bacterial decontamination during treatment, although this also depends on saturation degree and amino acid composition, respectively. The concentration of anti-oxidants naturally present in the oil is too low to impact the NTP inactivation potential. On the other hand, protein content needs to be rather high (7.1 ± 0.2 m%) in order to have a clear effect, although the effect is more pronounced when humid air is used for plasma generation (no bacterial reduction at high casein concentration and 75% RH). For these reasons, protein-rich and sugary food products seem to be better suited to be NTP treated compared to greasy foods. This could be fruits and vegetables, although their surfaces will also be of major importance. Furthermore, NTP might be advantageous compared to other (conventional or novel) technologies for the microbial decontamination of lean meats. For fish and meat products with higher fat content, the treatment dose needs to be increased. Nevertheless, it needs to be taken into account that a higher treatment dose might also induce a more intense nutrient loss (e.g., protein degradation), although, for solid foods, this will be limited to the surface of the product. Therefore, these changes to the nutritional value due to the current MSDBD plasma treatment should be investigated as such. Additionally, the relative humidity of the headspace at the moment of plasma treatment of packaged products is a critical factor for the efficiency of the NTP treatment, especially when both NTP and packaging technologies are integrated into one system. These considerations are of utmost importance when an (MSDBD) non-thermal plasma treatment is implemented in the production chain and show the need for an appropriate design.
Author Contributions
Conceptualization, K.D.B., A.N., B.D.M., N.D.G. and F.D.; methodology, K.D.B., A.V.R. and F.D.; validation, K.D.B., A.V.R. and F.D.; formal analysis, K.D.B. and A.V.R.; investigation, A.V.R. and A.N.; resources, A.N., B.D.M., N.D.G. and F.D.; data curation, K.D.B., A.V.R. and F.D.; writing—original draft preparation, K.D.B.; writing—review and editing, K.D.B., A.V.R., A.N., B.D.M., N.D.G. and F.D.; visualization, K.D.B. and A.V.R.; supervision, N.D.G. and F.D.; project administration, N.D.G. and F.D.; funding acquisition, B.D.M., N.D.G. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Special Research Fund of Ghent University, grant number 01G00120.
Data Availability Statement
The data presented in this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.7385615.
Acknowledgments
The authors would like to thank Danyang Liu (NutriFOODchem, Ghent University) for his help with oil and lipid handling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knorr, D.; Watzke, H. Food Processing at a Crossroad. Front. Nutr. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Alzamora, S.M.; López-Malo, A.; Tapia, M.S.; Welti-Chanes, J. Minimally Processed Foods. In Encyclopedia of Food and Health; Academic Press: Kidlington, Oxford, UK, 2016; Volume 3, pp. 767–771. ISBN 9780123849533. [Google Scholar]
- Barba, F.J.; Orlien, V.; Mota, M.J.; Lopes, R.P.; Pereira, S.A.; Saraiva, J.A. Implementation of Emerging Technologies. In Innovation Strategies in the Food Industry; Academic Press: Kidlington, Oxford, UK, 2022; pp. 121–143. [Google Scholar]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-Thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Khouryieh, H.A. Novel and Emerging Technologies Used by the U.S. Food Processing Industry. Innov. Food Sci. Emerg. Technol. 2021, 67, 102559. [Google Scholar] [CrossRef]
- COST Action CA19110 (PlAgri). WG5 Technical Roadmap—Key Food Applications and Standardized Procedures. 2022. Available online: https://plagri.eu/wg5-applications-of-plasma-processes-and-technologies-in-food-industry/ (accessed on 2 December 2022).
