UPLC-QTOF-MS-Based Metabolomics and Antioxidant Capacity of Codonopsis lanceolata from Different Geographical Origins
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Materials and Chemicals
2.2. Preparation of Samples for UPLC-QTOF-MS Analysis
2.3. UPLC-QTOF-MS Analysis and Data Processing
2.4. Preparation of the Ethanolic Extract of C. lanceolata
2.5. Determination of Bioactive Compounds
2.6. Biological Assays
2.6.1. Cell Culture
2.6.2. Determination of Intracellular ROS Scavenging Activity
2.7. Determination of Antioxidant Capacity
2.7.1. DPPH Radical Scavenging Assay
2.7.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7.3. Antioxidant Assay
2.8. Statistical Analysis
3. Results and Discussion
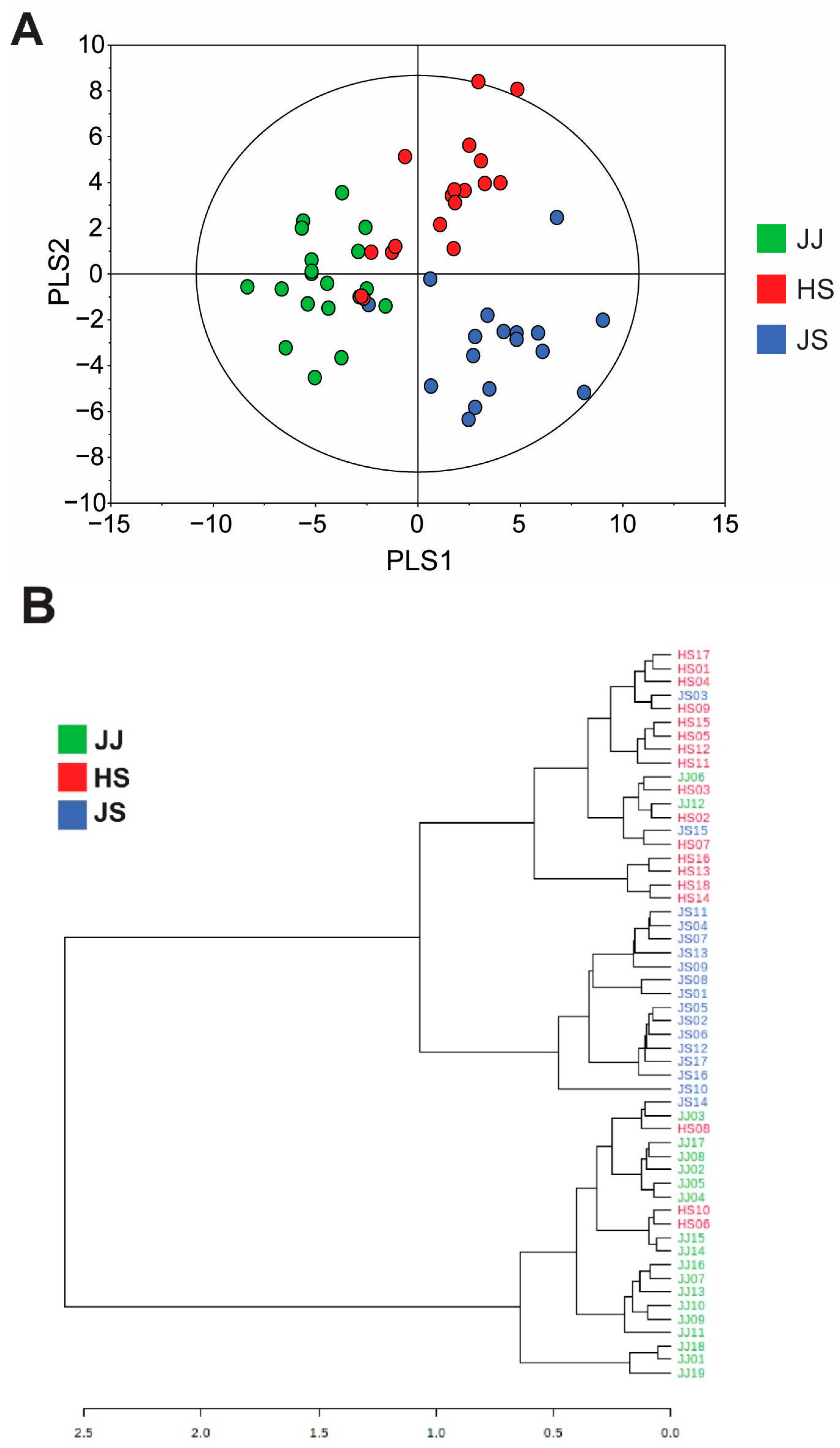
3.1. Metabolite Profiling of C. lanceolata Extracts Using UPLC-QTOF-MS
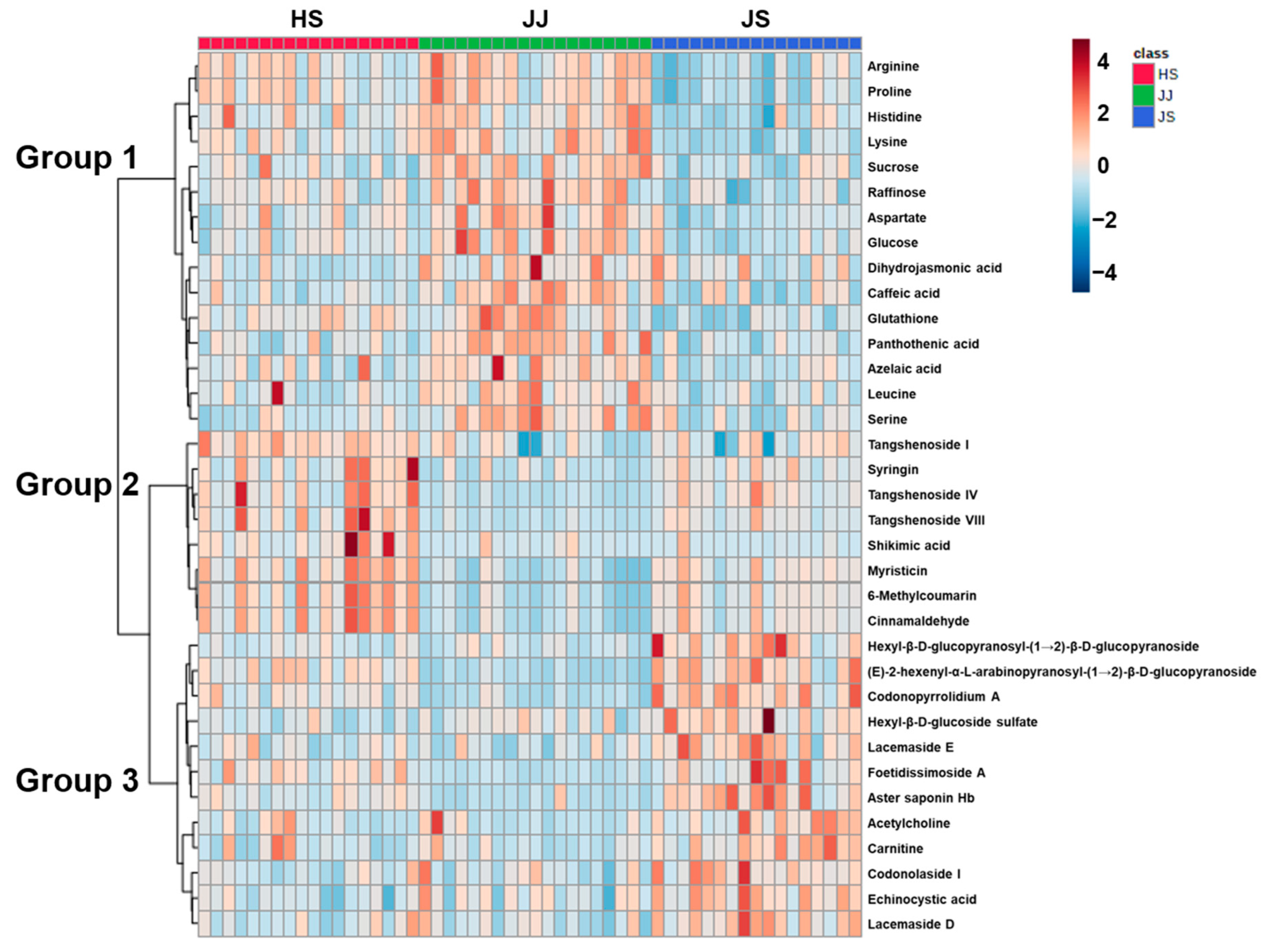
3.2. Differences in C. lanceolata Metabolites According to Geographical Origin
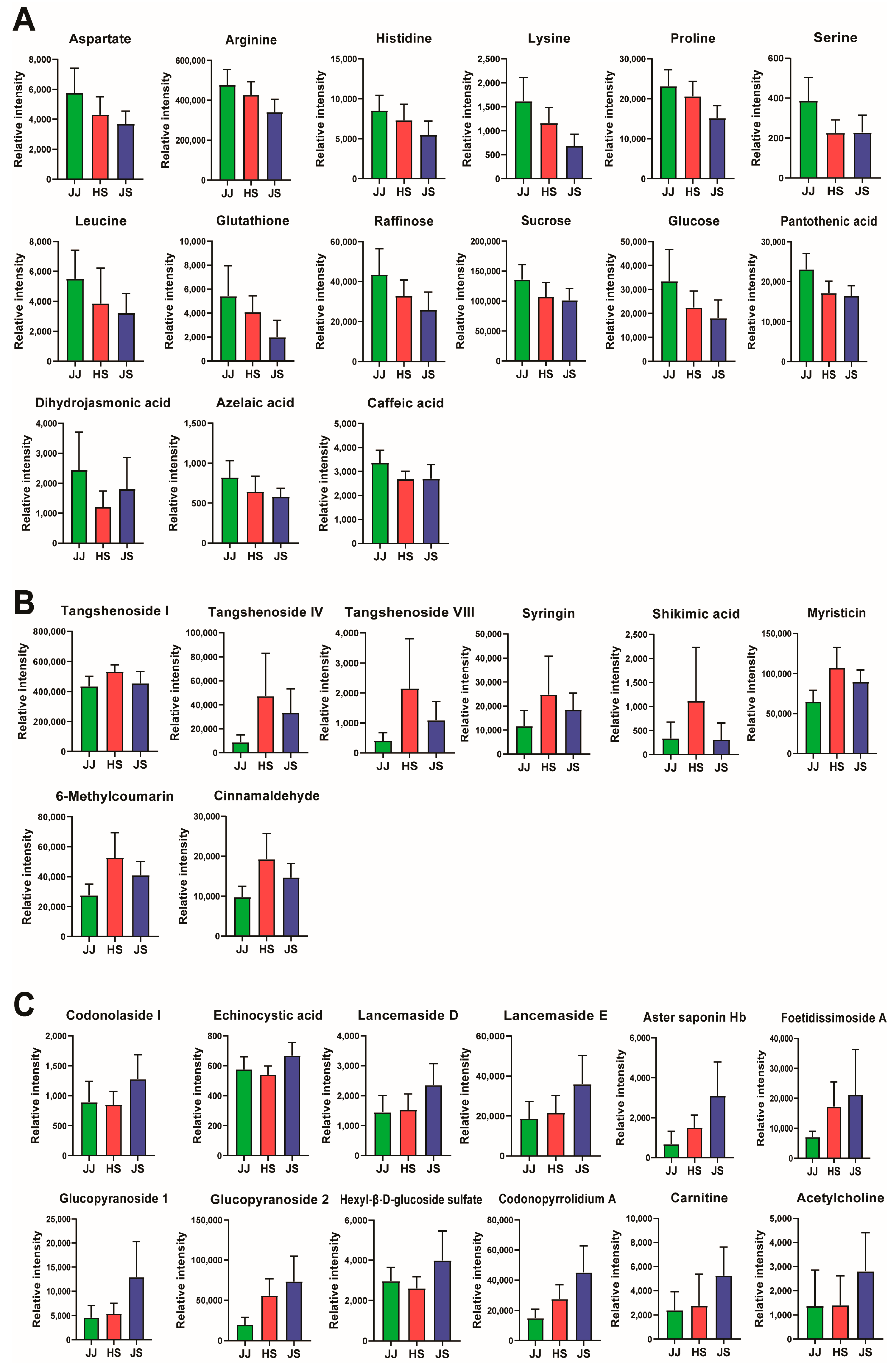
3.3. Determination of the Bioactive Compound Content in C. lanceolata
3.4. Determination of Antioxidant Capacity
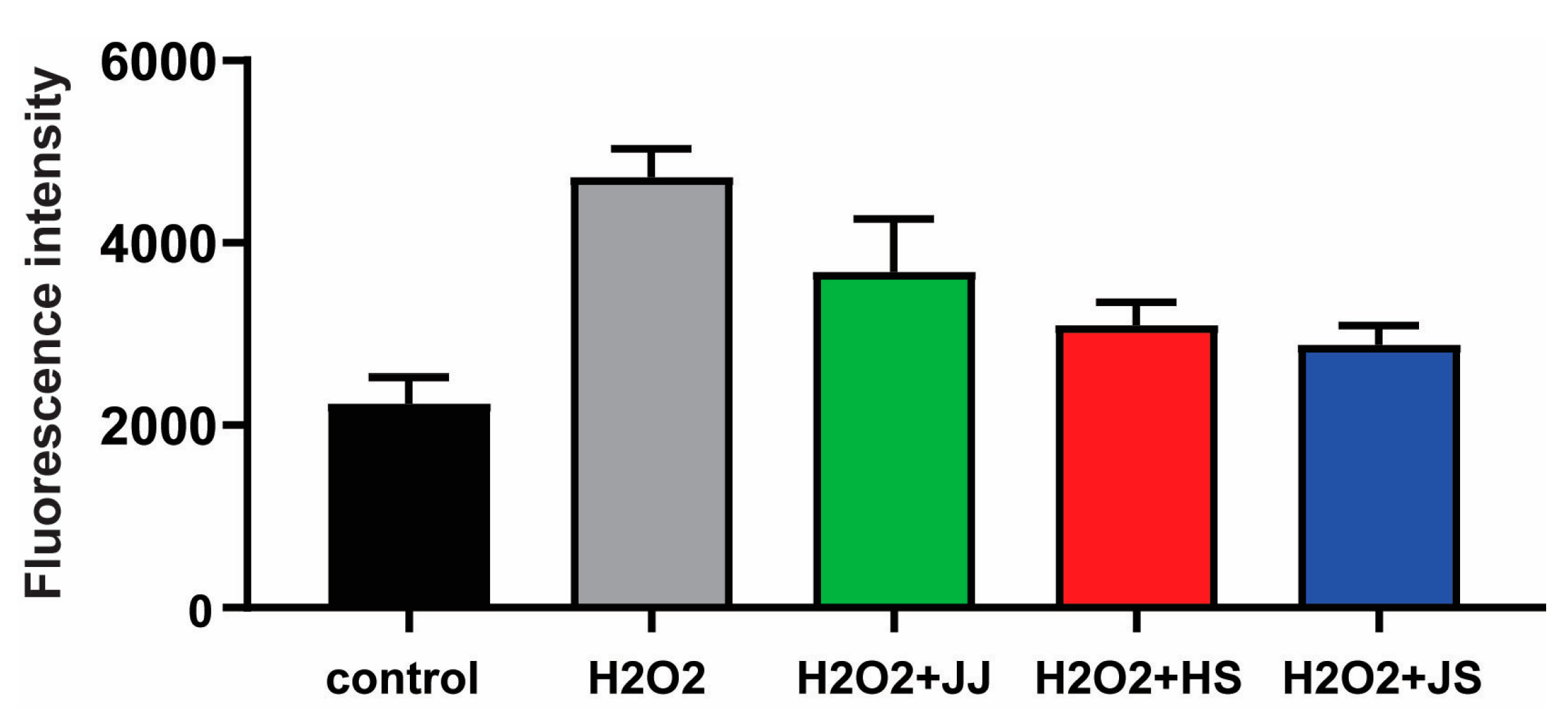
3.5. Cellular Antioxidant Capacity in H2O2-Stimulated HepG2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, B.S.; Kim, J.Y.; Jang, M.; Kim, G.-C.; Park, Y.-H.; Hwang, I.G. Quantitative analysis of tangshenoside I and lobetyolin from Korean deoduk (Codonopsis lanceolata). Korean J. Food Nutr. 2018, 31, 957–963. [Google Scholar]