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of Non-Thermal Atmospheric Pressure Plasma with Solid and Liquid Food Systems: A Review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Langmuir, I. Oscillations in Ionized Gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma-Catalysis: The Known Knowns, the Known Unknowns and the Unknown Unknowns. J. Phys. D Appl. Phys. 2016, 49, 243001. [Google Scholar] [CrossRef]
- Fridman, A. Introduction to Theoretical and Applied Plasma Chemistry. In Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008; pp. 1–11. [Google Scholar]
- Misra, N.N.; Ziuzina, D.; Cullen, P.J.; Keener, K.M. Characterization of a Novel Cold Atmospheric Air Plasma System for Treatment of Packaged Liquid Food Products; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012; Volume 3. [Google Scholar]
- Whitehead, J.C. The Chemistry of Cold Plasma. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Misra, N.N., Schlüter, O., Cullen, P.J., Eds.; Academic Press, 2016; pp. 53–81. ISBN 9780128013656. [Google Scholar]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for Medicine. Phys Rep 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, H.; Hinton, A.; Zhang, J. Influence of In-Package Cold Plasma Treatment on Microbiological Shelf Life and Appearance of Fresh Chicken Breast Fillets. Food Microbiol 2016, 60, 142–146. [Google Scholar] [CrossRef]
- Surowsky, B.; Bußler, S.; Schlüter, O.K. Cold Plasma Interactions With Food Constituents in Liquid and Solid Food Matrices. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Misra, N.N., Schlüter, O., Cullen, P.J., Eds.; Academic Press: Kidlington, Oxford, UK, 2016; pp. 179–203. ISBN 9780128013656. [Google Scholar]
- Kim, Y.-M.; Yun, H.-S.; Eom, S.-H.; Sung, B.-J.; Lee, S.-H.; Jeon, S.-M.; Chin, S.-W.; Lee, M.-S. Bactericidal Action Mechanism of Nonthermal Plasma: Denaturation of Membrane Proteins. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 77–83. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of Cold Plasma Technology for Microbiological Safety in Meat Industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Vleugels, M.; Shama, G.; Deng, X.T.; Greenacre, E.; Brocklehurst, T.; Kong, M.G. Atmospheric Plasma Inactivation of Biofilm-Forming Bacteria for Food Safety Control. IEEE Trans. Plasma Sci. 2005, 33, 824–828. [Google Scholar] [CrossRef]
- Roth, S.; Feichtinger, J.; Hertel, C. Characterization of Bacillus Subtilis Spore Inactivation in Low-Pressure, Low-Temperature Gas Plasma Sterilization Processes. J. Appl. Microbiol. 2010, 108, 521–531. [Google Scholar] [CrossRef]
- de Baerdemaeker, K.; van der Linden, I.; Nikiforov, A.; Zuber, S.; de Geyter, N.; Devlieghere, F. Non-Thermal Plasma Inactivation of Salmonella Typhimurium on Different Matrices and the Effect of Selected Food Components on Its Bactericidal Efficacy. Food Res. Int. 2022, 151, 110866. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Chen, C.K.; Hsu, C.L. Non-Thermal Atmospheric Gas Plasma for Decontamination of Sliced Cheese and Changes in Quality. Food Sci. Technol. Int. 2020, 26, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Song, W.-J.; Eom, S.; Kim, S.B.; Kang, D.-H. Antimicrobial Efficacy of Cold Plasma Treatment against Food-Borne Pathogens on Various Foods. J. Phys. D Appl. Phys. 2020, 53, 204003. [Google Scholar] [CrossRef]
- Ziuzina, D.; Han, L.; Cullen, P.J.; Bourke, P. Cold Plasma Inactivation of Internalised Bacteria and Biofilms for Salmonella Enterica Serovar Typhimurium, Listeria Monocytogenes and Escherichia Coli. Int. J. Food Microbiol. 2015, 210, 53–61. [Google Scholar] [CrossRef]
- Hayashi, N.; Yagyu, Y. Treatment of Protein Using Oxygen Plasma Produced by RF Discharge. Trans. Mater. Res. Soc. Jpn. 2008, 33, 791–794. [Google Scholar] [CrossRef]
- Sarangapani, C.; Ryan Keogh, D.; Dunne, J.; Bourke, P.; Cullen, P.J. Characterisation of Cold Plasma Treated Beef and Dairy Lipids Using Spectroscopic and Chromatographic Methods. Food Chem. 2017, 235, 324–333. [Google Scholar] [CrossRef]
- Gross, K.C.; Wang, Y.; Saltveit, M. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; United States Department of Agriculture: Washington, DC, USA, 2016; Volume 66. [Google Scholar]