- He, J.-Y.; Ma, N.; Zhu, S.; Komatsu, K.; Li, Z.-Y.; Fu, W.-M. The genus Codonopsis (Campanulaceae): A review of phytochemistry, bioactivity and quality control. J. Nat. Med. 2015, 69, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.E.; Lee, Y.G.; Cho, J.Y. Regulatory effects of Codonopsis lanceolata on gene expression of GM-CSF in macrophage-like cells. J. Ethnopharmacol. 2009, 123, 185–189. [Google Scholar] [CrossRef]
- Hossen, M.J.; Kim, M.Y.; Kim, J.H.; Cho, J.Y. Codonopsis lanceolata: A review of its therapeutic potentials. Phytother. Res. 2016, 30, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.E.; Choi, W.S.; Hong, E.K.; Lee, J.; Rhee, M.H.; Park, H.-J.; Cho, J.Y. Inhibitory effect of saponin fraction from Codonopsis lanceolata on immune cell-mediated inflammatory responses. Arch. Pharm. Res. 2009, 32, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-P.; Wang, H.; Yuan, Z. Triterpenoid saponins with anti-inflammatory activity from Codonopsis lanceolata. Planta Med. 2008, 74, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Seong, D.-H.; Park, D.-S.; Kim, S.-S.; Gou, J.-Y.; Ahn, J.-H.; Yoon, W.-B.; Lee, H.-Y. Chemical compositions of fermented Codonopsis lanceolata. J. Korean Soc. Food Sci. Nutr. 2009, 38, 396–400. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, W.S.; Chung, J.Y.; Chung, H.C.; Lee, H.Y. Enhancement of cosmeceutical activity from Codonopsis lanceolata extracts by stepwise steaming process. Korean J. Med. Crop Sci. 2013, 21, 204–212. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Song, G.; Kim, M.-H.; Do, S.-G.; Bae, K.-H. Increasement of antioxidative activity in Codonopsis lanceolata adventitious root treated by Methyl jasmonate and salicylic acid. J. Plant Biotechnol. 2013, 40, 178–183. [Google Scholar] [CrossRef]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K.; Castro-Perez, J.; Granger, J.H.; Johnson, K.A.; Smith, B.W.; Plumb, R.S. High resolution “ultra performance” liquid chromatography coupled to oa-TOF mass spectrometry as a tool for differential metabolic pathway profiling in functional genomic studies. J. Proteome Res. 2005, 4, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, F.; Feng, F.; Liu, W. Characterization, quantitation and similarity evaluation of Codonopsis lanceolata from different regions in China by HPLC-Q-TQF-MS and chemometrics. J. Food Compos. Anal. 2017, 62, 134–142. [Google Scholar] [CrossRef]
- An, Y.M.; Jang, H.-J.; Kim, D.-Y.; Baek, N.-I.; Oh, S.-R.; Lee, D.Y.; Ryu, H.W. Selecting marker substances of main producing area of Codonopsis lanceolata in Korea using UPLC-QTOF-MS analysis. J. Appl. Biol. Chem. 2021, 64, 245–251. [Google Scholar] [CrossRef]
- Woldegiorgis, A.Z.; Abate, D.; Haki, G.D.; Ziegler, G.R. Antioxidant property of edible mushrooms collected from Ethiopia. Food Chem. 2014, 157, 30–36. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar]
- Bak, M.-J.; Jeong, W.-S.; Kim, K.-B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int. J. Mol. Med. 2014, 34, 1516–1522. [Google Scholar] [CrossRef]
- Yang, F.; Song, Y.; Zhang, S.; Zhou, W. An LC-MS/MS method for determination of calceorioside B with cardiomyocyte protective activity in rat plasma and application to a pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 1619–1622. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ohta, S.; Komoto, N.; Ushijima, M.; Kodera, Y.; Hayama, M.; Shirota, O.; Sekita, S.; Kuroyanagi, M. Rapid identification of triterpenoid saponins in the roots of Codonopsis lanceolata by liquid chromatography–mass spectrometry. J. Nat. Med. 2008, 62, 423–429. [Google Scholar] [CrossRef]