- Bhat, M.Y.; Dar, T.A.; Rajendrakumar Singh, L. Casein Proteins: Structural and Functional Aspects. In Milk Proteins—From Structure to Biological Properties and Health Aspects; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook with CD-ROM, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Carrera, C.S.; Dardanelli, J.L. Water Deficit Modulates the Relationship between Temperature and Unsaturated Fatty Acid Profile in Soybean Seed Oil. Crop Sci. 2017, 57, 3179–3189. [Google Scholar] [CrossRef]
- Obando, M.; Soto, E.; de Meulenaer, B. Influence of Oxidized Oils on Digestibility of Caseins in O/W Emulsions. Eur. J. Lipid Sci. Technol. 2018, 120, 1700331. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, C.; Yoon, J. Kinetics and Mechanisms of DMSO (Dimethylsulfoxide) Degradation by UV/H2O2 Process. Water Res. 2004, 38, 2579–2588. [Google Scholar] [CrossRef]
- Pekárek, S. Non-Thermal Plasma Ozone Generation. Acta Polytech. 2003, 43, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Moiseev, T.; Misra, N.N.; Cullen, P.J.; Mosnier, J.P.; Keener, K.M.; Bourke, P. Influence of High Voltage Atmospheric Cold Plasma Process Parameters and Role of Relative Humidity on Inactivation of Bacillus Atrophaeus Spores inside a Sealed Package. J. Hosp. Infect. 2014, 88, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, F.; Stratakos, A.C.; Koidis, A.; Berardinelli, A.; Cevoli, C.; Ragni, L.; Mancusi, R.; Manfreda, G.; Trevisani, M. Atmospheric Cold Plasma Process for Vegetable Leaf Decontamination: A Feasibility Study on Radicchio (Red Chicory, Cichorium Intybus L.). Food Control 2016, 60, 552–559. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella Enterica, Shiga Toxin-Producing Escherichia Coli, and Listeria Monocytogenes by a Novel Surface Discharge Cold Plasma Design. Food Control 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Ziuzina, D.; Misra, N.N.; Han, L.; Cullen, P.J.; Moiseev, T.; Mosnier, J.P.; Keener, K.; Gaston, E.; Vilaró, I.; Bourke, P. Investigation of a Large Gap Cold Plasma Reactor for Continuous In-Package Decontamination of Fresh Strawberries and Spinach. Innov. Food Sci. Emerg. Technol. 2020, 59, 102229. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.J.; Park, S.; Alahakoon, A.U.; Kim, K.; Choe, W.; Jo, C. Evaluation of Pathogen Inactivation on Sliced Cheese Induced by Encapsulated Atmospheric Pressure Dielectric Barrier Discharge Plasma. Food Microbiol. 2015, 46, 46–50. [Google Scholar] [CrossRef]
- Critzer, F.J.; Kelly-Wintenberg, K.; South, S.L.; Golden, D.A. Atmospheric Plasma Inactivation of Foodborne Pathogens on Fresh Produce Surfaces. J. Food Prot. 2007, 70, 2290–2296. [Google Scholar] [CrossRef]
- Cataldo, F. On the Action of Ozone on Proteins. Polym. Degrad. Stab. 2003, 82, 105–114. [Google Scholar] [CrossRef]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Mechanisms of Nitric Oxide-Related Antimicrobial Activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Kato, T.; Okamoto, Y. Damage of Amino Acids and Proteins Induced by Nitrogen Dioxide, a Free Radical Toxin, in Air. Free Radic. Biol. Med. 1994, 16, 373–382. [Google Scholar] [CrossRef]
- Klein, B.; Vanier, N.L.; Moomand, K.; Pinto, V.Z.; Colussi, R.; da Rosa Zavareze, E.; Dias, A.R.G. Ozone Oxidation of Cassava Starch in Aqueous Solution at Different PH. Food Chem. 2014, 155, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lii, C.; Liao, C.-D.; Stobinski, L.; Tomasik, P. Effect of Corona Discharges on Granular Starches. J. Food Agric. Environ. 2003, 1, 143–149. [Google Scholar]
- Thirumdas, R.; Kadam, D.; Annapure, U.S. Cold Plasma: An Alternative Technology for the Starch Modification. Food Biophys. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Bie, P.; Pu, H.; Zhang, B.; Su, J.; Chen, L.; Li, X. Structural Characteristics and Rheological Properties of Plasma-Treated Starch. Innov. Food Sci. Emerg. Technol. 2016, 34, 196–204. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Deeyai, P.; Chaiwat, W.; Horrungsiwat, S.; Leejariensuk, K.; Suphantharika, M.; Fuongfuchat, A.; Dangtip, S. Modification of Tapioca Starch by Non-Chemical Route Using Jet Atmospheric Argon Plasma. Carbohydr. Polym. 2014, 102, 790–798. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, S.; Li, X.; Li, L.; Xie, F.; Chen, L. Effect of Oxygen Glow Plasma on Supramolecular and Molecular Structures of Starch and Related Mechanism. Food Hydrocoll. 2014, 37, 69–76. [Google Scholar] [CrossRef]
- Morent, R.; de Geyter, N.; Gengembre, L.; Leys, C.; Payen, E.; van Vlierberghe, S.; Schacht, E. Surface Treatment of a Polypropylene Film with a Nitrogen DBD at Medium Pressure. Eur. Phys. J. Appl. Phys 2008, 43, 289–294. [Google Scholar] [CrossRef]
- Kerr, R.W. The Action of Nitrogen Dioxide on Corn Starch and Its Fractions. J. Am. Chem. Soc. 1950, 72, 816–820. [Google Scholar] [CrossRef]
- Castanha, N.; Miano, A.C.; Jones, O.G.; Reuhs, B.L.; Campanella, O.H.; Augusto, P.E.D. Starch Modification by Ozone: Correlating Molecular Structure and Gel Properties in Different Starch Sources. Food Hydrocoll. 2020, 108, 106027. [Google Scholar] [CrossRef]
- Zhu, L.; Gunn, C.; Beckman, J.S. Bactericidal Activity of Peroxynitrite. Arch Biochem. Biophys. 1992, 298, 452–457. [Google Scholar] [CrossRef]
- Hernandez, P.; Sager, B.; Fa, A.; Liang, T.; Lozano, C.; Khazzam, M. Bactericidal Efficacy of Hydrogen Peroxide on Cutibacterium Acne. Bone Jt. Res. 2019, 8, 3–10. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold Atmospheric Plasma Activated Water as a Prospective Disinfectant: The Crucial Role of Peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Bruno, G.; Wenske, S.; Lackmann, J.W.; Lalk, M.; von Woedtke, T.; Wende, K. On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas. Biomolecules 2020, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.R.G.; Zavareze, E.D.R.; Helbig, E.; de Moura, F.A.; Vargas, C.G.; Ciacco, C.F. Oxidation of Fermented Cassava Starch Using Hydrogen Peroxide. Carbohydr. Polym. 2011, 86, 185–191. [Google Scholar] [CrossRef]
- Zhou, Y.; Park, H.; Kim, P.; Jiang, Y.; Costello, C.E. Surface Oxidation under Ambient Air-Not Only a Fast and Economical Method to Identify Double Bond Positions in Unsaturated Lipids but Also a Reminder of Proper Lipid Processing. Anal. Chem. 2014, 86, 5697–5705. [Google Scholar] [CrossRef]
- Kim, T.J.; Silva, J.L.; Chamul, R.S.; Chen, T.C. Influence of Ozone, Hydrogen Peroxide, or Salt on Microbial Profile, TBARs and Color of Channel Catfish Fillets. J. Food Sci. 2000, 65, 1210–1213. [Google Scholar] [CrossRef]
- Pryor, W.A.; Lightsey, J.W. Mechanisms of Nitrogen Dioxide Reactions: Initiation of Lipid Peroxidation and the Production of Nitrous Acid. Science 1981, 214, 435–437. [Google Scholar] [CrossRef]
- Hogg, N.; Kalyanaraman, B. Nitric Oxide and Lipid Peroxidation. Biochim. Biophys. Acta 1999, 1411, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Rød, S.K.; Hansen, F.; Leipold, F.; Knøchel, S. Cold Atmospheric Pressure Plasma Treatment of Ready-to-Eat Meat: Inactivation of Listeria Innocua and Changes in Product Quality. Food Microbiol. 2012, 30, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Abe, K. CHAPTER 1. Vitamin E: Structure, Properties and Functions. In Food Chemistry, Function and Analysis No. 11; Royal Society of Chemistry: Croydon, UK, 2019; pp. 1–11. ISBN 978-1-78801-240-9. [Google Scholar]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal Dielectric-Barrier Discharge Plasma-Induced Inactivation Involves Oxidative DNA Damage and Membrane Lipid Peroxidation in Escherichia Coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Benevides, C.M.D.J.; Veloso, M.C.D.C.; de Paula Pereira, P.A.; de Andrade, J.B. A Chemical Study of β-Carotene Oxidation by Ozone in an Organic Model System and the Identification of the Resulting Products. Food Chem. 2011, 126, 927–934. [Google Scholar] [CrossRef]
- Jiang, Y.; Loh, T.-P. Catalytic and Direct Methyl Sulfonylation of Alkenes and Alkynes Using a Methyl Sul-fonyl Radical Generated from a DMSO, Dioxygen and Copper System. Chem. Sci. 2014, 5, 4939–4943. [Google Scholar] [CrossRef]
- Bocci, V.; Zanardi, I.; Travagli, V. Answer on Letter to Professor E. I. Nazarov (from Velio Bocci). Available online: http://ozonetherapy.org/answer-bocci-nazarov/ (accessed on 2 December 2022).
- Bocci, V.; Zanardi, I.; Travagli, V. Oxygen/Ozone as a Medical Gas Mixture. A Critical Evaluation of the Various Methods Clarifies Positive and Negative Aspects. Med. Gas Res. 2011, 1, 6. [Google Scholar] [CrossRef]
- Kang, M.H.; Hong, Y.J.; Attri, P.; Sim, G.B.; Lee, G.J.; Panngom, K.; Kwon, G.C.; Choi, E.H.; Uhm, H.S.; Park, G. Analysis of the Antimicrobial Effects of Nonthermal Plasma on Fungal Spores in Ionic Solutions. Free Radic. Biol. Med. 2014, 72, 191–199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).