- Ma, X.-Q.; Leung, A.K.M.; Chan, C.L.; Su, T.; Li, W.-D.; Li, S.-M.; Fong, D.W.F.; Yu, Z.-L. UHPLC UHD Q-TOF MS/MS analysis of the impact of sulfur fumigation on the chemical profile of Codonopsis Radix (Dangshen). Analyst 2014, 139, 505–516. [Google Scholar]
- Li, M.; Hua, S.; Huang, X.; Yue, H.; Chen, C.; Liu, S. Non-targeted metabonomics to investigate the differences in the properties of ginseng and American ginseng based on rapid resolution liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J. Sep. Sci. 2021, 44, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-C.; Wu, Y.-F.; Yu, W.-Y.; Yu, B.; Ying, H.-Z. Polyacetylenes from Codonopsis lanceolata Root Induced Apoptosis of Human Lung Adenocarcinoma Cells and Improved Lung Dysbiosis. Biomed. Res. Int. 2022, 2022, 7713355. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lin, Z.; Yuan, Z. Tangshenosides from Codonopsis lanceolata roots. Phytochem. Lett. 2013, 6, 567–569. [Google Scholar] [CrossRef]
- Li, J.-P.; Liang, Z.-M.; Yuan, Z. Triterpenoid saponins and anti-inflammatory activity of Codonopsis lanceolata. Pharmazie 2007, 62, 463–466. [Google Scholar]
- Jung, Y.; Lee, J.; Kim, H.K.; Moon, B.C.; Ji, Y.; Hwang, G.-S. Metabolite profiling of Curcuma species grown in different regions using 1 H NMR spectroscopy and multivariate analysis. Analyst 2012, 137, 5597–5606. [Google Scholar] [CrossRef]
- Barneix, A.J.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar]
- Yun, D.-Y.; Kang, Y.-G.; Kim, E.-H.; Kim, M.; Park, N.-H.; Choi, H.-T.; Go, G.H.; Lee, J.H.; Park, J.S.; Hong, Y.-S. Metabolomics approach for understanding geographical dependence of soybean leaf metabolome. Food Res. Int. 2018, 106, 842–852. [Google Scholar] [CrossRef]
- Lunáčková, L.; Masarovičová, E.; Lux, A. Respiration rate and chemical composition of Karwinskia roots as affected by temperature. Biol. Plant. 2000, 43, 611–613. [Google Scholar] [CrossRef]
- Park, H.E.; Lee, S.-Y.; Hyun, S.-H.; Kim, D.Y.; Marriott, P.J.; Choi, H.-K. Gas chromatography/mass spectrometry-based metabolic profiling and differentiation of ginseng roots according to cultivation age using variable selection. J. AOAC Int. 2013, 96, 1266–1272. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yang, C.; Zhu, P.; Tian, C.; Liang, A. Protective effects of syringin against oxidative stress and inflammation in diabetic pregnant rats via TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 2020, 131, 110681. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Park, K.-T.; Choung, S.-Y. Codonopsis lanceolata and its active component Tangshenoside I ameliorate skeletal muscle atrophy via regulating the PI3K/Akt and SIRT1/PGC-1α pathways. Phytomedicine 2022, 100, 154058. [Google Scholar] [CrossRef] [PubMed]
- Sá, R.D.C.D.S.E.; Nalone Andrade, L.; dos Reis Barreto de Oliveira, R.; de Sousa, D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014, 19, 1459–1480. [Google Scholar]
- Kang, J.-K.; Chung, Y.-C.; Hyun, C.-G. Anti-Inflammatory Effects of 6-Methylcoumarin in LPS-Stimulated RAW 264.7 Macrophages via Regulation of MAPK and NF-κB Signaling Pathways. Molecules 2021, 26, 5351. [Google Scholar] [CrossRef]
- Babu, P.S.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde—A potential antidiabetic agent. Phytomedicine 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Yendo, A.C.; de Costa, F.; Gosmann, G.; Fett-Neto, A.G. Production of plant bioactive triterpenoid saponins: Elicitation strategies and target genes to improve yields. Mol. Biotechnol. 2010, 46, 94–104. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, J.; Yoo, D.S.; Lee, Y.G.; Byeon, S.E.; Hong, E.K.; Cho, J.Y. Protective effect of stress-induced liver damage by saponin fraction from Codonopsis lanceolata. Arch. Pharm. Res. 2009, 32, 1441–1446. [Google Scholar] [CrossRef]
- Solecka, D. Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol. Plant. 1997, 19, 257–268. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An overview. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 153–167. [Google Scholar]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.S.; Augusta, I.M.; da Conceição Braz, M.V.; Riger, C.J.; Prudêncio, E.R.; Sawaya, A.C.H.F.; Sampaio, G.R.; da Silva Torres, E.A.F.; Saldanha, T. Aroeira fruit (Schinus terebinthifolius Raddi) as a natural antioxidant: Chemical constituents, bioactive compounds and in vitro and in vivo antioxidant capacity. Food Chem. 2020, 315, 126274. [Google Scholar] [CrossRef] [PubMed]
| Area | Coordinates (N,E) | Average Annual Temperature | Age (Years) |
|---|---|---|---|
| Jeju | 33°29′58.636″ N 126°31′52.277″ E | 16.5 °C | 2–3 |
| Jeongseon | 37°22′50.718″ N 128°39′39.424″ E | 10.7 °C | 5–6 |
| Hoengseong | 37°29′30.325″ N 127°59′5.748″ E | 11.3 °C | 3–4 |
| Bioactive Compounds | C. lanceolata | p-Value | ||
|---|---|---|---|---|
| JJ | HS | JS | ||
| TPC (mg GAE/100 g dry weight) | 349.98 ± 71.70 | 409.62 ± 42.04 * | 426.98 ± 70.05 ## | 0.0057 |
| TFC (mg QE/100 g dry weight) | 185.49 ± 32.61 | 256.94 ± 33.30 **** | 281.00 ± 42.50 #### | <0.0001 |
| Assays | C. lanceolata | p-Value | ||
|---|---|---|---|---|
| JJ | HS | JS | ||
| DPPH radical scavenging assay (IC50 mg/mL) | 20.04 ± 5.72 | 13.89 ± 2.03 *** | 15.31 ± 2.59 # | 0.0006 |
| FRAP assay (µmol FE/g dry weight) | 22.53 ± 7.12 | 32.06 ± 6.55 *** | 31.22 ± 6.05 ### | <0.0001 |
| Antioxidant assay (µmol TE/g dry weight) | 8.19 ± 3.02 | 9.40 ± 2.05 | 10.14 ± 2.60 | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, M.; Jo, S.r.; Kim, Y.-C.; Kim, M.-S. UPLC-QTOF-MS-Based Metabolomics and Antioxidant Capacity of Codonopsis lanceolata from Different Geographical Origins. Foods 2023, 12, 267. https://doi.org/10.3390/foods12020267
Nam M, Jo Sr, Kim Y-C, Kim M-S. UPLC-QTOF-MS-Based Metabolomics and Antioxidant Capacity of Codonopsis lanceolata from Different Geographical Origins. Foods. 2023; 12(2):267. https://doi.org/10.3390/foods12020267
Chicago/Turabian StyleNam, Miso, Sae rom Jo, Young-Chan Kim, and Min-Sun Kim. 2023. "UPLC-QTOF-MS-Based Metabolomics and Antioxidant Capacity of Codonopsis lanceolata from Different Geographical Origins" Foods 12, no. 2: 267. https://doi.org/10.3390/foods12020267
APA StyleNam, M., Jo, S. r., Kim, Y.-C., & Kim, M.-S. (2023). UPLC-QTOF-MS-Based Metabolomics and Antioxidant Capacity of Codonopsis lanceolata from Different Geographical Origins. Foods, 12(2), 267. https://doi.org/10.3390/foods12020267




